Nephrotic syndrome in patients with cancer may be related to the primary malignancy or chemotherapeutic therapy. Solid organ cancers may cause membranous glomerulonephritis which is manifested by nephrotic syndrome; other less common histologic presentations include focal and segmental glomerulosclerosis and minimal change disease. In addition, chemotherapy agents may cause renal toxicity by affecting the small blood vessels, glomeruli, tubules, and interstitium. Tyrosine kinase inhibitors such as sunitinib may cause endothelial and podocyte damage leading to thrombotic microangiopathy affecting only the kidney and manifested by proteinuria and hypertension. We report a case of an elderly man with gastrointestinal stromal tumor (GIST) on treatment with sunitinib who had as a complication a thrombotic microangiopathy manifested with nephrotic syndrome and a hypertension of difficult control, which was finally controlled by stopping this drug but had a fatal outcome due to its malignancy.
El síndrome nefrótico en los pacientes con cáncer se puede asociar a su enfermedad de base o al tratamiento quimioterapéutico. El cáncer de órganos sólidos puede producir una glomerulonefritis membranosa que se manifiesta con síndrome nefrótico; otras presentaciones histológicas menos frecuentes son la glomeruloesclerosis focal y segmentaria y la enfermedad de cambios mínimos. Adicionalmente, los tratamientos quimioterapéuticos pueden causar toxicidad renal por afección de los pequeños vasos sanguíneos, los glomérulos, los túbulos y el intersticio. Los inhibidores de la tirosin quinasa como el sunitinib pueden causar daño endotelial y podocitario, produciendo una microangiopatía trombótica limitada a los riñones, que se manifiesta con proteinuria e hipertensión. Se presenta el caso de un hombre anciano con tumor de GIST que fue tratado con sunitinib y como complicación presentó una microangiopatía trombótica manifestada con síndrome nefrótico e hipertensión de difícil control, que se controló al suspender este medicamento pero con desenlace fatal por su neoplasia maligna.
Cancer may affect the kidney through to glomerular lesions (paraneoplastic glomerulopathies), or as a consequence of the toxic effects of chemotherapy, myeloablative radiation and direct involvement of the renal vasculature caused by the tumour cells.1,2 The drugs related with kidney damage include both tyrosine kinase receptor inhibitors and sunitinib.3,4
Sunitinib is a multi-targeted tyrosine kinase inhibitor that blocks the activity of multiple enzymes, including the vascular endothelial growth factor (VEGF), the signalling system involved in angiogenesis and tumour growth.2,5 Growing evidence reports the risk of this drug in small blood vessel damage and in the renal glomeruli, which is manifested clinically with arterial hypertension, proteinuria, nephrotic syndrome and acute renal failure.6 The most frequent kidney lesions observed are focal segmental glomerulosclerosis, thrombotic microangiopathy (TMA) and sometimes acute tubular necrosis and acute interstitial nephritis.1,3,5–10
We report a rare case of thrombotic microangiopathy limited to the kidney secondary to the use of sunitinib several months after initiation, that was documented by a kidney biopsy and which led to chronic kidney disease with nephrotic syndrome and difficult-to-control hypertension, which was finally controlled by stopping this drug but had a fatal outcome due to its malignancy
Case reportA 74-year-old man with a background of arterial hypertension and hypothyroidism, with normal baseline kidney function (creatinine 0.8 mg/dL, without proteinuria). In 2014, he was diagnosed with a gastrointestinal stromal tumor (GIST) in the distal oesophagus with multiple metastatic liver lesions. The patient was initially treated with 54 cycles of imatinib (tyrosine kinase inhibitor [TKI]). In 2018, it was observed progression of the oesophageal and liver tumoral lesions, with evidence of new lesions in the lungs, prompting a switch to sunitinib (a more powerful TKI) which was given from August 2018 until May 2020; in total, the patient received six cycles consisting of 50 mg/day of sunitinib for four weeks followed by two weeks rest.
In April 2020, the patient presented a progressive impairment of his renal function, with elevated serum creatinine, nephrotic range proteinuria, marked oedema of the lower extremities and eyelids that was associated with dyspnoea on exertion, orthopnoea, paroxysmal nocturnal dyspnoea and a reduction in diuresis. The complementary paraclinical analyses documented macrocytic anaemia, elevated LDH, negative direct Coombs test; serum complement components C3 and C4 within normal values (C3: 112 mg/dl y C4: 22 mg/dl), normal ADAMTS 13, without thrombocytopenia, without reticulocytosis or consumption of haptoglobin, negative cryoglobulins and the presence of 1% schistocytes in the peripheral blood smear. No compromise of other organs was found, the echocardiogram was normal; macrocytosis was associated with vitamin B12 deficiency (Table 1).
Laboratory tests.
| Haematological studies | Renal studies | Coagulation profile | |||
|---|---|---|---|---|---|
| Haemoglobin | 11.6 g/dl | Creatinine | 2.58 mg/dl | PPT | 32 s |
| Haematocrit | 35.5% | BUN | 31 mg/dL | PT | 12 s |
| Leukocytes | 5,150 mm3 | Proteins 24 h urine | 5.8 g | INR | 1.1 |
| Neutrophils | 3,930 mm3 | Urine analysis | Direct Coombs | Negative | |
| Platelets | 218,000 mm3 | pH | 5.5 | Endocrine profile | |
| Schistocytes | 1% | Density | 1,020 | TSH | 3.65 mIU |
| MCV | 109 Fl | Proteins | 500 mg/dl | Metabolic profile | |
| ESP: Schistocytes + and the presence of macrocytes | Erythrocytes | Negative | Albumin | 1.6 g/dL | |
| Liver studies | Leukocytes | Negative | Total cholesterol | 170 mg/dL | |
| AST | 24 U/L | Electrolytes in blood | HDL cholesterol | 35 mg/dL | |
| ALT | 14 U/L | Sodium | 145 mEq/L | Triglycerides | 199 mg/dL |
| Total bilirubin | 0.55 mg/dL | Chloride | 106 mEq/L | Fasting glycaemia | 115 mg/dL |
| Alkaline phosphatase | 64 U/L | Potassium | 3.7 mEq/L | HbA1C | 5.8% |
| LDH | 438 U/L | Calcium | 6.94 mg/dL | Vitamin B12 | 150 ng/mL |
| Haptoglobin | 160 mg/dL | Phosphorous | 5,2 mg/dL | ADAMTS 13 | 56% |
ALT, alanine transaminase; AST, aspartame transaminase; BUN, blood urea nitrogen; HbA1C, glycosylated haemoglobin; LDH, lactate dehydrogenase; TSH, thyroid-stimulating hormone; MCV, mean corpuscular volume.
In view of this clinical scenario, the diagnostic possibilities considered were membranous nephropathy secondary to the underlying oncological condition or TMA-type adverse drug (sunitinib) effect.
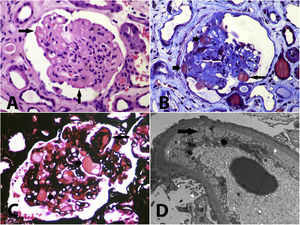
Initially, the hypertension was treated with amlodipine 10 mg/day, metoprolol 100 mg/day, furosemide 40 mg/day, prazosin 6 mg/day with adequate control of blood pressure values and improved diuresis. A kidney biopsy was performed, with findings of chronic thrombotic microangiopathy accompanied by secondary focal segmental glomerulosclerosis (Fig. 1). In addition, interstitial oedema, mononuclear inflammatory infiltrate and acute epithelial damage (tubulointerstitial nephritis), with the presence of organising thrombi in some arterioles. Due to the biopsy findings in a patient who totally refused to go on dialysis; in May 2020 the sunitinib was suspended, with a significant improvement in renal function, resolution of the nephrotic syndrome, control of arterial blood pressure values and no signs of microangiopathic haemolytic anaemia; the results of the last blood tests performed in September 2020 were creatinine 1.26 mg/dl, haemoglobin 11.7 g/dl, platelets 523,000 mm3, LDH 287 U/L, peripheral blood smear without the presence of schistocytes, albumin 3.5 g/dl and a proteinuria of 120 mg in 24 h urine. However, the patient presented progression of his liver tumour lesions with no possibility of additional treatment, whereupon the decision was taken to give palliative treatment and the patient died from malignant tumour lesions in October 2020.
(A) Glomerulus with solidified appearance, with loss of or reduction in capillary lumen, some capillary walls are thickened and there are hyaline segments (arrows) that may correspond to segments with irreversible damage and protein exudates or to intracapillary thrombi (haematoxylin-eosin, X400). (B) Glomerulus with ischaemic retraction, compaction and hyaline segments (short arrows), and a recent intracapillary thrombus (long arrow) (Masson trichrome, X400). (C) Intracapillary accumulations of hyaline or proteinaceous material (short arrows) that may correspond to segments of hyalinosis or to organising thrombi; a double contour is observed (long arrow) and the capillary lumen are narrow (methenamine silver, X400). (D) Diffuse podocyte injury with loss of pedicles, marked subendothelial oedema with loss of fenestrations (arrow); in other capillaries, double contours are detected, without electron-dense deposits (transmission electron microscopy, original magnification, X2.100).
Sunitinib is a TKI that affects the vascular endothelial growth factor receptor (VEGFR) and the platelet-derived growth factor receptor (PDGFR) pathways. Its use is becoming increasingly more widespread in cancer on account of its antiangiogenic properties, and it is indicated mainly in renal cell cancer, GIST and pancreatic neuroendocrine tumours.7
Renal toxicity related to TKI has been described with sunitinib5,10,11; in the literature, there are descriptions of patients with a preeclampsia-like syndrome with hypertension and proteinuria, with improvement following suspension or a reduction in the dose of sunitinib7,11; another patient with a diagnosis of GIST developed a TMA, albeit without kidney biopsy data12; subsequently, different cases of histologically documented cases of TMA were reported in patients on treatment with sunitinib5,8,13–15 and of acute interstitial nephritis secondary to sunitinib.16 Other histopathological renal lesions secondary to antiangiogenic treatment include IgA nephropathy, membranoproliferative glomerulonephritis and focal segmental glomerulosclerosis with some bias in the reports, since most of the patients in this context of kidney failure and cancer on chemotherapy do not undergo a kidney biopsy.6,14,17
VEGF is expressed constitutively by podocytes, and the VEGF receptors are present in the normal glomerular capillary endothelial cells. The pathogenesis of TMA in patients who receive anti-VEGF therapy is probably related to the perturbation of the signalling of the podocyte-endothelial VEGF axis18,19; in studies in animals that were given anti-VEGF antibodies, glomerular endothelial cell detachment and a reduction in nephrin production were observed.5
The exact mechanism whereby the anti-VEGF therapy causes proteinuria is not fully known, although several mechanisms have been proposed; one of them appears to be a direct consequence of VEGF inhibition, since the latter is expressed in the normal nephron, maintaining the filtration barrier and glomerular endothelial integrity.2,18 In addition, the inhibition of VEGF reduces the bioavailability of nitric oxide and prostaglandin I2, which may contribute both to an ischaemic lesion and a hyperfiltration lesion in non-ischaemic nephrons.14,20 Another possible mechanism is the increase in erythropoietin production, which increases blood viscosity, increasing the risk of glomerular microthrombosis.5
A review of published studies demonstrated a variety of approaches to the management of TMA associated with sunitinib ranging from the use of angiotensin receptor blockers13 to urgent plasma exchange and treatment suspension.12,21 The clinical spectrum of severity is broad, from isolated proteinuria to hypertension and neurological deficits. In the patient that we present, renal toxicity by sunitinib presented with progressive kidney failure, nephrotic syndrome and difficult-to-control arterial hypertension associated with TMA with limited compromised kidney function and improvement of the latter on suspension of this medication.
The incidence of renal toxicity associated with TKI could be greater due to the lack of renal biopsy data and the lack of systemic detection of de novo proteinuria. We suggest that patients receiving TKI be monitored and that renal function be evaluated, which includes the presence of proteins in urine and urinary sediment, considering to perform a kidney biopsy in patients with renal dysfunction since it excludes other aetiologies and would guide us towards the right therapy.
ConclusionThe approach to nephrotic syndrome in cancer patients must be integrated in order to evaluate whether it is associated with the underlying disease, the chemotherapy treatment or another cause. Sunitinib is being used increasingly more often in the treatment of cancer on account of its antiandrogenic properties. However, one of its side effects is thrombotic microangiopathy limited to the kidney, which may present with nephrotic syndrome and hypertension. Renal function should be monitored continuously in patients who receive this medication, which includes the presence of proteins in urine and the evaluation of urinary sediment. In the event of impaired renal function or the development of hypertension or proteinuria, consideration should be given to the suspension of sunitinib
Conflict of interestDr, Nieto-Ríos has given conferences on thrombotic microangiopathies sponsored by Alexion Pharma. The other authors declare no conflict of interest.