MYH9 related diseases are caused by mutations in the MYH9 gene and constitute a rare group of genetic entities. Its inheritance follows an autosomal dominant pattern. The MYH9 gene, encodes the nonmuscle myosin heavy chain IIA, expressed in different tissues and especially in podocytes and mesangial cells. The disorder is characterized by the presence of macrothrombocytopenia, leukocyte inclusions and a variable risk of developing renal failure, hearing loss and early-onset cataracts. We describe the case of a 27-year-old Caucasian woman, diagnosed initially with idiopathic thrombocytopenic purpura. After a detailed family history and the appearance of renal involvement and hearing loss, genetic testing allowed to make the diagnosis of nephropathy associated with MYH9 mutation. This case is an example of the delayed diagnosis of uncommon diseases and highlights the usefulness genetic testing. A review of the disease is provided.
Las enfermedades relacionadas con mutaciones del gen MYH9 son un grupo de patologías genéticas raras. Su herencia sigue un patrón autosómico dominante en donde el gen MYH9, codifica la cadena pesada de la miosina IIA no muscular que se expresa en diferentes tejidos pero especialmente en los podocitos y en las células mesangiales. Este trastorno se caracteriza por la presencia de macrotrombocitopenia, inclusiones leucocitarias y un riesgo variable de desarrollar insuficiencia renal, hipoacusia y cataratas en edad juvenil o adulta. Describimos el caso de una mujer de 27 años, de raza caucásica, diagnosticada inicialmente de púrpura trombocitopénica idiopática. Tras una detallada historia familiar y el desarrollo de síntomas clínicos posteriores con afectación renal e hipoacusia, se le realizó un estudio genético que nos permitió el diagnóstico de nefropatía asociada a la mutación en el gen MYH9. Este caso destaca el retraso del diagnóstico y la utilidad del estudio genético en pacientes con enfermedades muy poco frecuentes. Se procede a la revisión de la enfermedad en este artículo.
Diseases related to mutations of the MYH9 gene are a group of ultra-light genetic pathologies characterized by the presence of macrotrombocytopenia, leukocytic inclusions with a variable risk of developing renal failure, and hearing loss and cataracts in young or adult age.1 The Italian Registry for diseases associated with mutations in the MYH9 gene includes 108 affected individuals, which indicates that the prevalence of the disorder in Italy is at least 3:1,000,000. Since mild forms are identified incidentally and severe forms are often misdiagnosed, the actual prevalence may be higher.2
It is transmitted as autosomal dominant inheritance, that is to say that the mutated allele is dominant over the normal and a single mutated copy is sufficient to have expression of the disease, being able to affect men and women with equal probability. Therefore, each affected individual has a 50% chance to transmit the disease to their offspring.
Historical evolutionThe group of diseases caused by mutation in the MYH9 gene were grouped into four syndromes characterized by presenting macrotrombocytopenia associated with other diseases. Historically these syndromes corresponded with3–5:
- –
May-Hegglin anomaly.
- –
Fechtner syndrome.
- –
Epstein syndrome.
- –
Sebastian's syndrome.
Both the Epstein syndrome and the Fechtner syndrome, in which renal involvement is present, had been classically considered as variants of the Alport syndrome.6 Conversely, the Sebastian syndrome and May-Hegglin anomaly present without renal involvement, manifested only by the presence of leukocytic inclusions and ocular or auditory involvement.
These four disorders, characterized by thrombocytopenia with giant platelets, were classified according to the morphological aspects of the Döhle bodies and the different combinations of the other manifestations of mutation in MYH9: hearing loss, glomerular nephropathy and cataracts. However, because the phenotype of a person with a pathogenic variant in MYH9 often evolves over time, the clinical expression in an individual may change over time with the manifestation of a new features. In addition, the four syndromes do not define all the possible manifestations derived from the heterozygous pathogenic variants in MYH9. Finally, members of the same family may have different phenotypes and receive different diagnoses within the spectrum of diseases associated with mutations in the MYH9 gene. For these reasons, the pathologies associated with MYH9 have been proposed as a new nosological entity that includes all individuals with heterozygous pathogenic variants in MYH9 regardless of the appearance of the neutrophils and the clinical phenotype.7 The four historically known syndromes are genetically distinct from the Alport syndrome, the main differential diagnosis.8
Advances in the massive sequencing of genes have allowed in recent years the analysis and identification of different variants at the intronic level and even risk polymorphisms or haplotypes in MYH9, that are related with idiopathic focal and segmental glomerulosclerosis, hypertensive nephropathy and end-stage renal disease in both diabetics and non-diabetics patients.9–11 In Spain, the EPIRCE study showed that around 10% of the population has some degree of chronic kidney disease12–14 and Tavira et al. through the Spanish study RENASTUR, evaluated in depth the possible genetic causes of chronicity and demonstrated a certain association between the polymorphism rs3752462 of MYH9 and the risk of developing chronic kidney disease.15 These results are supported by a case-control study from China which describes the association of the polymorphism rs3752462 of MYH9 in patients with hypertension and chronic kidney disease.16 In animal models with variants in MYH9 that affects the cytoskeleton structure of the podocyte, it has been observed that the administration of nephrotoxic drugs (doxorubicin and adriamycin), HIV infection and arterial hypertension favor the development of albuminuria and renal function deterioration of.17–19
PathogenesisMYH9 is a gene composed of 40 exons, located in chromosome 22q and codes for the nonmuscle myosin heavy chain IIA chain (NMMHC IIA). This protein is part of the superfamily of motor proteins, present in all eukaryotic cells, and has important functions in the stabilization of the cytoskeleton. It is a hexamer enzyme with two light chains and two heavy chains that have an N-terminal and a C-terminal domain.20 The mutated NMMHC IIA is not expressed in platelets and it forms aggregates in leukocytes, which results in leukocyte inclusions that are characteristic of this disease. The formation of macro platelets could be explained by an early and ectopic production of platelets in the bone marrow interstitium. In studies conducted with mice megakaryocytes, it is considered that these changes could be due to an alteration in the Rho-associated kinase-myosin light chain (Rho-ROCK-MLC) pathway of thrombopoiesis regulated by the NMMHC IIA.21,22
Abnormalities of heavy chains myosin that are expressed in podocytes and mesangial cells explains the presence of proteinuria and the evolution to chronic renal disease in patients affected.1,17
Clinical characteristics of the pathologies associated with mutations in the MYH9 gene- (a)
Manifestations related to thrombocytopenia:
- –
Frequent hematomas.
- –
Excessive bleeding even after hemostasis measures (major or minor surgeries, treatment with platelet antiaggregants).23
- (b)
Sensorineural hearing loss. It may be presented between the 1st and 6th decade of life.24,25
- (c)
Eye involvement: early onset cataracts (it occurs at early-middle age and it is detected by slit lamp).26,27
Renal involvement: it is observed in 30% of patients with gene mutations. It is characterized by early onset proteinuria, with or without microhematuria, and rapid progression to chronic kidney disease. The nephropathy associated with mutation of the MYH9 gene usually progresses to terminal chronic kidney disease around the age of 30, although cases of more advanced age have o been described.1,14,28
There is family history of an autosomal dominant inheritance pattern. The absence of a family history of disease related to mutations in MYH9 does not exclude the diagnosis. The clinical expression in affected relatives may be very variable.
Analytical abnormalities and microscopic findings- –
Thrombocytopenia.
- –
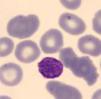
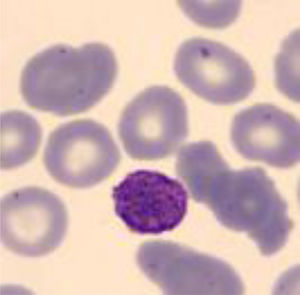
The peripheral blood smear demonstrates the presence of large platelets (mean platelet diameter >3.7μm and/or >40% platelets with diameter >3.9μm) or Döhle-like bodies in neutrophils cytoplasm. It should be noted that Döhle type bodies are present in 42–84% of patients with mutations in MYH9.29
- –
The immunofluorescence of a peripheral blood smear demonstrates typical MYH9 protein aggregates in the cytoplasm of neutrophils.4,30,31 In neutrophils of non affected individuals the MYH9 protein is evenly distributed.
- –
Elevation of serum creatinine levels indicating renal failure and risk of progression to end-stage renal disease.
- –
Chronic elevation of transaminases.32
- –
Urinalysis: proteinuria and microhematuria. Proteinuria is the earliest sign of renal involvement.33–35
If there is a high clinical suspicion, it should be performed an analysis of the MYH9 gene or the use of a panel of genes which also allows to make the differential diagnosis with other pathologies that are clinically similar.36
Phenotype–genotype correlationsThe identification of the MYH9 family-specific pathogenic variant may help to assess the risk of developing the non-congenital clinical manifestations of the disease.
Affected individuals with pathogenic variants involving the major domain of the MYH9 protein show more severe thrombocytopenia as compared with those with pathogenic variants that affect the extreme domain. The risk of developing kidney damage, hearing loss and cataract also depends on the specific MYH9 pathogenic variant.
- •
Replacement p.Asp1424His is associated with an intermediate or high risk of developing non-congenital manifestations of the disease. Most people affected with the variant p.Asp1424His develop kidney disease before age 60. The majority of patients affected by this variant will develop hearing loss within the 60 years of age and the risk of cataracts is higher than with other genotypes.
- •
The substitutions p.Asp1424Asn and p.Glu1841Lys, as well as the nonsense or frameshift pathogenic variants, are associated with a low risk of developing non-congenital manifestations of the disease. In individuals with these pathogenic variants, thrombocytopenia is usually the only manifestation of the disease throughout life.37
The absence of thrombocytopenia in other members of the family does not exclude the presence of a mutation in MYH9 because the frequency of de novo variants is high (35% of the probands).38
The differential diagnosis must take into account the acquired and congenital forms of thrombocytopenia, as well as the nephropathies related to collagen IV:
- (a)
Acquired thrombocytopenia. Differentiating MYH9 from acquired forms of thrombocytopenia may be difficult, in fact several patients with MYH9 disease have been misdiagnosed with idiopathic thrombocytopenic purpura (ITP) (which is autoimmune). This led to the administration of treatments (immunosuppressive drugs and splenectomy) that are ineffective. If there is no family history of thrombocytopenia, evaluation of peripheral blood smear is a simple and effective method to identify individuals with MYH9 disease and separate these from ITP patients, since platelets are significantly larger in people with MYH9 disease than in patients with IT. To be precise, an average diameter of platelets >3.7μm distinguishes MYH9 from PTI with a 86% sensitivity and 87% specificity. Moreover, a proportion of platelets with a diameter >3.9μm (approximately half of a red blood cell) greater than 40% differentiates MYH9 from PTI with 85% sensitivity and 87% specificity.39
- (b)
Congenital thrombocytopenia40:
- –
Bernard-Soulier syndrome (BSS) (OMIM 231200).
- –
Gray platelet syndrome (OMIM 139090).
- –
Cytopenia linked to X related to mutations in the GATA1 gene.
- –
Thrombocytopenia associated with mutations in the MPL and MECOM genes.
- (c)
Nephropathies related to Collagen IV, including X-linked and autosomal (dominant and recessive) forms of Alport syndrome. The spectrum of Alport's disease is very broad, some patients with autosomal dominant inheritance may only present persistent microscopic hematuria, which rarely progresses to renal failure. By contrast, patients with Alport syndrome linked to X or a recessive inheritance have hematuria, proteinuria, progressive renal failure and end-stage renal disease. They also present extrarenal abnormalities, including progressive sensorineural hearing loss (usually present at the end of childhood or early adolescence), anterior lenticone, maculopathy, corneal endothelial vesicles, and recurrent corneal erosion. Platelet defects have not been described in Alport's disease. Therefore, when a nephropathy is associated to macrothrombocytopenia, pathologies related to mutations in the MYH9 8 gene must be taken into consideration.
The differential diagnosis with Alport syndrome is often difficult, since both entities may present as a hereditary glomerular disease with proteinuria, progressive renal failure and bilateral sensorineural hearing loss. The presence of thrombocytopenia will suggest the diagnosis of MYH9.
Treatment and prevention- (a)
Hematological disorders.
- –
Mild or moderate Bleeding: Local measures (compression, cauterization, application of gauze with tranexamic acid) are the first-line treatment for mucocutaneous bleeding and are often sufficient to control the bleeding.
- –
Platelet transfusions.
- –
Eltrombopag. It interacts with human trompoetin by inducing the proliferation and differentiation of megakaryocytes from progenitor cells of the bone marrow. This oral drug has been reported to increase platelet counts and reduce the hemorrhagic tendency in most cases of patients with mutations in MYH9.41,42
- –
Antifibrinolytics. Some authors recommend the systemic administration of antifibrinolytic agents, such as tranexamic acid or epsilon-aminocaproic acid, to treat mild or moderate mucocutaneous bleeding.20
- –
As a prophylaxis, the use Desmopressin shortens the bleeding time in certain surgeries in patients with MYH9 disease. Since not all individuals respond to treatment, thus a trial dose is recommended to identify patients that may benefit from this treatment for the prevention of bleeding associated to invasive procedures or future episodes of bleeding.37,43
- (b)
Hearing loss. The use of cochlear implants in patients with severe hearing loss improves verbal communication skills.44–46
- (c)
Eye involvement. Opacity of the lens are treated with surgery.
- (d)
Renal involvement.
- –
Proteinuria is usually improved by the use of inhibitors of the renin angiotensin aldosterone system.47
- –
Dialysis or kidney transplant are the treatment for patients with end-stage renal disease.
- (e)
Liver involvement. The elevation of liver enzymes does not require any specific treatment.
- (f)
Women in reproductive age.
- –
Oral contraceptives are effective in preventing or controlling menorrhagia; however, they increase the risk of thrombosis, which is also described in patients with MYH9 gene mutations. Thus, the balance between the risks and benefits of oral contraceptives should be taken into consideration.3
- –
Births should be handled as in women with other forms of thrombocytopenia. Pregnant women whose more severe thrombocytopenia and bleeding history before pregnancy, will have a higher incidence of bleeding related to childbirth. In vaginal deliveries in women with severe thrombocytopenia, an increased risk of neonatal intracranial hemorrhage should be considered.39
- (g)
Genetic advice. The inheritance is of autosomal dominant transmission, therefore each individual has a 50% chance of transmitting the disease to their offspring. Prenatal diagnosis and preimplantation diagnosis are possible, as long as the mutation in the MYH9 gene in the parent is known.
A 27-year-old woman had been diagnosed of thrombocytopenia during childhood in the context of hemorrhagic diathesis. At diagnosis, she ha platelets count between 3000 and 10,000/microL with frequent hemorrhages. Initially she was thought to have idiopathic thrombocytopenic purpura. Treatment with glucocorticoids and intravenous gamma globulin was started without improvement in the platelet count.
Five years after the diagnosis, splenectomy was performed with no improvement in thrombocytopenia. Due to persistent thrombocytopenia, a peripheral blood study was performed observing platelets with macrodismorphy (giant platelets with abnormalities in the granulation), dysmorphic red blood cells with Howell-Jolly bodies that could be attributable to the lack of spleen, and no alterations in the white series.
Due to the frequent hemorrhages and anemia, she required continuous treatment with oral iron and platelet transfusions in each surgical act.
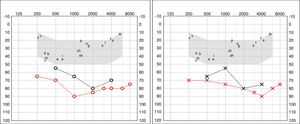
Four years later, she was diagnosed with bilateral sensorineural hearing loss. The hearing loss progressed slowly and she had to use bilateral hearing aids and at the age of 26 she was referred to a specialized center and a cochlear implant was placed in the left ear. The logoaudiometric results after the cochlear implant were excellent (Figs. 1–3). In the preoperative study, a thromboelastogram was performed to evaluate the risk of bleeding, the result was normal despite thrombocytopenia. The extension of blood showed large platelets with anomalies in the granulation. No cytoplasmic inclusions were observed in neutrophils (Fig. 4). The ophthalmological study was normal.
At the same time, it was observed a decrease in total serum proteins was observed at 55g/L (normal values 64–83), in serum albumin was 27g/L(normal values 34–48). She ha a proteinuria 2.7g/24h, with serum creatinine of 0.9g/dl and eGFR by CKD-EPI 86ml/min/1.73m2. She was referred to the Nephrology Service to evaluate a nephrotic syndrome with mild edema in the lower extremities and associated hypercholesterolemia. Given the risk of bleeding due to severe thrombocytopenia, the renal biopsy was not performed and she was started on lisinopril 5mg.
Family history was revised, drawing the attention that her mother also had idiopathic thrombocytopenia, although less marked than in the patient, with platelet counts between 40–50,000/l (normal values: 140,000–350,000). The mother also presented progressive bilateral hearing loss from childhood with bilateral hearing aids but without signs of nephropathy. The presence of familial hearing loss was confirmed, highlighting affectation of the great-grandfather, grandfather, great-aunts and direct aunt of the mother's branch. Given the suspicion of familial disease, a genetic study was carried out using a panel of 140 genes associated with kidney diseases.
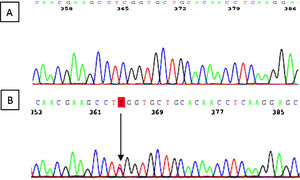
The variant c.287C> T (p.Ser96Leu) was identified in exon 2 of the MYH9 gene. It is a “missense” variant that results in the change of the amino acid serine to leucine in the 96th place of the myosin 9 protein. The secondary genetic study of the 54-year-old mother revealed the same genetic variant (Fig. 5).
Genetic analysis is specially importance in this disease, because as previously mentioned, the existence of a correlation genotype-phenotype may be relevant for prognosis. Authors such as Pecci et al. proposed that mutations in the N-terminal domain had been associated with a higher incidence of nephropathy, hearing loss and more severe thrombocytopenia with respect to C-terminal domain mutations.2,48 In our case, the patient presented a mutation in exon 2 of the MYH9 gene (c.287C>T), which determine the substitution of the amino acid serine for leucine (p.Ser96Leu) in the N-terminal domain of the protein. The location of the mutation can explain the clinical presentation of the disease, however there is high phenotypic variability within individuals with the same mutation, as it is the case of our patient who presents a much more serious clinical expression than her mother. More recent studies by Pecci et al. published suggest that not all patients with mutations in the N-terminal domain share the same prognosis.37 The influence of environmental factors or variants on other additional genes that interact with MYH9 may determine the progression of the disease as it seems to be our case.
Despite treatment with ramipril, the patient presented progressive deterioration of renal function. One year after the diagnosis the proteinuria increased to 3.6g/24h and the glomerular filtration rate decreased to 48ml/min/1.73m2. At present, the patient is in multidisciplinary follow-up to detect possible complications related to her disease.
This case reveals the importance of conducting a thorough family history, especially when there is a high suspicion of low prevalence family diseases. It would be advisable to obtain a detailed family history with special emphasis on renal, auditory and ophthalmological clinical manifestations when evaluating a child with thrombocytopenia. In the presence of unexplained thrombocytopenia with a compatible family history, it would be advisable to perform a urine test to rule out proteinuria; if there is evidence of proteinuria, it is advisable to initiate early treatment with inhibitors of the renin-angiotensin-aldosterone system, to slow down the progression of kidney disease.47
ConclusionsThe diagnosis of patients with nephropathy associated with mutations in MYH9 is not easy, as it is the case of our patient, being a genetic disease unfrequent and underdiagnosed. The use of genetic diagnosis by means of gene panels is of special relevance since it allows to achieve a personalized, reliable diagnosis. In addition, it allows the secondary diagnosis of other members of the family and offers the patient the possibility of genetic counseling before gestation.
New lines of research are needed regarding the role of the MYH9 gene in kidney diseases as well as the search for new therapeutic strategies that allow to improve the prognosis of affected individuals.
Key concepts- 1.
Nephropathy due to MYH9 mutation in presents a pattern of autosomal dominant inheritance.
- 2.
It is a disorder characterized by the presence of macrotrombocytopenia, hipoacusia, cataracts and nephropathy with proteinuria and variable risk of developing kidney failure.
- 3.
Groups of pathologies known as: May-Hegglin anomaly, Fechtner syndrome, Epstein syndrome and Sebastian syndrome are grouped as by the name of diseases associated with MYH9.
- 4.
There are some polymorphisms in MYH9 that are associated with an increased risk of developing chronic kidney disease.
- 5.
Differential diagnosis should be made with other causes of thrombocytopenia and/or collagen IV nephropathies.
- 6.
If there is kidney involvement with proteinuria, treatment with inhibitors of the renin angiotesin system could slow the progression to end-stage renal disease.
- 7.
Although the location of the mutation could explain the clinical presentation of the disease, there is high genotype-phenotype variability of individuals with the same mutation.
The authors declare that they have no conflicts of interest.
To Ricard Pellejero, responsible for the Library of the Fundació Pyuigvert, for his assistance in the search of the bibliographic material.
Please cite this article as: Furlano M, Arlandis R, del Prado Venegas M, Novelli S, Crespi J, Bullich G, et al. Nefropatía asociada a mutación del gen MYH9. Nefrologia. 2019;39:133–140.