Patients with advanced chronic kidney disease (ACKD) have a high prevalence of malnutrition. The dietary restrictions that we usually apply in terms of macro and micronutrients force our patients to follow dietary guidelines that deviate from healthy patterns.
ObjetivesTo determine if a personalized nutritional intervention program, minimizing the usual restrictions would be justified in case it improved the evolution of kidney disease compared to standard treatment.
Secundary objetivesTo determine changes in nutrient intakes and in anthropometric and biochemical parameters, as well as quantify episodes of hyperkalemia.
Material and methodsA single-center, randomized and controlled educational intervention clinical trial was conduct in patients from the ERCA outpatients clinic at the Complejo Hospitalario Universitario de Albacete. 75 patients were included, assigning 35 to a Control group and 40 to the Intervention group with 1-year follow-up. The nutritional status was determined using anthropometric data, body composition by Bioimpedance, blood and urine biochemical parameters and a 24-h recall questionnaire. The nutritional intervention was carried out in three different ways: individual, collective and telephone recall.
ResultsAt the beginning of the study, the BMI showed a situation of weight excess with a mean of 28.83 kg/m2 (5.4) in men and 26.96 kg/m2 (4.09) in women. 70% of our patients had overweight. The abdominal circumference was 105.3 cm (10.2) and 92.3 cm (13.7) for men and women respectively without significant changes throughout the study. The percentage of fat mass (FM) was high in both groups for men and women throughout the study. We did not find biochemical parameters of malnutrition and only significant differences were observed in glomerular filtration rate (GFR), which increased in the intervention group. No patient presented any episodes of hyperkalemia during the study. The energy intake in both groups showed an inadequate distribution of macronutrients with a poor intake of carbohydrates (CH) that was supplemented with an excess of fat. In the case of micronutrients, we did observe an increase in potassium and fiber intakes with a decrease in sodium and phosphorus in the intervention group.
ConclusionsMalnutrition is not exclusively an intake defficit and encompasses both the problems derived from a deficit and an excess of nutrients intake. Un to 70% of our patients showed weight excess and a fat mass higher than desirable. The implementation of an individualized nutritional education program, including a vegetables and fiber rich diet, less atherogenic, not only did not cause electrolyte alterations but also slowed the progression of kidney disease.
El paciente con enfermedad renal crónica avanzada (ERCA) presenta una elevada prevalencia de malnutrición. Las restricciones dietéticas que aplicamos habitualmente en cuanto a macro y micronutrientes obligan a nuestros pacientes a seguir pautas dietéticas alejadas de los patrones saludables.
ObjetivoDeterminar si un programa de intervención nutricional personalizado minimizando las restricciones habituales estaría justificado si mejorase la evolución de la enfermedad renal comparado con el tratamiento estándar.
Objetivos secundariosDeterminar los cambios en las ingestas de nutrientes y en los parámetros antropométricos y bioquímicos así como los episodios de hiperpotasemia.
Material y métodosSe realizó un ensayo clínico de intervención educativa, unicéntrico, randomizado y controlado en los pacientes de la consulta ERCA del Complejo Hospitalario Universitario de Albacete. Se incluyeron 75 pacientes asignando 35 en un grupo control y 40 en el grupo de intervención con seguimiento a 1 año. La situación nutricional se determinó mediante datos antropométricos, composición corporal por Bioimpedancia, parámetros bioquímicos en sangre y orina y cuestionario de recuerdo de 24 horas. La intervención nutricional se realizó de 3 formas: individual, colectiva y recuerdo telefónico.
ResultadosAl inicio del estudio el IMC mostró una situación de exceso de peso con una media en hombres de 28,83 kg/m2 (5,4) y de 26,96 kg/m2 (4,09) en mujeres. El 70% de nuestros pacientes mostraron exceso de peso. La circunferencia abdominal fue de 105,3 cm (10,2) y 92,3 cm (13,7) para hombres y mujeres respectivamente sin cambios significativos a lo largo del estudio. El porcentaje de masa grasa (MG) fue elevado tanto hombres como en mujeres durante todo el estudio. Los parámetros bioquímicos no mostraron una situación de malnutrición y solo se observaron diferencias significativas en el filtrado glomerular (FG) que aumentó en el grupo intervención. Ningún paciente presentó episodios de hiperpotasemia durante el estudio. La ingesta energética mostró en ambos grupos una inadecuada distribución de macronutrientes con una pobre ingesta de hidratos de carbono (HC) que se suple con un exceso de grasa. Para los micronutrientes sí observamos en el grupo intervención un aumento en las ingestas de potasio y fibra con una disminución de las de sodio y fósforo.
ConclusionesMalnutrición no es sinónimo exclusivamente de desnutrición y engloba tanto los problemas derivados del déficit como del exceso de ingesta de nutrientes. El 70% de nuestros pacientes mostraron exceso de peso y un porcentaje de masa grasa mayor del deseable. La aplicación de un programa de educación nutricional individualizado realizando una dieta rica en vegetales y fibra, menos aterogénica, no provocó alteraciones electrolíticas y supuso un enlentecimiento en la progresión de la enfermedad renal.
Nutritional and dietary management is fundamental in the treatment of patients with chronic kidney disease (CKD) throughout the course of their disease. The nutritional requirements in macro and micronutrients varies as the kidney disease progresses, and the modifications in the diet of our patients will be intensified as the patient approaches the most advanced stages of CKD (stages 4 and 51).
The kidney patient is very likely to be malnourished at some point in their disease. According to numerous studies that also include dialysis patients, the prevalence of malnutrition in CKD ranges from 12% to 80%,2,3 reaching higher figures in some of the series. Prevalence of poor nutrition in non-dialysis advanced chronic kidney disease (ACKD) ranges between 12% and 28%, although the number of studies is smaller and number of patients is less.
In recent years, the term protein energy wasting (PEW), which is defined as the pathological state in which there is a decrease in protein and energy stores, gives equal importance to catabolism and malnutrition.7,8 There is evidence that malnutrition in CKD implies an increase in morbidity and mortality, in the number of hospital admissions, in the length of hospitalizations, in the number of complications of infectious aetiology, and in mortality of cardiovascular origin.9
However malnutrition in kidney patients, not only includes malnourishment. On many occasions the terms malnutrition and poor nutrition are confused and they are used as synonyms. Malnutrition refers to deficiencies, excesses or imbalances, in a person's intake of energy and/or nutrients. We refer to a much broader problem that not only encompasses malnutrition, but also other entities related to food intake such as overweight and obesity.10,11 In fact, the percentage of obesity is increasing in our patients just as it is in the general population, with alarming data indicating that more than half of patients with ACKD present excess of weight.
Nutritional advice is recommended since the initial stages of CKD. At this initial stage, a dietary intervention is recommended in patients with excess weight in order to reduce the weight which should improve renal hyperfiltration and reduce the progression of CKD.12–14
In the most advanced stages of CKD (ACKD) nutritional advice is used as a renoprotective and antiproteinuric measure trying to slow down the progression of CKD and maintain an adequate nutritional status.15
Although it is recommended that ACKD clinic should be multidisciplinary, in many centers the main nutritional advice is still given by the nephrologist without the collaboration of a nutrition expert, being forced to give nutritional advice to very complex malnourished patients. The measures that we usually apply to our patients are, the restriction of energy in the form of macronutrients for cases of overweight and obesity with associated cardiovascular risk factors and also restrictive diets in micronutrients such as phosphorus, calcium, sodium and potassium.16,17 Restrictions in protein intake are recommended, since there are studies that show that they reduce proteinuria and can improve GFR.18 Several studies have suggested that half of the protein in the diet can be provided in the form of vegetable proteins, thus reducing the bioavailability of phosphorus and the production of uremic toxins involved in the progression of CKD.19,20 Despite this, it is not the usual practice to look for vegetables as source for protein intake. Regarding water intake, on many occasions we recommend a fluid restriction, despite the fact that many times diuresis of the patient would probably allow a higher intake.21
The recommendations of the World Health Organization (WHO) are for a low-salt Mediterranean diet pattern, but in routine practice, nephrologists do not recommend plant-based diets because of fear to hyperkaliemia and that they have low nutritional content.
It is likely that the nutritional advice that we have usually offered could not guarantee adequate caloric and mineral support, furthermore it may interfere with the quality of life of our patients. Currently, the trend is to limit restrictions and perform an individualized dietary advice. Some randomized controlled trials show that in patients with CKD stages 3 and 4 who receive general nutritional advice, or even diets rich in fruits and vegetables do not present an increase in serum potassium or episodes of hyperkalemia as compared with those taking bicarbonate or resins in restrictive diets.22,23 Therefore we wonder if the nutritional requirements of kidney patients are so different from the general population and what would happen if we released the diet of our patients with close monitoring and adapting the nutritional advice individually towards diets richer in vegetables and fiber with less atherogenic effect. Thus, the purpose of this study was to assess the nutritional status of the patient that we follow in our ACKD outpatient clinic and determine whether an individualized nutritional education program with less dietary restriction of the diet would improve the anthropometric nutritional parameters, the biochemistry values and the progression of renal failure as comparison with the usual nutritional recommendations that the patients are usually receiving in our clinic.
Patients, material and methodsThe study included patients from 18 to 80 years with ACKD treated in the nephrology outpatient clinic of the General University Hospital of Albacete.
This is a single-center, randomized, open, controlled clinical trial of educational intervention, whose study population is patients from the ACKD clinic not on dialysis. The study was approved by the Ethics and Research Committee of our hospital.
As inclusion criteria, patients had to be over 18 years of age, without prior renal replacement therapy or control by a nutritionist. Exclusion criteria were having any active infectious, inflammatory, or tumor and inability to obtain informed consent.
The sample size was calculated using Epidat (V3.1), software developed by the Epidemiology Service of the Directorate Xeral de Saude Government of the Ministry of Health (Xunta de Galicia) with the support of the Organization Pan American Health (PAHO-WHO) and the CES University of Colombia.
A sample of 75 patients were randomly distributed as a control group including 35 patients and an intervention group of 40.
Patients of both groups were evaluated in the same way. Demographic data and associated comorbidities were collected at baseline.
Nutritional assessment was performed through a detailed clinical history. Mean intakes of energy (kcal), carbohydrates (CH) measured in g and expressed as % of total kcal as well as fat, protein (g/kg/day), saturated fat (%), sodium (mg), potassium (mg), phosphorus (mg), fiber (g), vitamins C (g), D (mcg) and E (g), were obtained from 24-h recall questionnaires carried out on three different days, including one weekend. The analysis of this dietary information was performed using the Odimet® nutrition software, a dietary metabolic organizer designed by the University Clinical Hospital of Santiago de Compostela, Spain.
The variables used for anthropometric assessment were weight (kg) and height (cm), body mass index (BMI) in kg/m2 was obtained, abdominal circumference (AC) (cm), considering that a perimeter ≥ 102 cm in men and ≥88 cm in women was considered abdominal obesity. In addition, multifrequency bioimpedance (BCM Fresenius®) was performed to assess body composition. The percentage of fat-free mass (FFM) was measured with respect to the total body mass (% FFM) which is considered adequate if it is above 40%−50%, this value is useful as an isolated measurement and to assess evolution and the percentage of fat mass (% FM), useful for both a single measurement and to assess evolution and obesity is considered if exceeds 25% in men and 33% in women.
The variables measured in blood and urine were (units and normal ranges in serum by our laboratory are shown): urea (10−50 mg/dL), creatinine (0.7−1.2 mg/dL), glomerular filtration rate (GFR) (mL/min/1.73 m2), 24-h proteinuria (g/day), bicarbonate (22−26 mmol/L), total lymphocytes (1−4 × 103/mcl), transferrin (200−360 mg/dL), albumin (3.5−5.2 g/dL), total protein (6.6−8.7 g/dL), prealbumin (20–40 mg/dL), CRP (0−5 mg/L), sodium (135−145 mmol/L), potassium (3.5−5.1 mmol/L), calcium (8.6–10.2 mg/dL) and phosphorus (2.6−4.5 mg/dL).
The patients were evaluated troughout one year. Biochemistry were obtained at the first visit and at 3, 6 and 12 months.
Patients who started renal replacement therapy with hemodialysis, peritoneal dialysis or kidney transplant were excluded from the study.
Description of the work performed:
A recruitment period of six months was required to reach the adequate sample size.
Informed consents were given to all participants for their signature.
Data collection was carried out at months 0, 3, 6 and 12. The nutritional intervention consisted of four individual sessions, eight group sessions (which included cooking workshops) and eight telephone reminders. The sessions dealt with basic aspects of nutrition, macronutrients and micronutrients, interpretation of food labeling, allergies and cooking techniques adapted to their needs. The exchange system adapted to the tastes and preferences of each patient was explained and a book with nutritional composition tables adapted to kidney patients edited for this study was delivered.24
The control group receive the recommendations on lifestyle and the usual restrictions that are usually specified in the ACKD outpatient clinic.
Statistical analysis: For the description of the sample, measures of central tendency (mean and standard deviation) have been used in the case of metric variables and measures of frequency (absolute frequency and percentage) in the case of qualitative variables. The differences between groups, when it comes to quantitative variables, have been evaluated using the Student's t-test (parametric tests) or the Mann-Whitney U-test (non-parametric tests).
Intergroup comparisons of qualitative variables were made using the χ2 test, using Fisher's exact statistic on those occasions in which the expected frequency was less than 5. In the case of intragroup comparisons, to assess the progression throughout the study, results in the four visits in the metric variables were evaluated using the general linear model for repeated measures (parametric tests) and the Friedman statistic (non-parametric test). The Cochran Q test for related samples was used for intragroup comparisons of qualitative variables throughout the four visits.
Significance was considered if the P value was <.05.
The statistical analyzes of the study have been performed with the R® software (version 4.0.1), developed by the Department of Statistics, University of Auckland, New Zealand.
ResultsDemographic and clinical characteristicsAfter 12 months from the initiation of the study there were 57 patients left, 21 in the control group (eight dropouts, one transplanted and five on dialysis) and in the intervention group there were 36 patients (one died, one abandoned and two started dialysis). The difference observed between the two groups in the number of patients that started dialysis or had a transplant was due to the greater deterioration of the GFR in the control as compared to the intervention group. However we do not know if the dropouts in the control group were due to the lack of close attention as in the intervention group. These results were taken into account when analyzing the data.
The mean age was 56.9 years. The distribution by sex was 53% men and 47% women in the intervention group and 60% and 40% in the control group, respectively.
In both groups there was a high comorbidity with an age-adjusted Charlson index of 4.1 (SD 1.74), with at least three associated comorbidities (Table 1).
Epidemiological characteristics and associated comorbidity expressed as a percentage (%), except age, Charlson index and in number of comorbidities that are numerical.
| Total sample (n 75) | Intervention Group (n 40) | Control group (n 35) | P-value | |
|---|---|---|---|---|
| Age (years) | 56.9 | 57.3 | 56.6 | ns |
| Sex (M/F %) | 56.5/43.5 | 53/47 | 60/40 | ns |
| Charlson index | 4.2 (SD: 1.7) | 4.1 (SD: 1.74) | 4.3 (SD: 1.69) | ns |
| Number of comorbidities | 3.2 (SD: 1.03) | 3.1 (SD: 1.2) | 3.3 (SD: 0.9) | ns |
| HTN (%) | 94.7 | 95 | 94.3 | ns |
| Dyslipidemia (%) | 81.3 | 75 | 88.6 | ns |
| DM (%) | 25.3 | 27.5 | 22.9 | ns |
| PVD (%) | 10.7 | 7.5 | 14.3 | ns |
| CVD (%) | 1.3 | 2.5 | 0 | ns |
| IHD (%) | 10.7 | 10 | 11.4 | ns |
| Hyperuricemia (%) | 77.3 | 72.5 | 82.9 | ns |
| Altered mineral metabolism (%) | 62.3 | 56.1 | 68.6 | ns |
| Anemia (%) | 42.7 | Four. Five | 40 | ns |
H: man; M: woman; HTN: arterial hypertension; DM: diabetes mellitus; PV: peripheral vascular disease; CVD: cerebrovascular disease; IHD: ischemic heart disease; SD: standard deviation; ns: not significant.
At the beginning of the study, the mean GFR was 19.05 mL/min/1.73 m2 (SD: 5.22). At baseline no significant differences were observed between the two groups in anthropometric data and body composition, laboratory parameters, and mean dietary intakes.
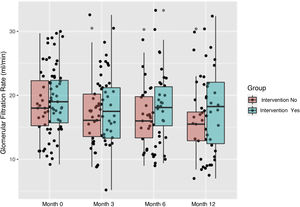
Evolution of kidney functionAt the end of the study, serum creatinine was lower and the GFR was higher in the intervention group that control (P < .05). Fig. 1 shows the increase in GFR in the intervention group from 19.2 to 20.7 mL/min/1.73 m2 and the decrease in the control group from 18.9 to 15.3 mL/min/1.73 m2 with significant differences between the two groups (P < .015).
Anthropometry and body compositionWeight remained stable with minimal decrease in both groups. The mean weight in men was 83.3 kg (SD: 10.86) at baseline and 80.3 kg (SD: 11.3) at the end of the study; in women it was 66.5 kg (SD: 12.55) and 62.6 kg (SD: 9.7), at baseline and at the end of the study respectively.
In both groups, the BMI at the initiation of the study reflected a situation of overweight with an average of 26.3 kg/m2 in the intervention group and 27.9 kg/m2 in the control group; hardly any changes were observed. Men had a BMI of 28.7 (SD: 8.5) and women 26.7 (SD: 4.7), with no significant differences between them. When we categorize according to BMI in low weight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2) and obesity (>30 kg/m2) no differences were observed between the groups at the beginning and at the end of the study. At the beginning of the study, the percentage of patients with a BMI in the overweight range was 38.2% and with obesity with a BMI > 30 was 31.6%.
Abdominal circumference did not change significantly. An expected difference was observed between gender at the beginning, which was not significant. The values were 105.3 cm (SD: 10.2) in men and 92.3 cm (SD: 13.7) in women.
Bioimpedance measurements showed a percentage of FM (%) men of 28.7% (SD: 8.5) at the beginning and 26% (SD: 8.1) at the end of the study. In women, the FM was 37.4% (SD: 10.2) at baseline and 32.6% (SD: 13.2) at the end. There were no significant changes in any group.
The fat-free mass FFM (%) did not undergo significant changes throughout the study either. There were observed expected differences by sex, but the differences were not significant nor their change throughout the study. For men, the FFM (%) was 59.1 (SD: 12.6) at the beginning and 63% (SD: 11.7) at the end. For women, the FFM (%) was 48.2 (SD: 14.8) at baseline and 55% (SD: 19.3) at the end.
Biochemical parametersThe values of the biochemical parameters at the beginning of the study are shown in Table 2. No significant differences were observed throughout the study in any group, in terms of laboratory values measured for urea, 24-h proteinuria, total lymphocytes, transferrin, albumin, total proteins, prealbumin, CRP, sodium, potassium and phosphorus. Bicarbonate increased in the intervention group bit it was not significant and without changes in pH.
Biochemistry at the beginning of the study. Next to each parameter, the reference value of the laboratory is displayed.
| Total (n 75) | Control group (n 35) | Intervention group (n 40) | P-value | |
|---|---|---|---|---|
| Urea (10−50 mg/dL) | 109.91 (SD: 27.23) | 105.23 (SD: 24.10) | 114.6 (SD: 30.37) | ns |
| Creatinine (0.7−1.2 mg/dL) | 3.25 (SD: 0.89) | 3.27 (SD: 0.82) | 3.24 (SD: 0.99) | ns |
| GFR (mL/min/1.73 m2) | 19.05 (SD: 5.22) | 18.95 (SD:5.37) | 19.16 (SD: 5.08) | ns |
| Proteinuria 24 h (g/day) | 2.55 (SD: 6.58) | 3.9 (SD: 10.6) | 1.46 (SD: 1.58) | ns |
| Na (135−145 mmol/L) | 140.97 (SD: 2.22) | 140.77 (SD: 2.66) | 141.72 (SD: 2.37) | ns |
| K (3.5−5.1 mmol/L) | 4.98 (SD: 0.50) | 4.97 (SD: 0.50) | 4.98 (SD: 0.58) | ns |
| Bicarbonate (22−26 mmol/L) | 22.37 (SD: 2.98) | 23.18 (SD: 2.69) | 21.86 (SD: 3.30) | ns |
| P (2.6−4.5 mg/dL) | 3.83 (SD: 0.7) | 3.82 (SD: 0.7) | 3.87 (SD: 0.67) | ns |
| Total protein (6.6−8.7 g/dL) | 6.88 (SD: 0.50) | 6.88 (SD: 0.55) | 6.9(SD: 0.42) | ns |
| Albumin (3.5−5.2 g/dL) | 4.18 (SD: 0.3) | 4.13 (SD: 0.4) | 4.23 (SD: 0.27) | ns |
| Prealbumin (20−40 mg/dL) | 28.12 (SD: 5.80) | 28.7 (SD: 5.30) | 27.5 (SD: 6.01) | ns |
| CRP (0−5 mg/L) | 5.54 (SD: 3.56) | 4.95 (SD: 6.73) | 6.49(SD: 0.42) | ns |
| Total lymphocytes (1−4 × 103/mcl) | 1,813.43 (SD: 710) | 1703 (SD: 610.9) | 1884.27 (SD: 786.02) | ns |
GFR: glomerular filtration rate, Na: sodium, K: potassium, P: phosphorus, CRP: C-reactive protein, SD: deviation standard, ns: not significant.
The intakes of macro and micronutrients in a previous analysis were compared according to whether the patient was diabetic or not, and it did not show any difference, so they have been ignored in the results.
Energy intake was 22 kcal/kg/day (SD: 6.43) for the sample, remaining stable in both groups without significant changes. The distribution of energy in the different macronutrients is detailed below.
The intake of CH were a 45.14% (SD: 11.2) of the diet. In the intervention group, it initially increased and then decreased to baseline levels after the second visit. It did not change in the control group.
The mean protein intake was 0.92 g/kg/day (SD: 0.3). Protein intake remained stable in the two groups.
Mean fat intake was 35.7% (SD: 3.9) with a 9.8% (SD: 4) of saturated fat. Initially, fat intake decreased in the intervention group to gradually rise to baseline values at the end of the study; similar changes were observed in the intake of saturated fat. Monounsaturated fats increased slightly in the intervention group and decreased in the control group. The polyunsaturated did not change significantly.
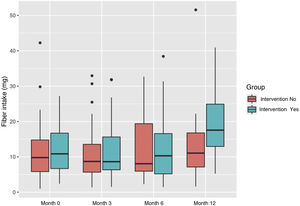
The average fiber intake was 11.98 g (SD: 7.74). There was an increase in consumption in the intervention group starting from 12.25 g (SD: 7.09) and finished the study with a mean consumption of 19.9 g (SD: 9.60). In the control group it was 11.67 g (SD: 8.51) and 13.4 g (SD: 10.42), at the beginning and end respectively (Fig. 2).
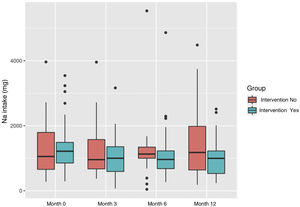
Daily sodium intake decreased in the intervention group from a daily consumption of 1358.2 mg (SD: 750.20) to 983.6 mg (SD: 576.48). In the control group it increased from 1325.9 (SD: 847.56) to 1480.81 mg (SD: 1169.52) (Fig. 3).
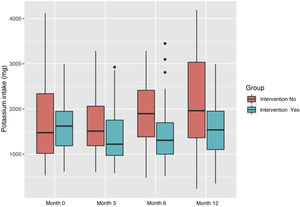
Regarding potassium, in the intervention group showed a decrease in potassium intake at the end of the study, from 1608 mg (SD: 557.06) to 582.56 (SD: 538.18). In the control group it was 1093.77 mg (SD: 831.39) at baseline and 1093.77 mg (SD: 1093.77) at the end (Fig. 4).
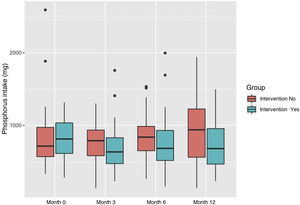
As far as the daily intake of phosphate, the intervention group reduced its consumption of 813.88 mg (SD: 286.06) to 723.57 mg (SD: 324.72), and increased in the control group from 833 mg (SD: 430.15) to 948.67 mg (SD: 497.13) (Fig. 5).
The intake of vitamins A, C, D and E did not change significantly.
Frequency of consumptionDespite not finding significant differences, we did observe a trend of increasing the consumption of vegetables and fruits in the intervention group; in this group, the frequency of vegetable consumption was 1.52 (SD: 0.65) at the beginning, increasing to 2.03 (SD: 1.13) at the end. In the control group, the mean vegetable consumption was 1.62 (SD: 0.94) at the beginning and 1.5 (SD: 0.65) at the end.
For fruit consumption, the portions were 1.68 (SD:) and 1.45 (SD: 7.74) at the beginning and end in the intervention group and 1.53 (SD: 1.14) at baseline and 1.68 (SD: 1, 14) at 12 months in the control group.
DiscussionNutritional advice is essential in the treatment of CKD since malnutrition associated with kidney disease is associated to a worse prognosis and increased morbidity and mortality.25 Renal patients have usually been labeled as having protein-calorie malnutrition; the majority of studies are in dialysis patients that report a prevalence of malnutrition-malnutrition ranging from 12% to 80%.3,26 To date there are very few studies describing the prevalence of malnutrition in ACKD not on dialysis, and the values reported are between 18%–28%.4–6 In 2018, a Spanish study in 186 patients reported that, after performing a subjective global assessment (GSA), protein energy wasting criteria (PEW), a three-day dietary record, anthropometric parameters and bioimpedance, a 27.9% of patients had values in the malnutrition range.6 In our study we found that the predominant profile is malnutrition due to excess weight in almost 70% of patients with and a percentage of fat mass greater than desirable. Regarding fat distribution, android obesity predominates, taking as reference of cardiovascular risk an abdominal circumference of 88 cm in women and 102 cm in man, our results clearly showed a situation risk.
The estimated prevalence of excess of weight in adults in Spain, according to data from the WHO (2017), by BMI, 61.6% are overweight (37.8% overweight and 23.8% obese).27 The nutritional profile in CKD is changing according to the change in the pattern of society due to the obesity and overweight epidemic, which in turn is the cause of the subsequent development of cardiovascular diseases, high blood pressure, dyslipidemia, insulin resistance and increased in the incidence of some types of cancer that are significant load to our health system close to saturation.
In CKD, a dietary intervention that achieves weight loss produces an increase in GFR during an average period of two years, regardless of the type of dietary change.13,28 The treatment of obesity could generate debate since in some studies have shown the beneficial effects of being overweight on dialysis survival, a phenomenon known as “reverse epidemiology”, and which also affects variables such as blood pressure or cholesterol, among others29; but it is not so clear whether this beneficial effect can be sustained in the long term.30 Again, most studies are in the dialysis population and not in the previous stages of CKD. Our results showed a significantly slower progression of kidney disease in the intervention group with a higher GFR, compared to the control group; however, this improvement in GFR was not accompanied by significant weight loss. We think that the lower deterioration in the intervention group could be due to lifestyle changes and probably to the improvement in their quality of life.
The WHO recommends a lifestyle based on the Mediterranean diet and a low-salt diet in the general population, and especially in patients with cardiovascular risk, obesity and overweight. It is a dietary model rich in fats from natural plant sources (virgin olive oil and nuts), with abundant consumption of minimally processed plant-based foods (vegetables, fruits, whole grains and legumes), low meat consumption (especially red or processed meats), moderate consumption of fish and frugality in meals. The PREDIMED trial demonstrated that a high-fat Mediterranean diet supplemented with virgin olive oil or nuts, implemented in a primary cardiovascular prevention setting, resulted in a 30% reduction in clinical events of cardiovascular disease31; it was also found a significant protection of the Mediterranean diet against diabetes. However, similar to our results, the preliminary study did not find weight loss associated with improvement in cardiovascular events.
Expert groups created by the National Institutes of Health and WHO recommend that overweight and obese adults lose 10% of their initial weight, and the primary treatment should be a lifestyle intervention (National Institute of Health, 1998; WHO, 1998). In addition, according to the American Association of Dieticians, such intervention to lose weight should include a hypocaloric diet, the practice of physical activity and behavioral therapy. Prevention should be carried out with an adequate protein intake and exercise.
Some studies in dialysis patients already compare the typical recommendations of the diet of patients with CKD with those of a Mediterranean diet pattern.32 But this is not the usual practice. Patients with ACKD need around 30–35 calories kcal/kg/day,33 but we limit intake with typical restrictions to control weight, potassium or phosphorus. Thus, in the majority of patients with CKD we observe a poor energy pattern with few carbohydrates from vegetables and excessive consumption of fats.34 Patients with ACKD spontaneously tend to ingest low energy and proteins that can reach <0.7 g/kg/day, which is below the minimum recommended.35 However, it is recommended a decrease in protein intake in order to preserve kidney function, without knowing, on many occasions, what the actual intake of that specific patient. The protein requirements of patients with CKD are controversial, because most studies have few patients and their duration is too short, but a protein intake between 0.6−0.8 is recommended g/kg/day in stages prior to the start of dialysis and restrictions of up to 0.66 g/kg/day are accepted, with at least half of these proteins having high biological value.36 Once dialysis has started, the nutritional status is more vulnerable and the the indications on protein intake are changed, patients are recommended to increase their intake to 1–1.2 g/kg/day to need even 1.5 g/kg/day in situations of PEW or high catabolic states on dialysis.37,38 Our results agree with the inadequate distribution of dietary macronutrients observed in CKD patients. We observe a poor energy intake with few CH and an excess of fat. We think that one of the reasons for this decrease in CH could be the restriction of the consumption of fruit, vegetables, grains and legumes, by recommending a diet low in potassium. On the other hand, it is necessary to make up for these deficiencies with other food groups that can increase the overall intake of fats.
Protein restriction is often supported by the advice to reduce phosphorus intake, as protein-rich foods are usually also rich in phosphorus. This organic phosphorus is found in foods of animal and vegetable origin, but its absorption is different, for example, phosphorus from legumes and dried fruits, as it is in the form of phytic acid and humans lack the enzyme phytase, intestinal absorption can be reduced by half.39 But once again, vegetables are the foods we restrict in our patients' diets for fear of hyperkalemia. Our patients did modify their micronutrient intake and we observed an increase in potassium and fiber intakes, accompanied by a reduction measured by 24-h recall of daily intakes of sodium and phosphorus, according to the objectives set in the intervention. Although these differences were not statistically significant, it does seem to us that they follow a trend and that they may have clinical relevance, especially since they do not translate into an increase in plasma potassium levels.
Another strong point of the Mediterranean diet or healthier patterns of diet with abundant consumption of vegetables would be the increased intake of fiber. Fiber intake recommendations in our patients should be similar to those of the general population (around 20−35 g/day). In CKD there is a dysregulation in the intestinal microbiota in favor of proteolytic bacteria (Clostridium and Bacteroides), which produce toxic substances such as ammonium, thiols, phenols and indoles that accumulate in the patients with reduced kidney function, to the detriment of saccharolytic bacteria (bifidobacteria and Lactobacillus) that preferentially ferment carbohydrates, giving rise to short-chain fatty acids such as acetate, propionate and butyrate, which are beneficial. This dysbiosis with a high nitrogen/carbohydrate ratio, typical of a low fiber diet, promotes protein fermentation leading to an increase in uremic toxins. Increasing fiber intake involves eating fruit, vegetables or legumes, which are also restricted in this population.40–42 Again there is conflict on this point, since if we recommend a diet rich in fiber it will also be rich in fruits, vegetables and legumes that contain potassium.
The treatment of food with prolonged soaking and double and even triple boiling, traditionally recommended to reduce the amount of potassium, produces a significant loss of the rest of the vitamins and minerals, also modifying the qualities of the food, with loss of flavor and textures. Some studies have shown that simple boiling is enough to achieve the necessary potassium reduction in many of the most common vegetables used in our diet.43
In non-dialysis ACKD, the scarcity of studies, the fear of hyperkalaemia and the lack of nutritional education of patients make the management of dietary recommendations very complex.44 It seems that the risk of hyperkalemia and the fear of an insufficient supply of nutrients from diets with more vegetables, make the diet of kidney patients to move away from healthy patterns. According to the latest recommendations of the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines,45 dietary potassium restriction is a valid strategy to treat acute hyperkalaemia, but there is no direct evidence to support the current recommendation to restrict potassium in the diet of patients with CKD as a rule, however there is no evidence that increasing potassium intake or liberalizing potassium restrictions in patients with advanced CKD is safe. It is hypothesized that potassium restriction as a general strategy to prevent hyperkalaemia in people with CKD may deprive patients of the benefits associated with high-potassium diets. For this reason, it is increasingly important to move from the traditional recommendations of individual nutrients to a comprehensive management of the diet.46 With a nutritional education program and freeing the diet, we can also improve the quality of life considerably.47 Our study aimed specifically to demonstrate that individualized nutritional advice and nutritional education for patients with kidney disease with the consequent relaxation of restrictions could improve the nutritional profile of patients with ACKD, without favoring hyperkalaemia.
After nutritional education and freeing the diet, vegetable consumption showed to be increased in the consumption frequency questionnaires, fiber consumption also increased in the intervention group and, even so, during the study, potassium intake decreased in the intervention group and increased in the control group. Plasma potassium levels, despite not being restricted, remained stable in both groups. In our study, the increased consumption of fruit and vegetables, as well as vegetable fats, although it did not reduce weight, did not lead to an increase in it, nor were cases of hyperkalaemia observed, probably due to the reduction of other products that are also rich in potassium such as some dairy or processed foods, since it has been shown that the quality of the fats ingested influences our health more than the quantity.
Our patients were not malnourished by classical biochemical parameters. They did not have low levels of albumin, prealbumin, transferrin, bicarbonate, cholesterol, or total lymphocytes. What we have found in terms of analytical parameters are differences in creatinine and glomerular filtration rates. Regarding proteinuria, there were no significant changes in any group, remaining stable.
ConclusionsThe concept of malnutrition is not exclusively synonymous with undernutrition and encompasses both the problems derived from the deficit and excess intake of nutrients and the existence of overweight or obesity does not exclude a nutritional deficit. The current values for non-dialysis ACKD are not good, reflecting the global epidemic of overweight and obesity that we are experiencing. Nutritional advice for kidney patients is complex due to the multiple restrictions that have been carried out on a regular basis, which distances us from healthy eating patterns, such as the Mediterranean, and also worsens quality of life, which is associated with increased morbidity and mortality in these patients. Therefore, should we change the current diet of our patients to a pattern of Mediterranean diet rich in vegetables? The answer, as observed in our study, is that we are in the process of changing it. It seems reasonable to modify the usual recommendations towards healthier diets, provided that nutritional therapy in patients with CKD is individualized to avoid possible risks. This change may lead to a reduction in the progression of kidney disease with little risk of hyperkalemia if the patient is under close control. In addition, physical exercise should be recommended, including strength training as possible for each patient. More multicenter studies with multidisciplinary teams are necessary to assess long-term results and thus be able to improve the nutrition of our patients.
FinancingThis work has not received none type of financing. It is part of the doctoral thesis of Dr. María Martínez Villaescusa.
Conflict of interestsThe authors declare that they have no conflict of interest.