Background: In postoperative critically-ill patients who develop Acute Kidney Injury (AKI) it is important to focus on survival and quality of life beyond hospital discharge. The aim of the study was to evaluate outcome and quality of life in patients that develop AKI after major surgery. Methods: This retrospective study was carried out in a Post- Anaesthesia Care Unit with five intensive care beds during 2 years. Patients were followed for the development of AKI. Preoperative characteristics, intra-operative management and outcome were evaluated. Six months after discharge, these patients were contacted to complete a Short Form-36 questionnaire (SF-36) and to have their dependency in Activities of Daily Living (ADL) evaluated. Chi-square or Fischer's exact test were used to compare proportions between groups. A “t test” and a paired “t test” for independent groups was used for comparisons. Results: Of 1597 patients admitted to the PACU, 1200 patients met the inclusion criteria. One hundred–fourteen patients (9.6%) met AKI criteria. Patients with AKI were more severely ill, stayed longer at the PACU. Among 71 hospital survivors at 6 months follow-up, 50 completed the questionnaires. Fifty-two percent of patients reported that their general level of health was better on the day they answered the questionnaire than 12 months earlier. Patients that met AKI criteria after surgery had worse SF-36 scores for physical function, role physical and role emotional domains. Six months after PACU discharge, patients that met AKI criteria were more dependent in Instrumental-ADL but not in Personal-ADL. Conclusions: Patients that develop AKI improved selfperception of quality of life despite having high rate of dependency in ADL tasks. For physical function and role physical domains they had worse scores than PACU patients that did not develop AKI.
INTRODUCTION
Acute kidney injury (AKI) is commonly seen in the perioperative period and in the Intensive Care Unit (ICU). It is associated with a prolonged hospital stay and high morbidity and mortality1-5.
To date, there is no universally accepted definition for acute kidney dysfunction. Varying terms, including acute renal failure, renal insufficiency, kidney injury, and renal impairment, and various definitions (e.g., percent or absolute increments of creatinine, or decrements of urine output) have been used in previous publications. Furthermore the term acute kidney injury has been put forth as the preferred nomenclature to replace acute renal failure with the understanding that the spectrum of AKI is broad and includes different degrees of severity.
We used the proposed definition by the Acute Kidney Injury Network (AKIN) that was formed to facilitate the development and execution of initiatives to ensure the best outcomes for patients with acute kidney injury6,7. These criteria were based on accumulating evidence that even small alterations in serum creatinine are associated with severe consequences2,8-10.
Acute Kidney Injury occurs in approximately 1-5% of all hospitalized patients and is increasingly prevalent1,11 and is known to be an independent predictor of poor in-hospital outcome1,2,12.
It is devastating to both patients and anaesthesiologists, when patients with no evidence of renal dysfunction preoperatively, develop AKI after surgery. Various studies have been published determining AKI incidence in specific patient populations: hospitalized patients13, ICU patients14,15, after cardiac surgery16,17 patients with sepsis and patients on renal replacement therapy18,19.
We conducted a study in patients that met AKI criteria after major surgery to evaluate outcome and quality of life. In order to accomplish these tasks we tried to evaluate the longterm health status, incorporating measures of quality of life, functional status and hospital discharge location that are now recognized as significant markers of morbidity in survivors of critical illness20,21.
The long-term quality of life and functional status for survivors of critical illness specifically characterized by severe Acute Renal Failure have been described in various studies 22-27.
Several questionnaires have been validated to study Health-Related Quality of Life (HRQOL)28-32, mostly multi-item scales. The Short-Form General Health Survey (SF-36) was developed during the Medical Outcomes Study to measure generic health concepts that are relevant across age, disease and treatment groups33. It is a self-completed questionnaire covering all aspects of HRQOL, it shows good reliability and validity33,34, and it has been used for various groups of patients including post-discharge Intensive Care Unit (ICU).
The ability to care for oneself and live independently is considered a measure of functional outcome after hospitalization35. Functional status refers to the level of involvement in activities and is often used as a synonym for performance in ADL36. ADL appraisal scales consider functional and instrumental activities. The ability of patients to handle these activities has been assessed by generic or disease-specific tests. Katz’s ADL Scale36 and the Lawton Instrumental ADL37 have been used for critical care survivors.
The aim of the present study was to evaluate outcome, quality of life and autonomy in ADL in patients that develop AKI after major surgery and to identify its determinants.
METHODS
The Institutional Review Board approved the study and waived the requirement for informed consent for the retrospective review of medical records. This retrospective cohort study was carried out in the multidisciplinary Post-Anaesthesia Care Unit with five intensive care beds, at the Hospital São João, an 1100-bed community teaching hospital in Porto, Portugal.
Patient population
All postoperative patients admitted to the PACU, aged 18 years or more, who underwent scheduled or emergency surgery between 1 March 2006 and 1 March 2008 with an overnight admission and more than 12 hours of PACU stay were eligible to the study. Patients readmitted during the study period were enrolled in relation to the time of their first admission. Excluded were patients submitted to cardiac or pulmonary surgery.
The PACU admits all surgical patients, with the exception of cardiothoracic.
Patients with abnormal renal function preoperatively (defined as creatinine blood levels higher than 2.0 mg/dL) were excluded.
Primary outcome
The primary outcome was the development of AKI during PACU stay.
Patients were classified as having AKI criteria according to the definition proposed by the Acute Kidney Injury Network7 if they had an increment of Scr >0.3 mg/dL or >50% increase within any 48 hr interval and/or an episode of <0.5 mL/kg/hr urine output for >6 hrs despite fluid challenge of >500 mL of normal saline, when appropriate.
Covariates
The following variables were recorded on admission to the PACU: age, gender, body mass index (BMI), American Society of Anesthesiologists physical status (ASA-PS)38, emergency or scheduled surgery, preadmission comorbilities (specifically ischemic heart disease, congestive heart failure, cerebrovascular disease, hypertension, renal insufficiency, diabetes, hyperlipidemia) and duration and type of anesthesia. Intraoperative and PACU data were collected as well as hospital length of stay (LOS). Mortality was recorded for all patients. The Acute Physiology and Chronic Health Evaluation (APACHE) II39 and the Simplified Acute Physiology Score II (SAPS II)40 were calculated using standard methods except that they were modified to excluded creatinine increments and the number and types of organ failures.
Adapting a classification scheme developed by Lee and colleagues41, we calculated the Revised Cardiac Risk Index (RCRI), assigning one point for each of the following risk factors: high-risk surgery, ischemic heart disease, cerebrovascular disease (defined as history of transient ischemic attack or history of cerebrovascular accident) and diabetes mellitus requiring insulin therapy.
Physiologic data were recorded using customized data entry forms. Included was Serum Creatinine (Scr) that was recorded for each day and for PACU admission and at least every patient had two Scr determinations. These data were also recorded 24 hours before meeting criteria for AKI, at the time of AKI, 24 hours after AKI, and >48 hours after AKI.
We’ve also recorded PACU and hospital LOS. For mortality we have recorded PACU mortality, hospital mortality and mortality at 6 months after PACU discharge.
Functional capacity
Functional capacity before surgery was evaluated within the first 24 hours after PACU admission in terms of the patient’s ability to handle personal and instrumental ADL. All eligible consenting patients were interviewed directly by a trained investigator. When the patient was unable to respond, the questionnaire was completed by a close family member living in the same household as the patient. This evaluation was repeated along with the SF-36 questionnaire six months after PACU discharge.
Quality of life
HRQOL was assessed by the SF-3634. The survey contains 36 questions that evaluate eight health domains considered to be important for patient well-being and health status. These domains reflect physical health, mental health, and the impact of health on daily functioning. The eight multiple-item domains encompass physical functioning (ten items), social functioning (two items), role limitations caused by physical problems (four items), role limitations caused by emotional problems (three items), mental health (five items), energy and vitality (four items), pain (two items) and general perception of health (five items). There is one further unscaled item relating to self-reported changes in the respondent’s health status during the past year. For each item, scores are coded, summed and transformed to a scale from 0 (worst possible health state measured by the questionnaire) to 100 (best possible health state). Scores can be aggregated to measures representing a physical health summary scale (consisting of physical functioning, physical role, pain and general health) and a mental health summary scale (vitality, social functioning, emotional role and mental health)28.
The answers to the question about self-reported changes in health status (“compared to one year ago, how you would rate your health in general now?”) were dichotomized as: better, about the same or worse than one year ago.
To minimize distress to the next of kin, each patient’s records were checked on the hospital information system after 6 months to ascertain whether he or she was still alive. A copy of a formal letter was sent to all known survivors accompanied by a return envelope and a validated Portuguese SF-36 self-report form42,43. This version has been validated for the population of the city of Porto from which the subjects of this report were drawn44.
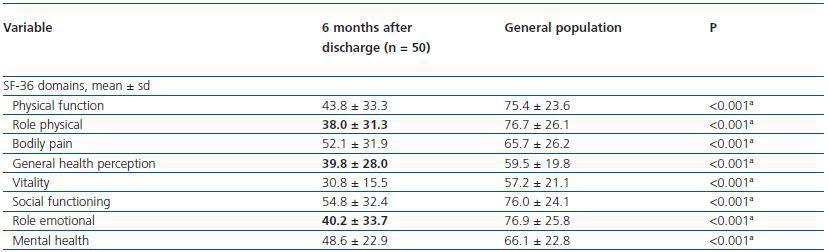
Scores for all domains obtained for patients that developed AKI were compared with this published40 urban population values obtained for population of Porto.
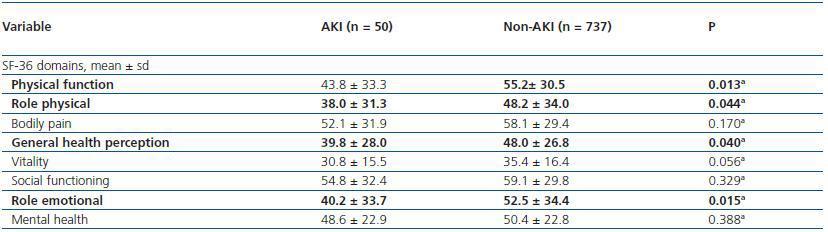
We also compared scores in all SF-36 domains obtained for AKI patients with scores obtained for PACU patients that didn’t develop AKI after surgery.
The questionnaire used to assess dependency was based on the Katz Index of Independence in ADL22 and Lawton Instrumental ADL scale. The Lawton Instrumental ADL scale is an easily to administer assessment instrument that provides self-reported information about the functional skills necessary for living in the community. Deficits in the instrumental Lawton scale were scored and a summary score ranging from 0 (low function, dependent) to 7 (high function, independent) was obtained. The Katz ADL scale assesses basic personal activities of daily living and ranks adequacy of performance in six functions. Dependency in each personal activity was evaluated and a summary score ranging from 0 (independence in all activities) to 6 (dependency in all activities) was obtained. The personal ADL (P-ADL) considered were bathing, dressing, going to the toilet, transferring from bed to chair, continence and feeding. The instrumental ADL (I-ADL) considered were cleaning, food shopping, public transportation and cooking. Answers were categorized into two groups: able or unable to perform each activity and group of activities. Patients were considered dependent if they were dependent in at least one I-ADL or PADL activity.
Outcome measures
Considered outcome endpoints were: 1) Functional capacity and ADL. Patients were considered dependent if they were dependent in at least one I-ADL or P-ADL activities. 2) Quality of life. Quality of life was evaluated at 6 months after PACU discharge. 3) Mortality. Patients were considered survivors if they were alive 6 months after PACU discharge.
Statistical methods
Descriptive analyses of variables were used to summarize data and the Mann-Whitney U test was used to compare continuous variables between two groups of subjects; chisquare or Fischer’s exact test were used to compare proportions between two groups of subjects.
We used a significance level of 0.05 (two sided) for all statistical tests except when multiple comparisons were made. In such cases we controlled the values for multiple comparisons to reduce the risk of type II error and the entry criterion of P <0.001 was used.
A “t test” for independent groups was used for comparison to population means. The SF-36 scores for AKI population were compared with the general population using a paired “t test”. Every patient in the AKI population was paired prospectively with a demographically matched patient from the control population.
To evaluate the determinants of mortality and dependency in at least one ADL we have used a multiple logistic regression analysis with an entry criterion of P <0.001 and independent variables: age, gender, BMI, ASA-PS, type and magnitude of surgery, co-morbidities and RCRI score, type of anaesthesia, intra-operative fluid administration and length of anaesthesia, temperature at admission to the PACU, Troponin I at admission, PACU LOS, SAPS II scores, APACHE II scores and PACU and hospital LOS.
SPSS for Windows version 16.0 (SPSS, Chicago, IL) was used to analyze the data.
RESULTS
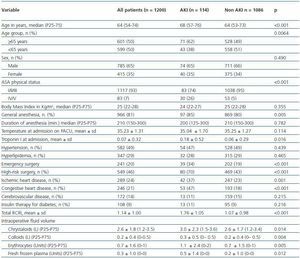
A total of 1597 patients were admitted to the PACU during the study period and 1200 patients met the inclusion criteria and were followed for the development of AKI after PACU admission. Eighty patients were excluded because they had abnormal renal function preoperatively (defined as Scr higher than 2.0 mg/dL), 196 stayed less than 12 hours and did not had an overnight admission, 52 because they had less than 18 years and 44 were admitted more than once to the PACU, 13 were admitted after pulmonary surgery and 12 were not surgical patients.
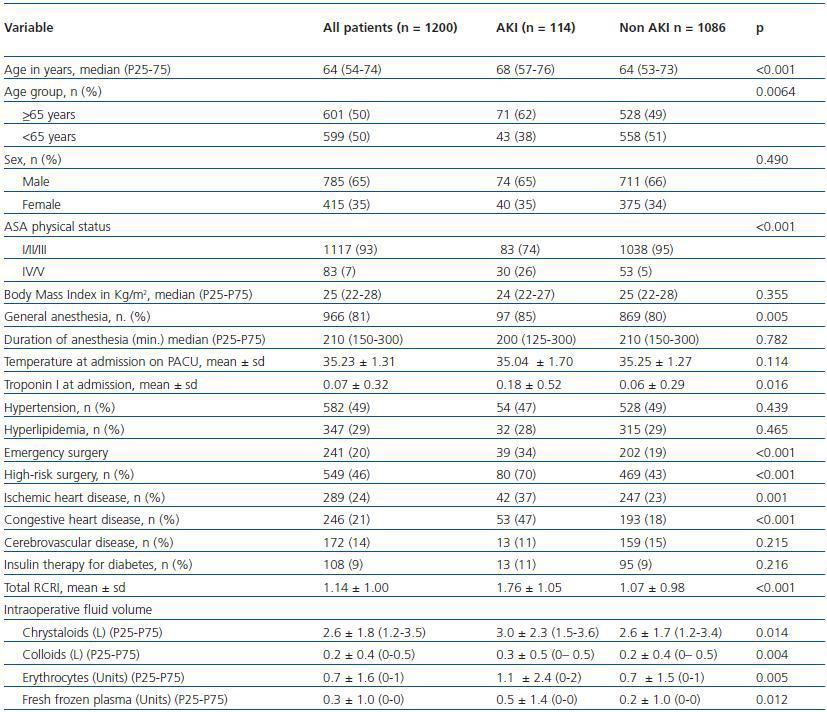
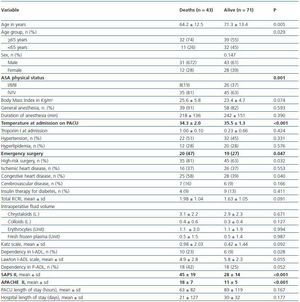
One-hundred fourteen patients (9.6%) developed AKI. The characteristics of patients with and without AKI are summarized in table 1. Patients with AKI were older (median age 68 versus 64 years, p <0.001), had higher ASAphysical status (26% versus 5%, p <0.001), were more likely to have been submitted to general anesthesia (85% versus 80%, p = 0.005), had higher Troponin I at admission (0.18 ± 0.52 versus 0.06 ± 0.29, p = 0.016) were more likely to have been submitted to emergency surgery (34% versus 19%, p <0.001) and high risk surgery (70% versus 43%, p <0.001), had more frequently ischemic heart disease (37% versus 23%, p = 0.001) and congestive heart disease (47% versus 18%, p <0.001), had higher RCRI scores (1.76 ± 1.05 versus 1.07 ± 0.98, p <0.001) and had higher volume of intraoperative fluids administered (3.0 ± 2.3 versus 2.6 ± 1.7, p = 0.014 for litres of crystalloids; 0.3 ± 0.5 versus 0.2 ± 0.4, p = 0.004 for litres of colloids; 1.1 ± 2.4 versus 0.7 ± 1.5, p = 0.005 for units of erythrocytes; 0.5 ± 1.4 versus 0.2 ± 1.0, p = 0.012 for units of fresh frozen plasma).
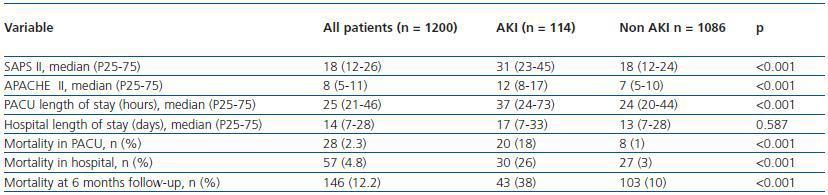
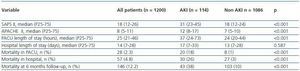
Table 2 shows the severity of disease scores and outcome for patients with and without AKI. Patients with AKI were more severely ill (median SAPS II 31 versus 18, p <0.001 and median APACHE II 12 versus 7, p <0.001), stayed longer at the PACU (median LOS 33 hours versus 20 hours, p <0.001). The unadjusted mortality rate at 6 months follow-up of patients with AKI was 38%, nearly 4 times the mortality rate of those without AKI (38% versus 10%, p <0.001). The increased mortality observed among patients with AKI was even greater for hospital mortality (26%, versus 3%, p <0.001) and PACU mortality (18% versus 1%, p <0.001).
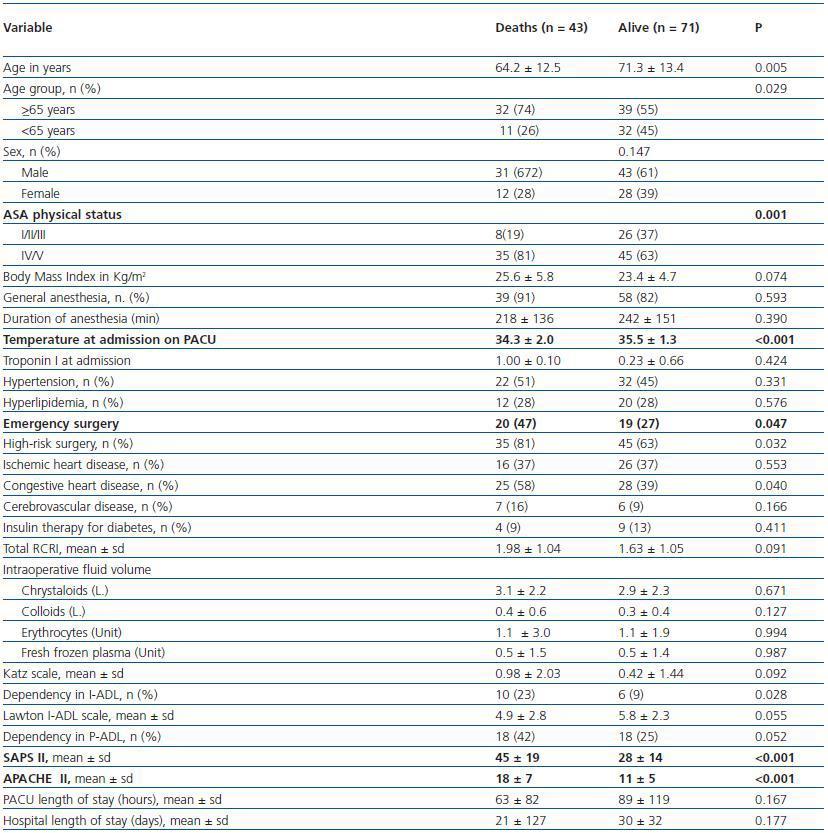
Table 3 presents the characteristics of PACU patients according to mortality at 6 months follow-up study. Patients with postoperative AKI criteria that have died until 6 months follow-up had higher scores of ASA-PS (81% versus 63% were ASA IV or V patients) and higher severity of disease scores (45 ± 19 versus 28 ± 14 for mean SAPS II scores and 18 ± 7 versus 11 ± 5 for mean APACHE II scores with p <0.001 for both scores) and had lower temperatures at admission to the PACU (34.3 ºC ± 2.0 versus 35.5 ºC ± 1.3 ºC, p <0.001).
Median PACU length of stay were higher in patients that met AKI criteria compared with those without AKI criteria (37 hours versus 24 hours, p <0.001).
Twenty patients (18%) died during PACU stay, ten died before hospital discharge and thirteen died before follow-up at 6 months.
Of the remaining 71 patients, 21 (30%) did not answer the questionnaires at 6 months follow-up but were known to be alive.
Response rate to the questionnaires was 70%. There were no significant differences in background and ICU variables between respondents and non-respondents.
FUNCTIONAL CAPACITY AND ADL
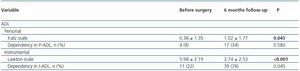
Focusing in patients that met AKI criteria, six months after discharge from ICU, 78% were dependent in at least one activity in I-ADL and 34% in at least one P-ADL (table 4).
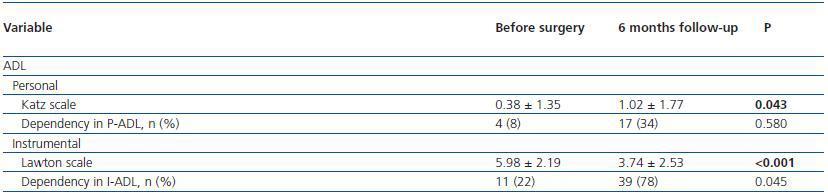
In these patients, dependency in I-ADL was significantly more frequent after surgery and Lawton scales were lower, indicating more dependency, after surgery. Dependency in PADL was not different after surgery but Katz scales were higher, indicating more dependency, 6 months after PACU discharge.
In the univariate analysis patients that met AKI criteria and had dependency in at least one ADL were older than those that were not dependent (OR 9.0, 95%CI 1.69 - 47.84, p = 0.010); there were no more differences in all other studied variables.
QUALITY OF LIFE MEASURES
Fifty-two percent of patients that met AKI criteria stated that their level of health in general was better on the day they completed the SF-36 questionnaire, while 36% considered it to be worse than previously (6 months before PACU discharge). In this group of patients there was no statistically significant relationship between the different studied variables and a worse self -reported general level of health.
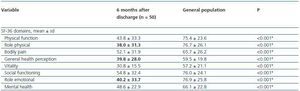
Compared to values observed for the urban population of Porto, the SF-36 sub scores of all patients that developed AKI criteria were worse for all domains (table 5).
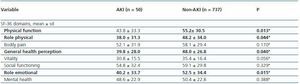
Patients that met AKI criteria had worse SF-36 scores for physical function, role physical, general health perception and role emotional domains when compared to PACU patients that didn’t met AKI criteria (table 6).
DISCUSSION
This is the first study to examine whether the Acute Kidney Injury Network’s interim consensus definition for AKI is associated with clinically meaningful outcomes in a cohort consisting of 2 years admissions of intensive care patients to a PACU.
In our study patients that met AKI criteria were 9 times as likely to die during hospitalization and almost 4 times as likely to die at 6 months after discharge. These patients that met AKI criteria stayed longer at PACU and were more severely ill. These associations were expected and previous studies that examined the impact of acute renal failure in intensive care population documented increased mortality and prolonged length of stay using AKI criteria14 or other definitions for acute renal failure7,13. These associations were also encountered after cardiac surgery17.
Not surprisingly, that patients that died and met AKI criteria had higher severity of disease scores and more co morbidities as can be supposed by higher ASA-PS. What is interesting is that temperature at admission was a determinant of mortality 6 months after PACU discharge. In a study about predictors of core hypothermia after surgery, Kongsayreepong et al.45 found that although core hypothermia on arrival tended to be a significant predictor of mortality the sample size may not have been large enough to prove this and the authors themselves published a study were this correlation could not be met46.
Acute kidney injury (AKI) is a heterogeneous syndrome encompassing a broad spectrum of insults and changes in function that occur acutely to the kidneys47. This syndrome is increasingly encountered in sick hospitalized patients, in particular those admitted to intensive care12,48,49. The development of AKI undoubtedly has important implications on both short- and long-term morbidity and mortality50.
The 2005 AKIN consensus definition was chosen understanding that kidney dysfunction is a continuum of disease, and in order to study it most broadly, the initial interim diagnosis should be as sensitive as possible. The definition for AKI was not intended to be final. Rather, it was proposed with the presumption that it would be tested and refined based on scientific data. Our data indicate that the AKI definition is an excellent beginning. Using only an increase in Scr >0.3 mg/dL within 48 hrs as the sole criterion for AKI has the added advantage of not requiring measurement of hourly urine output, a task not routinely done outside an ICU. Using these criteria Barrantes et al.14 (in a critically ill population) and Tian et al.10 (in a medical wards population) concluded that AKI was associated with poor outcomes.
The study by Chertow et al.2 first demonstrated that a change of Scr >_0.3 mg/dL any time during hospitalization was associated with increased mortality. Our data support not only that such small changes of creatinine are associated with meaningful differences in outcome but also that acute increments >_0.3 mg/dL predict outcomes. This clinically practical definition of AKI benefits from its simplicity and robust association with patient outcomes and therefore may be most appropriate for future epidemiologic and therapeutic studies.
With the present study we have examined the impact of the development of AKI after surgery on quality of life and independence in activities of daily living.
The patients in our study had higher degrees of dependency in instrumental ADL after surgery and they have scores indicating more dependency in Katz and Lawton scales. Our results indicate that 6 months after PACU discharge, in patients that met AKI criteria after surgery, 78% were dependent in at least one ADL activity and when we compare this degree of dependency with the previous status we saw that they are significantly more dependent.
These results are somehow worst that those reported by Maynard SE et al.22 in their study about quality of life in patients requiring renal replacement therapy that concluded that “almost half of patients became dependent in at least one ADL”.
It seems paradoxical that these patients, despite being more dependent, stated that their quality of life was better than before surgery. We think this could also be explained by their expectation of better health when they agreed to surgical intervention.
To study the impact of the procedure in quality of life we have used the self evaluated health transition item of SF36 questionnaire. This item is not used in scoring the scales and has been shown to be useful in estimating average change in health status during the year prior to its administration51.
Measuring changes in health status we found an improved subjective perception of quality of life 6 months after PACU discharge in patients that met AKI criteria, among the patients who completed the study. Other studies on patients after ICU discharge have reported similar findings using different tools52,53 and Gopal et al.54 have reported the same finding in patients with renal failure treated with renal replacement therapy. Because self-perception of health may reflect anticipation of future health after a surgical procedure, it is not surprising that these patients have similar scores to other patients subjected to surgical procedures and admitted to the PACU. Comparisons with a general (taken as “control”) population are difficult to interpret because our patients were all submitted to a surgical procedure and after surgery met AKI criteria. Thus, our finding that quality of life was worse in these patients than in the general population was not totally unexpected. The comparison to other PACU patients with similar demographic characteristics and from the same urban area seemed more appropriate for establishing comparisons with ours. We found that AKI patients had lower scores for physical domains and they also had a worst general health perception and role emotional.
This study has several limitations. The small sample size may limit the ability to document real differences among our subgroups of patients.
Because of the retrospective nature of this study we could not ascertain the therapeutic responses to the development of AKI and there were no gold standard for fluid challenge and we presume that practitioners may have varied considerably in their approach.
The outcomes we selected – mortality, length of PACU stay, quality of life and dependency in ADL activities – may not necessarily capture all relevant consequences of AKI and we did not know how frequent was the development of chronic kidney disease and the future need for replacement renal therapy.
We did not apply the SF-36 questionnaire before surgery so it was not possible to compare quality of life of patients before and after surgery. Nevertheless, we used the SF-36 question about self-reported changes in health status (“compared to one year ago, how would you rate your health in general, now?”) to conclude that the level of health in general was better for most patients on the day they completed the SF-36 than before surgery.
In summary, this study supports the interim consensus definition of AKI is clinically valid, in that it independently predicts meaningful clinical outcomes and that patients perceive their quality of life as improved six months after surgery, although they are more dependent in ADL activities.
COMPETING INTERESTS
The authors did not use funds for the research and have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
All people listed as authors contributed to the preparation of the manuscript and no person or persons other than the authors listed have contributed significantly to its preparation.
Each listed author participated in the work to the extent that they could all publicly defend its content. They all read the manuscript before its submission for publication and are prepared to sign a statement stating they had read the manuscript and agree to its publication.
Table 1. Patient characteristics and outcomes
Table 2. Severity of disease scores, PACU and Hospital Length of stay and Mortality
Table 3. Characteristics of patients according to mortality at 6 months follow-up
Table 4. Dependency and self-reported changes in health in general, 6 months after PACU discharge for
patients that met AKI criteria (n = 50)
Table 5. SF-36, 6 months after PACU discharge for patients that develop AKI and for general population
Table 6. SF-36 6 months after PACU discharge