La inmunosupresión basada en los inhibidores de la calcineurina (INC) ha hecho posible la reducción del rechazo agudo en el trasplante renal y el aumento de la supervivencia del injerto a corto pero no a largo plazo, siendo la muerte del receptor con injerto funcionante y la disfunción crónica del injerto sus principales causas. Los fármacos inhibidores de la m-Tor son inmunosupresores con capacidad antiproliferativa y antimigratoria, lo que les confiere un potencial papel protector en la disfunción del injerto renal, una mejora del perfil cardiovascular y una reducción en el desarrollo de neoplasias; todo ello podría, teóricamente, preservar la función renal y la vida del paciente a largo plazo en un grupo seleccionado de pacientes. Por otro lado, el uso de monoterapia en un paciente facilita su adherencia a cualquier tratamiento crónico. El objetivo del estudio es evaluar el uso de inhibidores de la m-Tor en monoterapia a largo plazo en un grupo seleccionado de receptores de trasplante renal de bajo riesgo inmunológico mediante un estudio observacional y prospectivo, desarrollado en el período 2001-2011. Los pacientes en tratamiento con inhibidores de la m-Tor junto con micofenolato mofetilo o prednisona fueron evaluados inmunológicamente de cara a ser incluidos en el protocolo de monoterapia con inhibidores de la m-Tor. Se incluyeron los pacientes considerados de bajo riesgo inmunológico: sin antecedentes de episodios previos de rechazo agudo, ausencia de anticuerpos antidonante específico y una producción de ATP inferior a 520 ng/dl. La valoración inmunológica se repitió a los 3 y 12 meses del inicio de la monoterapia. La población del estudio fueron 47 receptores de trasplante renal procedente de donante cadáver en tratamiento con inhibidores de la m-Tor en monoterapia, 34 con sirolimus y 13 con everolimus, con una edad media de 55,6 ± 12,5 (23-75 años), 25 varones y 22 mujeres. En el momento de incluirse en el estudio, 18 de ellos (38,2%) recibían prednisona y 29 (61,7%) micofenolato mofetilo, procediéndose a la suspensión de ambos fármacos. Un total de 5 pacientes habían recibido inducción sin INC, mientras que 42 pacientes fueron convertidos desde regímenes con INC por efectos secundarios de éstos o neoplasias. El seguimiento medio de los pacientes en monoterapia fue de 46,9 meses (IC 95%: 38,8-55,0). Durante su evolución, 7 de los 47 receptores incluidos en protocolo (11,5%) precisaron cambio la inmunosupresión por diferentes efectos adversos del tratamiento, sin pérdida del injerto durante el siguiente año. A lo largo del seguimiento, 4 pacientes (8,5%) perdieron el injerto debido en un caso a una muerte súbita con injerto funcionante y en tres casos a rechazo crónico. Se objetivó un episodio de rechazo agudo (2,1%) que respondió favorablemente al tratamiento con esteroides y reconversión a FK 506 y micofenolato mofetilo. Este paciente no desarrolló durante el seguimiento anticuerpos antidonante específico y mantuvo un nivel de activación linfocitaria bajo, con una producción de ATP de 170 ng/dl. Durante el estudio, ningún receptor desarrolló anticuerpos antidonante específico y todos mantuvieron los niveles de activación linfocitaria por debajo de 520 ng/dl. La supervivencia del injerto y el paciente fue del 100% al año y del 88,7 y 95,7%, respectivamente, a los cinco años. El porcentaje de pacientes que se mantuvieron en monoterapia fue de 97,9% al año y del 70,5% a los cinco años. La función renal mejoró en los 36 pacientes que se mantuvieron en monoterapia, pasando la creatinina media en plasma de 2,16 ± 1,05 mg/dl a 1,49 ± 0,56 mg/dl (p = 0,001) al final del seguimiento, y el filtrado glomerular estimado de 39,23 ± 25,23 a 52,23 ± 23,20 ml/mn (p = 0,001), con un incremento no significativo de la proteinuria de 306,6 ± 400 a 418,1 ± 514,1 mg/24 h (p = 0,17). El uso de factores eritropoyéticos e inhibidores de la enzima convertidora de angiotensina/antagonistas de los receptores de la angiotensina II se incrementó significativamente con el tratamiento m-Tor, pero no así el peso corporal, el uso de hipolipemiantes e hipotensores o el porcentaje de receptores con diabetes mellitus. Durante el seguimiento no se detectó ningún caso de falta de adherencia al tratamiento. Podemos concluir que la monoterapia con inhibidores de la m-Tor es una inmunosupresión eficaz a largo plazo en un grupo seleccionado de receptores de trasplante renal.
Calcineurin inhibitors have reduced acute rejection rates and improved short-term graft survival, but without any improvement in long-term outcomes, since calcineurin inhibitors cause nephrotoxicity and death with a functioning graft. mTOR inhibitors have antiproliferative and anti-angiogenic effects with no nephrotoxicity. These properties could improve patient and graft long-term survival rates in select transplant recipients. In addition, monotherapy always diminishes the rate of non-compliance in chronic patients. We examined the evolution of 47 low immunological risk kidney transplant recipients with mTOR inhibitor monotherapy. The mean age was 45±10 years (range: 18-69 years), with 25 males y 22 females. We performed an immunological evaluation before and at 3 and 12 months after starting monotherapy by detection of donor-specific antibodies by microsphere cytometry and the determination of lymphocyte activity with production of ATP by CD4+ T-lymphocytes activated by PHA mitogen. We considered patients to be of low immunological risk when the patient had an ATP production less than 520ng/dl and no history of acute rejection episode or donor-specific antibodies. Initially, 5 patients received immunosuppression induction without calcineurin inhibitors (mycophenolate, prednisone, mTOR inhibitors and anti-CD25), and 42 were converted to mTOR inhibitors due to secondary effects of calcineurin inhibitors or malignancies. A total of 34 recipients had received sirolimus and 13 everolimus. Eighteen out 47 patients (38.2%) received prednisone and 29 (61.7%) mycophenolate with mTor before starting monotherapy. The mean follow-up period after starting monotherapy was 46.9 months (95% CI: 38.8-55.0 months). At the end of the follow-up, 7 out of 47 recipients (11.5%) had to change immunosuppression without losing their grafts after 1 year, due to heavy proteinuria in 2 cases, pulmonary infection in 1, acute rejection in 1, hepatotoxicity in 1, vasculitis in 1 and a temporary inclusion on dialysis after acute pyelonephritis in 1. Four out of 47 patients (8.5%) lost their grafts, as a result of chronic rejection in 3 cases, and as a result of death with a functioning graft in 1. The rate of acute rejection was 2.1%, one episode, which was solved with steroid pulses and switching from mTOR inhibitors to tacrolimus and mycophenolate. No patients developed donor-specific antibodies, and all of them maintained an ATP production less than 520ng/dl. The rates of graft and recipient survival were both 100% at 1 year, and 88.7% and 95.7% at 5 years. The percentages of patients on monotherapy were 97.9% and 70.5 % at 1 and 5 years, respectively. At the end of the follow-up, 36 out of 47 recipients remained on mTOR inhibitor monotherapy. Serum creatinine and glomerular filtration rates improved significantly, from 2.16±1.05mg/dl to 1.49±0.56mg/dl (P=.001) and from 39.23±25.23ml/min to 52.23±23.20ml/min (P=.001), respectively. Proteinuria increased but not significantly, from 306.6±400mg/24h to 418.1±514.1mg/24h (P=.17). The patients treated with mTOR inhibitors received significantly more erythropoietin and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers than before starting mTOR, but there was no change in the treatment with statins or hypotensive agents. Body weight and the percentage of diabetic recipients were similar during the study. No cases of non-compliance were observed during the follow-up. The present study supports the safety and efficacy of monotherapy with mTOR inhibitors in select kidney transplant recipients.
Immunosuppression therapy based on calcineurin inhibitors (CNI) has produced a reduction in the incidence of acute rejection in kidney transplants and an increase in short-term graft survival rates.1
However, long-term transplant survival has not increased in a similar manner,2 with death of patient with a functioning graft3 and chronic graft dysfunction4 being the primary causes of graft loss. This is a logical consequence, since CNI increase the typical cardiovascular risk factors,3 including renal failure due to graft dysfunction.4 In addition, CNI have no protective effect against the development of cancer in the recipient.5,6
mTor inhibitor drugs are immunosuppressants that have no nephrotoxic effects and have the capacity for preventing cell proliferation and migration by blocking the intracellular signalling pathway that regulates the proliferation of activated T-cells.7 This confers these cells with a potential protective role against renal graft dysfunction, improves patient cardiovascular profiles and reduces the rate of cancer by also substantially reducing rates of angiogenesis.7 However, these properties also limit its early use in transplant recipients,8 due to increased rates of surgical complications caused by the interference of these drugs with the scarring process9 and higher acute rejection rates than in patients treated with immunosuppression regimens based on CNI,10 although it should be considered for use later in patient evolution.
In addition, and in contrast to CNI, mTor selectively expand the sub-population of regulatory T-lymphocytes (CD4+, CD25, highFOX3+), inducing the regulatory function of T-lymphocytes, naive CD4+, and act synergistically along with costimulation blocks to induce tolerance to the kidney transplant.11,12
Theoretically, these drugs could preserve long-term renal function and patient survival in select cases.
This evidence justifies the conversion to mTor in patients that have no contraindications and can tolerate these drugs, since the rate of removal from treatment due to side effects can reach 25%.13
In addition, monotherapy in these patients favours adherence to treatment,14 which facilitates proper administration of chronic immunosuppression therapy in transplant recipients.
The objective of our study is to evaluate the use of mTor inhibitors as a long-term monotherapy in select low immunological risk kidney transplant recipients.
MATERIAL AND METHODS
Ours was an observational and prospective study carried out during 2001-2011 in a renal transplantation centre.
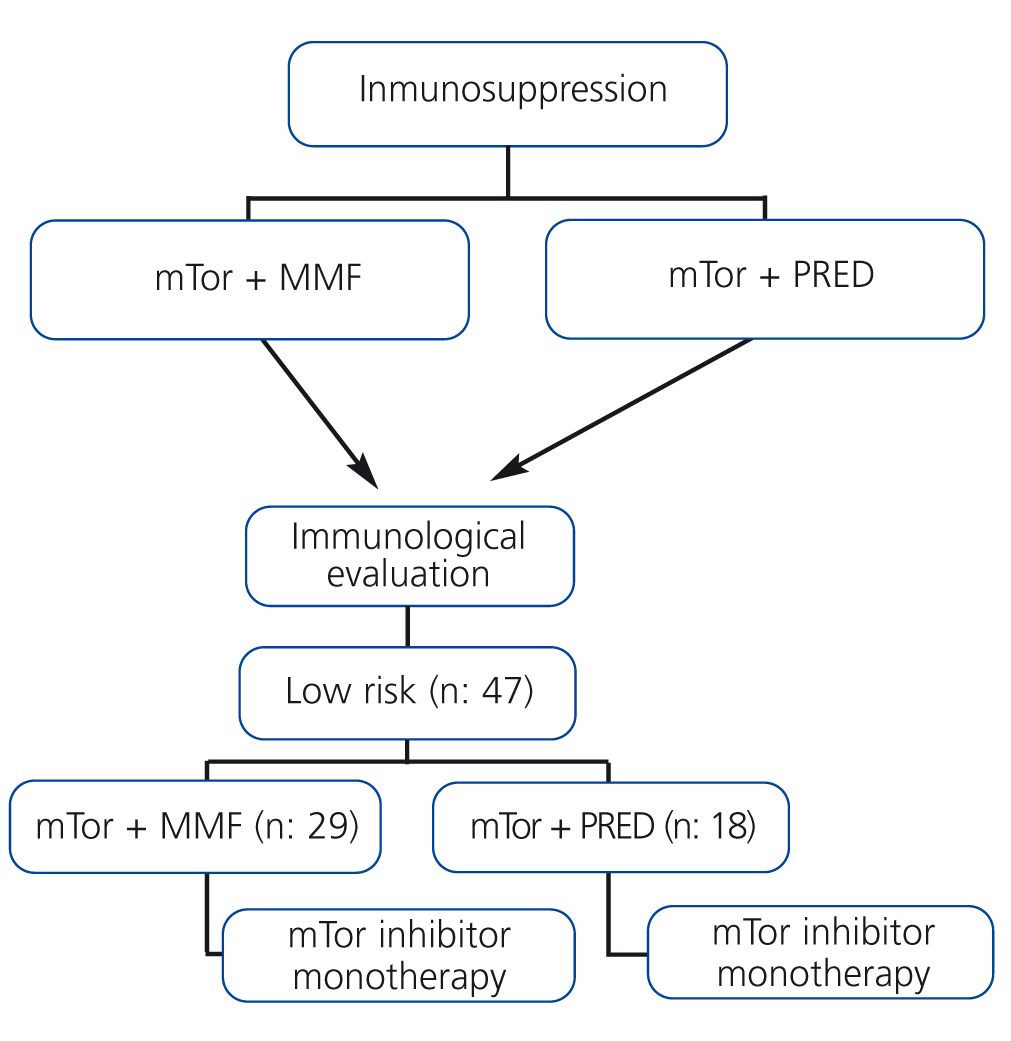
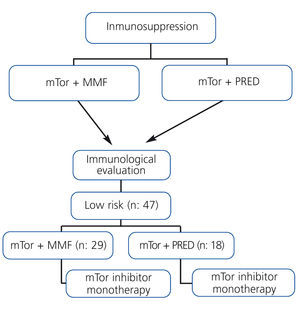
Patients on treatment with mTor along with mycophenolate mofetil or prednisone were evaluated immunologically for inclusion in the monotherapy protocol using mTor (Figure 1).
The immunological evaluation consisted of:
- Measuring donor-specific antibodies using microsphere cytometry (Luminex®).
- Evaluation of lymphocyte activity: production of ATP in cultures of T-lymphocytes and CD4+ activated by PHA mitogen (ImmuKnow CyleX®).
Patients were included in the monotherapy treatment protocol when they complied with the following characteristics: no history of acute rejection within the previous year, no donor-specific antibodies and production of ATP <520ng/dl, implying low immunological risk.
In all patients, we measured the presence of donor-specific antibodies along with the production of ATP at 3 and 12 months after the start of the monotherapy protocol.
The study population consisted of 47 recipients of kidney transplants from cadaveric donors, with a mean age of 55.6±12.5 years (range: 23-75 years). All patients were considered to be of low immunological risk, and 25 patients were male, 22 female. Sirolimus was administered in 34 cases and everolimus in 13. Upon inclusion in the study, 18 of the 47 patients (38.2%) received treatment with prednisone and 29 (61.7%) with mycophenolate mofetil along with mTor, and both drugs were subsequently suspended (Figure 1).
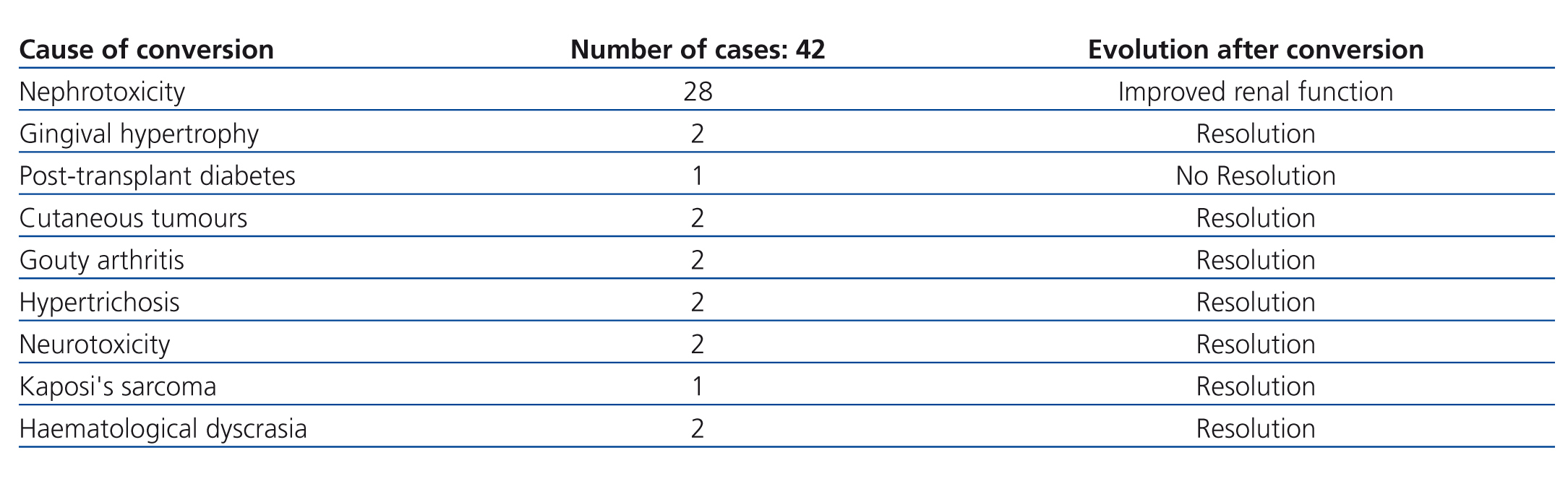
Of the 47 patients, 5 (10.6%) had initially received induction immunosuppression therapy without CNI, consisting of anti-CD25 + mTor + mycophenolate mofetil + prednisone. In 3 of these cases, this treatment was required due to a sub-optimal donor, due to a history of lymphoproliferative disease in one case, and due to a previous history of neurotoxicity from CNI in the last case. The other 42 patients (89.4%) had received treatment with CNI, with conversion to mTor after a mean 20.8 months following transplantation (95% confidence interval [CI]: 14.4-27.1 months) due to various causes that are summarised in Table 1. The conversion from CNI to mTor involved halving the CNI dose during the first week of starting mTor therapy and suspending the CNI in the second week, with sirolimus levels at a mean 10-16ng/dl and everolimus at 6-9ng/dl.
The plasma concentrations of sirolimus and everolimus were determined using immunoassay techniques (ACMIA auto-analyser Dimension XPand®) and high-resolution chromatography-mass spectrometry, respectively. The sensitivity limit of these techniques was 1.7ng/dl and 0.5ng/ml, respectively.
Renal function in recipients on monotherapy was measured using plasma creatinine and estimated glomerular filtration rate values (MDRD-4) at the moment of removal of the CNI therapy, or in the case of induction without CNI, when renal function stabilised post-transplantation and at the end of follow-up.
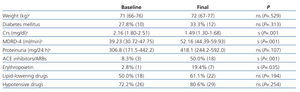
We also evaluated the effect of the mTor therapy on different clinical and laboratory parameters, such as body weight, diabetes mellitus (DM) and the use of medications to treat anaemia, dyslipidaemia, proteinuria and blood pressure, and we compared values between the start of treatment with mTor and at the end of treatment.
Adherence to treatment was evaluated through a personal interview and by measuring drug levels during each follow-up session.
Continuous variables are expressed as mean and 95% CI, or as median and interquartile range, depending on the distribution of the data. For the description of categorical variables, the number and percentage of patients per response category have been used.
We used statistical techniques to ensure compliance with statistical assumptions, prior to using the corresponding parametric tests to compare means and proportions. In the case of non-compliance with established assumptions, we used the corresponding non-parametric tests.
We compared variables between groups using ANOVA tests in continuous variables or the equivalent for non-parametric variables, depending on the inherent characteristics for each study variable, and McNemar tests for analysing categorical variables from related samples.
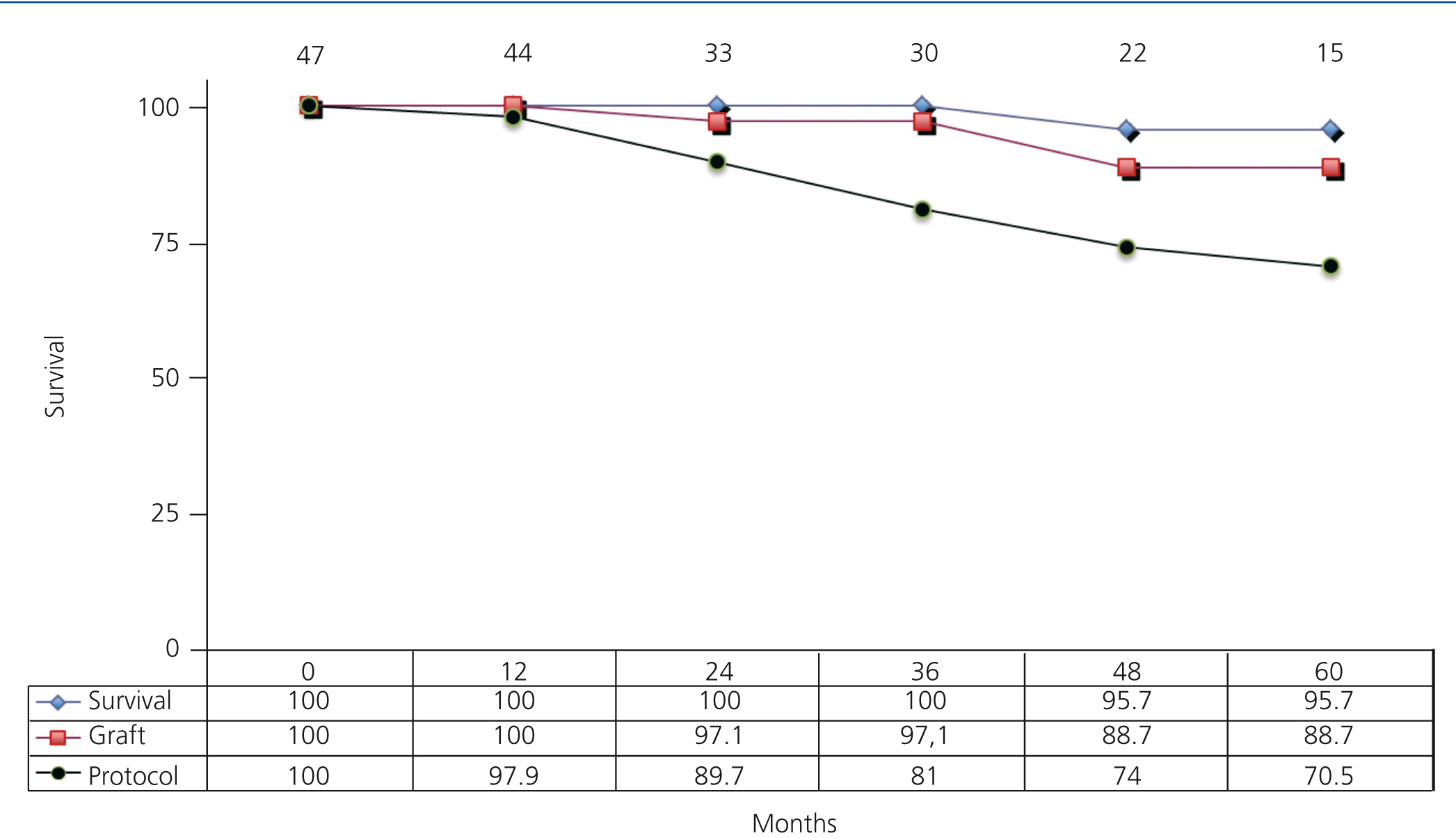
Graft survival, recipient survival, and the percentage of patients that were maintained on mTor inhibitor monotherapy were evaluated using Kaplan-Meier survival curves.
All statistical analyses were carried out using SPSS statistical software, version 19.0. In all statistical tests applied to the study variables, a statistical significance (α) of 0.05 was used.
RESULTS
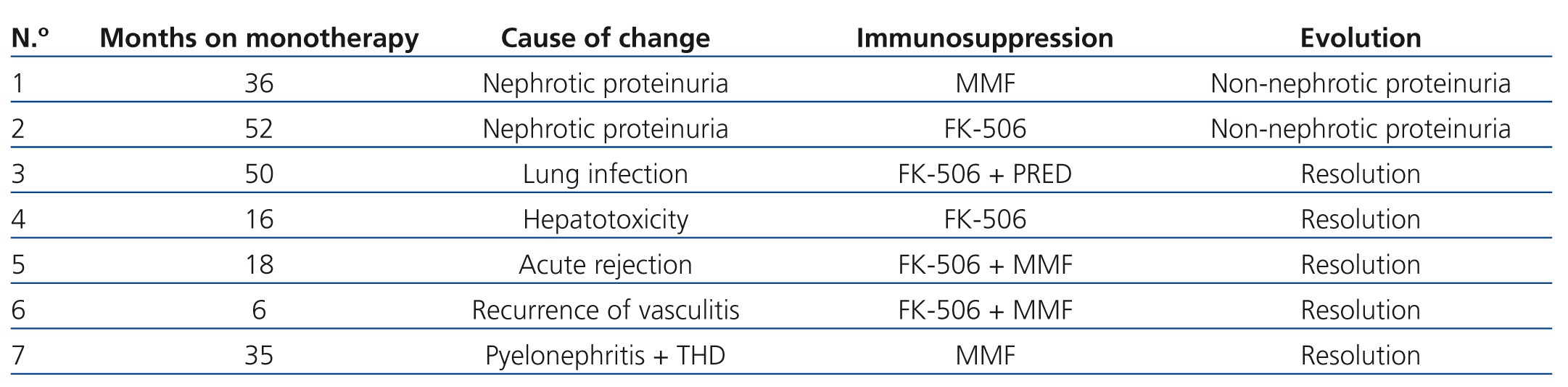
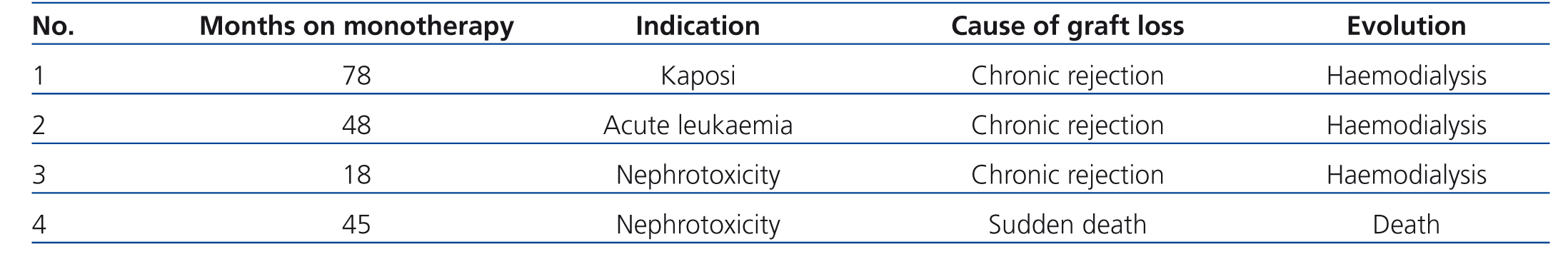
During the study period, 7 of the 47 recipients included in the monotherapy protocol (11.5%) required transfer to a different immunosuppression regimen due to various causes, summarised in Table 2, without graft loss during at least the year following reconversion. Another 4 patients lost their transplanted kidneys due to sudden death with a functioning graft in one case, and chronic rejection in three cases, two of which were due to underlying pathologies that impeded changes to immunosuppression therapy (cases 1 and 2 in Table 3), whereas the conversion to mTor in the third patient involved severe renal failure (case 3, Table 3).
The mean serum level of sirolimus at the end of follow-up was 12.5ng/ml (95% CI: 11.1-13.9ng/ml), whereas the mean level of everolimus was 6.7ng/ml (95% CI: 4.3-9.1ng/ml).
The mean follow-up period of patients on monotherapy was 46.9 months (95% CI: 38.8-55.0 months).
We observed one case of acute rejection (2.1%) that was histologically diagnosed and that responded favourably to treatment with steroids and reconversion to tacrolimus and mofetil (Table 2). This patient did not develop donor-specific antibodies during the follow-up period and maintained a low lymphocyte activation level, producing 170ng/dl ATP.
During follow-up, no recipients developed donor-specific antibodies, and lymphocyte activation levels were maintained below 520ng/dl.
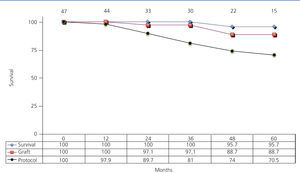
Graft and patient survival were both at 100% after one year, and were 88.7% and 95.7% after five years, respectively. In addition, 97.9% of patients remained on monotherapy after one year and 70.5% after five years (Figure 2).
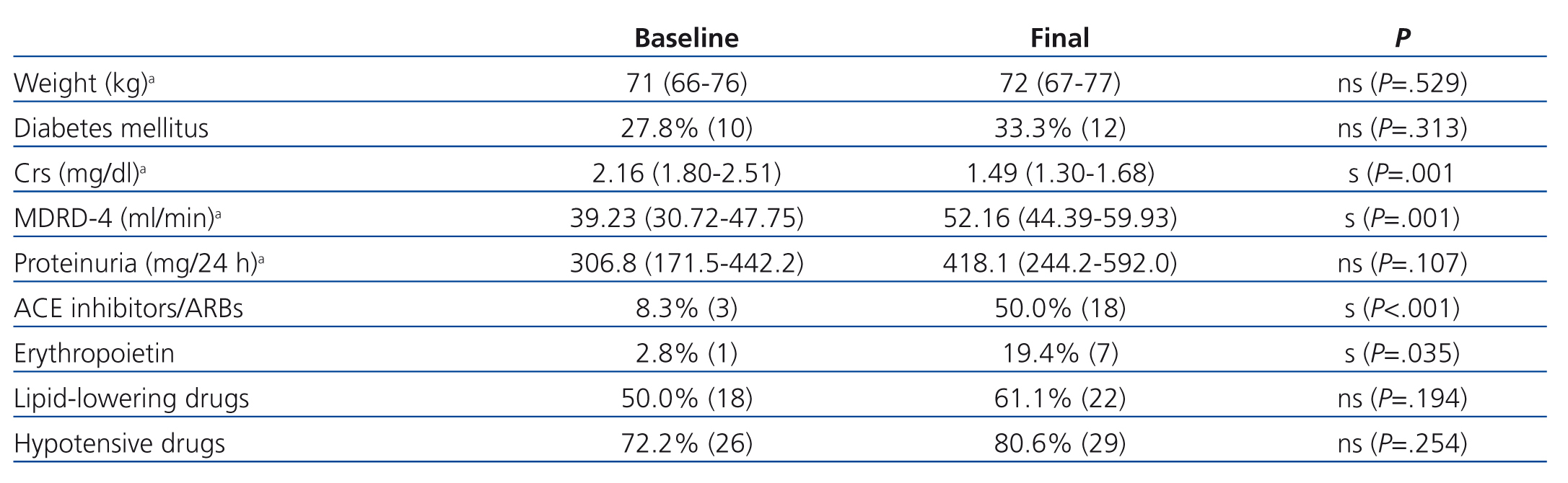
Renal function improved significantly during follow-up in 36 patients that remained on monotherapy, with a non-significant increase in proteinuria (Table 4). We did not observe any significant differences in the evolution of these parameters between patients receiving sirolimus and those receiving everolimus.
The use of erythropoietin and angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs) increased significantly during the follow-up period, but body weight, the use of lipid-lowering drugs and hypotensive drugs, and the percentage of recipients with DM did not (Table 4).
During follow-up, we detected no cases of non-adherence to treatment.
DISCUSSION
The patients in our study treated with mTor inhibitor monotherapy had a 100% rate of adherence to treatment, with no evidence of secondary side effects that would prevent from taking the drug.13 This was to be expected, since the patients included in the monotherapy protocol already had received treatment with mTor with good tolerance, although this was in conjunction with other immunosuppressant drugs. As such, patients that did not tolerate the drug well would have stopped taking it, and therefore could not have been included in the study (Figure 1).
The determining pathology in the conversion from CNI to mTor in patients that required it was resolved in all cases, except for one patient who developed post-transplant de novo DM (Table 1). Despite the expectations surrounding mTor, these drugs can increase the risk of post-transplant DM. In this context, an American study published by Johnston involving a sample of thousands of patients demonstrated that the risk of post-transplant de novo DM increased significantly in patients treated with mTor,15 for which it comes as no surprise that our patient with post-transplant DM maintained this pathology despite conversion from tacrolimus to mTor. We must also point out that the percentage of diabetic patients in our study did not increase significantly during follow-up (Table 4).
Proteinuria was the primary adverse side effect responsible for the removal of patients from the monotherapy protocol with mTor.13 Although it was initially believed that proteinuria was the consequence of removal from CNI, it appears to be in fact an independent effect of the administration of mTor, which in part could be due to glomerular hyper-filtration.16 In this context, two recipients required reconversion to CNI due to proteinuria, with a partial response to conversion (cases 1 and 2, Table 2).
In addition, we would like to bring attention to the significant increase in our study of the use of ACE inhibitors and ARBs following the use of mTor, in an attempt to reduce proteinuria that is induced by these drugs. In two cases, it is improbable that the mTor treatment caused the pathology leading to removal of the patient from the protocol, since hepatotoxicity is a very rare side effect observed in mTor,17 and this patient also had other associated factors that could have been responsible for this pathology (case 4, Table 2). As regards the other recipient (case 3, Table 2), this case involved a pulmonary infection with good response to antibiotics and not a pneumonitis associated with mTor,18 for which the percentage of patients that would have needed removal from mTor in our study would have been lower. However, the rate of removal of patients from the treatment protocol was similar to that of a related study.19
Only one patient in our study developed an episode of acute rejection during the follow-up period (case 5, Table 2), who responded favourably to treatment with steroids and reconversion to tacrolimus and mycophenolate mofetil. This case involved clinical/immunological dissociation, since this patient did not develop donor-specific antibodies during follow-up and maintained low levels of lymphocyte activation, not indicating that the patient probably would experience an episode of acute rejection. The fact that none of the recipients in our study developed donor-specific antibodies and that all maintained low levels of lymphocyte activation during the time on monotherapy, along with the low overall rate of acute rejection observed, suggests sufficient immunosuppressive power of mTor in monotherapy.7
It is also notable that not only did the patients in our study fail to experience a deterioration in renal function that would be expected with the passage of time after transplantation,2 but renal function even improved, a clear indicator of no nephrotoxic effects to accompany the adequate immunosuppression achieved with these drugs.7
A total of four patients lost their transplants during the monotherapy treatment, with changes to immunosuppression contraindicated in two of these cases (cases 1 and 2, Table 3),8 since it was a priority in both to stop the progression of the disease that had led to their inclusion in the treatment protocol.20,21 As regards the last case (case 3, Table 3), the conversion to mTor occurred at a point of advanced chronic renal failure that is currently a contraindication against conversion.22
The use of concomitant medication, above all erythropoietin and ACE inhibitors/ARBs, has increased significantly over the course of the study period, reflecting the presence of known side effects of treatment with mTor, such as increased anaemia, proteinuria, and dyslipidaemia, although these treatments have been well tolerated and have facilitated controlling the adverse effects produced by mTor treatment.13
Despite the fact that monotherapy with mTor is theoretically an attractive treatment option, there are surprisingly very few publications regarding this topic. One study by Pinto et al. was described in a retrospective, multi-centre trial involving 138 patients, with a mean follow-up time of 29 months, in which the rate of rejection was 1.4% and the rate of removal of the immunosuppressant regimen was 14%, with maintained renal function and increased proteinuria; results that are comparable to our own. On the other hand, there is very little information regarding the immunology of these patients that would guide the indications of including a patient on mTor inhibitor monotherapy.19
This study was observational, for which conclusions are limited due to the absence of a control group, but the experience gained was original, and may be useful for daily clinical practice.
We can conclude that monotherapy with mTor is an effective form of long-term immunosuppression in select kidney transplant recipients.
Conflicts of interest
The authors have no conflicts of interest to declare.
Table 1. Reasons for converting from anticalcineurinic inhibitors to mTor inhibitors
Table 2. Reasons for changing immunosuppression therapy in patients treated with mTor inhibitor monotherapy. Time of follow-up, final immunosuppression and evolution
Table 3. Reasons for graft loss in patients treated with mTor inhibitor monotherapy. Indications for inclusion and time of patient evolution
Table 4. Variations in weight, percentage of diabetes patients, Crs, MDRD-4, proteinuria and in use of concomitant drugs during the conversion to mTor and at the end of follow-up
Figure 1. Protocol for inclusion in mTOR inhibitor monotherapy
Figure 2. Actuarial patient and graft survival rates and maintenance on the treatment protocol of patients receiving mTor inhibitor monotherapy