The new drugs developed for the treatment of anemia in chronic kidney disease patients, together with their mechanisms of action are reviewed. At present, many of them are already in advanced stages of clinical trials and is expected to be incorporated into the therapeutic arsenal in the coming years. The potential benefits and possible limitations are also described.
Se revisan los nuevos fármacos desarrollados para el tratamiento de la anemia en la enfermedad renal crónica, junto con sus mecanismos de acción. En la actualidad, muchos de ellos se encuentran ya en fases avanzadas de ensayos clínicos y es de esperar que se incorporen al arsenal terapéutico en los próximos años. Se describen las potenciales ventajas y sus posibles limitaciones.
Anaemia is a common complication in advanced chronic kidney disease (ACKD) and its severity increases as kidney function decreases. The introduction of treatment with recombinant human erythropoietin (EPO) (epoetin) three decades ago entirely changed the magnitude of the problem.1Table 1 shows the most important events in the treatment of this condition to date.1–7 Throughout this time, the treatment of anaemia has been based on the use of erythropoiesis-stimulating agents (ESAs), which included epoetin and its analogues, together with the administration of iron by oral or parenteral route.8 The analogues of erythropoietin include darbepoetin and CERA (continuous erythropoietin receptor activator). Darbepoetin is composed of an EPO that includes two sialic acid molecules, giving it a longer half-life, while CERA is a pegylated EPOβ, with an even greater half-life. In recent years, agents that are biosimilar to epoetin have been incorporated into the therapeutic arsenal, as they are less expensive.
The objective of this review is to raise awareness of the drugs that are currently in different clinical trial phases and that may constitute the basis of treatment for renal anaemia in the coming years. Table 2 shows a simple classification of the different ESAs that we will review.
EPO-mimetic agentsThis group includes the peptide molecules that act on EPO receptors in a similar way to endogenous EPO. The first EPO-mimetic on the market is peginesatide (Hematide®), composed of 2 peptide chains of 21 amino acids each, bound to a polyethylene glycol group. Its half-life is estimated at 80h and it may be administered as a monthly injection; there are no differences between intravenous or subcutaneous routes. This drug was approved by the FDA in 2012, only for use in haemodialysis patients. As it does not require genetic technology for its manufacture, it is a less expensive product than epoetin. Preliminary studies demonstrated other potential advantages such as the absence of immunogenicity, and it may therefore be used in cases of pure red cell aplasia; its greatest efficacy is in patients who are more resistant to other ESAs.
In January 2013, 2 prospective, controlled and randomised studies were simultaneously published for peginesatide in patients with ACKD. The first (EMERALD Study) was carried out in the U.S.A. and in Europe. It included patients on haemodialysis and was compared to EPO-α administered 1–3 times/week. The main conclusion was that peginesatide, administered once a month, resulted in haemoglobin levels equivalent to those obtained with epoetin.6
The second study (PEARL Study) was also developed in the same countries and it compared the efficacy of monthly peginesatide to darbepoetin every two weeks in patients with ACKD without dialysis. The results showed a similar efficacy between the 2 drugs at the end of 52 weeks. However, sudden death was 7 times greater in the peginesatide group, and the mortality rate due to unknown causes was twice as high.7 Post-marketing data have shown serious hypersensitivity reactions with several deaths,9 which lead the FDA to withdraw its approval. Therefore, it seems reasonable to assume that in a market as highly competitive as ESAs, the future of peginesatide is poor.
There are other fusion proteins with EPO-mimetic properties currently in the initial study phases.
Agents that stimulate endogenous erythropoietinProlyl-hydroxylase inhibitors (PHIs)Patients with ACKD have a relative EPO deficiency, and although serum levels may be normal, they are inappropriate for the haemoglobin concentrations they present.10 Furthermore, there may be hepatic production of EPO, which may be stimulated during episodes of liver disease, as it was demonstrated in patients on haemodialysis many years ago.11 Moreover, there are EPO-producing cells that remain in the kidneys and other tissues, with a yield sufficient to maintain patients on haemodialysis without anaemia and no need for ESAs. This situation is more common in cases on a long period of renal replacement therapy, in patients with polycystic disease and in cases of liver disease due to hepatitis virus C.12
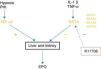
It has been known for many years that the inhabitants of the Andean altiplano have adapted to the state of hypoxia in which they live; in the same way that high altitude affects patients on haemodialysis.13 Low oxygen pressure induces the expression of the hypoxia-inducible factor (HIF), a transcription factor that regulates the expression of genes involved in erythropoiesis in response to changes in partial oxygen pressure. It is a heterodimeric protein with 2 components: hypoxia-sensitive HIF-α and HIF-β, which is an inactive component of the same molecule. The former has 3 subunits, which are called HIF-1α, HIF-2α and HIF-3α. HIF-1α is involved in the synthesis of EPO in embryonic states, losing its relevance after birth. It is also involved in processes of angiogenesis, mediated by VEGF, as well as in the anaerobic metabolism of glucose. Recently, it has been observed that this factor is also capable of contributing to the growth of renal cysts.14
HIF-2α is the most important subunit and is responsible for the changes in height and the genetic changes in the population that lives with these conditions. It is expressed in a large number of cells in the body, including the endothelial cells, hepatocytes, cardiomyocytes, glial cells, pneumocytes and renal peritubular interstitial cells. It is responsible for controlling EPO synthesis in adults and iron metabolism. Experimentally, it is known that HIF-2α inactivation followed by phlebotomy produces anaemia that cannot be recovered from, despite normal renal function, which gives an idea of how relevant a role it plays. For these reasons, HIF-2α becomes an important objective for pharmacological treatment. HIF-3α is the least important subunit and seems to play an inhibitory role.
In situations of normoxia, the participation of HIF-2α is not necessary, and it is therefore inactivated by hydroxylation through a proteosomal degradation process induced by prolyl-hydroxylase (PH). At least three PH subtypes are known, called PHD1, PHD2 and PHD3. In contrast, in situations of hypoxia, where an increase in the number of RBCs is needed to improve oxygen transport, HIF-2α stabilisation is produced by inhibition of the PH family. Therefore, HIF-2α becomes the main regulator of hypoxia-induced erythropoiesis.15
Currently, a family of drugs that act as PH inhibitors (PHIs) is being developed for clinical use. These new molecules are capable of increasing erythropoiesis by 2 mechanisms: first by stabilising HIF-2α levels, which likewise stimulates the synthesis of renal and hepatic EPO; second, through an improvement in iron metabolism, blocking the effect of hepcidin. Therefore, PHIs act physiologically, using the same mechanisms of action the body uses to adapt to less oxygen pressure in high altitudes.16
Hepcidin, synthesised by the liver, plays an important role in anaemia associated with inflammation, as it contributes to decreasing iron bioavailability. It acts through ferroportin degradation, which is a protein that acts as the principle exporter of iron from all cells. It must be recalled that iron from food is absorbed in the duodenum and subsequently passes through blood circulation, where it is bound to transferrin. From there, it is deposited in the liver cells and the reticuloendothelial system. Subsequently, in those cases in which erythropoiesis needs to be activated, it will again enter the circulation and be incorporated into the bone marrow. Therefore, the absence of ferroportin induced by hepcidin prevents iron from exiting the cells, which gives rise to iron sequestration in duodenal enterocytes, hepatocytes and macrophages. Physiologically, hepcidin inhibition occurs in cases of anaemia, iron deficiency, hypoxia (induced by HIF-2α) or due to genetic alterations, such as in the case of haemochromatosis. In contrast, hepcidin stimulation mainly occurs in cases of inflammation, which contributes to the development of anaemia in these cases.
Preliminary phase 1 data have already shown the increase in haemoglobin after treatment with FG-2216 in 12 patients on haemodialysis and in 6 healthy controls.16Table 3 includes some of the PHIs recently evaluated in phase 2 clinical studies. The initial results from clinical trials have now become available. Thus, experimentally, Molidustat is capable of stimulating EPO and reticulocyte production, without increasing blood pressure. Its effect is dose-dependent and it is administered by oral route. A daily dose of 2.5mg/kg obtains an effect equivalent to the administration of 100IU/kg epoetin.17 Roxadustat was compared to placebo in a controlled and randomised study. It was observed that a dose of 1mg/kg administered 2 times a week obtains a similar response on days 1 and 29, and on both days peak plasma levels of endogenous EPO were reached around 8h after administration. The effect on haemoglobin levels is also dose-dependent, with optimal doses at 1.5mg/kg. No advantages were found at greater doses, and administration 3 times a week is preferable to 2. In contrast, there is a significantly greater reduction of hepcidin levels if it is administered in doses of 2mg/kg 3 times per week.18 Similar results have recently been described with Daprodustat in stage 3–5 ACKD patients regarding the increase of haemoglobin and reticulocytes number, as well as in the decrease of hepcidin.19 Roxadustat maintain haemoglobin levels in patients on haemodialysis previously treated with epoetin, and also decrease hepcidin levels.20
Therefore, PHIs constitute a new therapeutic group for the treatment of renal anaemia with a series of advantages such as the capacity to increase haemoglobin levels through HIF stabilisation, thereby preventing fluctuations. They can be administered orally, enable improvement of iron mobilisation and present a good safety profile. As such, this may be a good alternative in the future for the management of anaemia in patients with ACKD, especially if the price is competitive as compared to ESAs, which require recombinant engineering processes for their manufacture. Nevertheless, more long-terms studies are needed to ensure that the potential effect of HIF on angiogenesis does not present adverse effects.
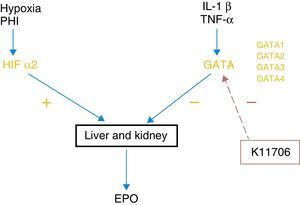
GATA inhibitorsErythropoiesis control by HIF-2α is negatively regulated by GATA, which is a transcription factor that inhibits EPO expression in the liver and kidneys. The main stimuli of this system are some of the known proinflammatory cytokines, such as IL-1, IL-6 or TNFα (Fig. 1). There are 4 subtypes of GATA and it seems that GATA-2 is the one that acts most potently on the regulation EPO synthesis. Thus, just as drugs that stabilise HIF-2α cause an increase in EPO synthesis, drugs that are capable of blocking the GATA system can act in a similar way.
Currently, at least 2 specific GATA inhibitors are known. Animal studies have shown that K7174, which suppresses the activity of GATA-1, -2 and -3, is capable of increasing the synthesis of EPO previously inhibited by inflammatory cytokines.21 K11706 is a much more potent inhibitory factor of GATA-2 and -3. In vitro experience has shown that its oral administration reverses the decrease in haemoglobin, reticulocytes and colony forming units-erythroid (CFU-E) induced by cytokines such as IL-1β, or TNF-α.22 Therefore, this group of inhibitors may improve inflammation-induced erythropoiesis. Nevertheless, more advanced-phase clinical studies are needed to be able to show that they may have a role in human clinical practice.
Agents with other mechanisms of actionAnti-hepcidin agentsIn addition to the inhibitory effect of hepcidin produced by PHIs that was described above, a specific anti-hepcidin drug has recently been developed. It was initially tested in monkeys,23 but the first publication in humans is now available. This drug, known as Lexaptepid, is capable of increasing serum iron levels and transferrin saturation in subjects who have previously developed an increase in hepcidin induced by endotoxaemia. The treatment was well tolerated.24 Therefore, treatment with anti-hepcidin drugs represents another new path for the management of anaemia associated with inflammation.
Anti-activin agentsActivin is a protein formed by 2 similar monomers bound by disulphide bonds, which belongs to the TGF-β superfamily. It is mainly produced in the ovarian follicles and gonads, and its most important function is to regulate the menstrual cycle by controlling FSH secretion. It also controls spermatogenesis and participates in healing processes and in the regulation of insulin secretion. In the bones, it acts as an inhibitor of bone growth by stimulating osteoclasts and inhibiting osteoblasts. It has 2 types of receptors, type I and type II.
Recently, an activin receptor type IIA antagonist called Sotatercept was developed. In a phase 1 trial for the treatment of osteoporosis in postmenopausal women it demonstrated an improvement in bone formation markers and a decrease in bone resorption, but as a side effect there was an increase in haemoglobin levels and in reticulocyte count.25 Following these findings, a large number of clinical trials have been started for the treatment of anaemia associated with neoplastic processes, metastasis or ACKD.
Preliminary (unpublished) data have shown in a small sample of patients on haemodialysis that Sotatercept produces a dose-dependent improvement in haemoglobin levels; the best response is attained with 0.7mg/kg. An added effect was bone formation stimulation, with an improvement in structure and density, assessed by quantitative computed tomography, together with an improvement in progression of vascular calcification assessed by the Agatston score. These findings make Sotatercept a promising molecule with the capacity to favourably treat the standard complications of ACKD patients, such as anaemia, vascular calcification and bone alterations.
In sum, new molecules have been developed with the capacity to stimulate erythropoiesis by mechanisms different to current ESAs. Some of them can be administered orally and they have shown good tolerance to date. In addition to the possibility of reducing treatment costs, these molecules mark the start of a new encouraging era of anaemia management in patients with ACKD.
Key concepts- •
New drugs for the treatment of renal anaemia, currently in advanced clinical trials, are showing very encouraging results for the coming years.
- •
PHIs stimulate erythropoiesis through HIF stabilisation and hepcidin inhibition.
- •
Activin inhibitors such as Sotatercept may act by improving anaemia, delaying vascular calcification and improving bone structure and density.
The authors declare they received no funding of any type for this review.
Conflicts of interestThe authors declare that they have no conflicts of interest regarding the content of this review.
Please cite this article as: López-Gómez JM, Abad S, Vega A. Nuevas expectativas en el tratamiento de la anemia en la enfermedad renal crónica. Nefrologia. 2016;36:232–236.