Introducción: Paricalcitol, un activador selectivo de los receptores de la vitamina D, se usa con éxito en el tratamiento del hiperparatiroidismo secundario a la enfermedad renal crónica (ERC). Además, se ha propuesto que reduce la proteinuria en pacientes con ERC. Sin embargo, poco se sabe de su efecto sobre las pérdidas proteicas peritoneales en pacientes en diálisis peritoneal (DP). Objetivos: Analizar la eficacia de paricalcitol en formulación oral en el control del hiperparatiroidismo secundario en pacientes en DP y comprobar su efecto sobre las pérdidas proteicas urinarias y en el efluente peritoneal. Material y métodos: Estudio prospectivo, de 12 meses de seguimiento, sobre una cohorte de pacientes en DP. La intervención fue la introducción de paricalcitol para el tratamiento de su hiperparatiroidismo secundario. Paricalcitol se dosificó en función de la hormona paratiroidea (PTH); así, si la PTH era menor de 500 pg/ml, cada paciente recibió una cápsula de 1 mg/día; si la PTH era mayor de esa cifra, recibieron la dosis de 2 mg/día. Se analizaron datos epidemiológicos, clínicos y analíticos. Resultados: 38 pacientes (edad 56 ± 19 años, 55 % mujeres, 16 % diabéticos, tiempo en técnica 14 ± 10 meses) fueron incluidos en el estudio. Treinta y tres de ellos recibieron 1 mg/día de paricalcitol; el resto, 2 mg/día. El empleo de paricalcitol se asoció a un descenso de la PTH del 30,7 ± 6,8 % (p < 0,001) tras los 12 meses de tratamiento, sin cambios en los niveles de calcio (8,82 ± 0,96 vs. 9,02 ± 0,91; p = 0,153) y fósforo (4,78 ± 0,63 vs. 4,93 ± 0,77; p = 0,693). Los pacientes no modificaron el tratamiento concomitante con captores del fósforo a lo largo del período de estudio; tampoco cambió la dosis de cinacalcet, aunque menos pacientes precisaron su uso a la finalización del estudio. Los niveles basales de PTH fueron predictores independientes de su descenso (b = 0,689, p = 0,018), no influyendo el resto de los parámetros analizados. A lo largo del período de estudio se produjo un descenso de la proteinuria (0,79 ± 0,41 vs. 0,64 ± 0,36 g/día, p = 0,034) sin cambios en la función renal (7,2 ± 1,1 vs. 6,3 ± 0,9 ml/min, p = 0,104), en el porcentaje de pacientes que tomaba inhibidores del sistema renina angiotensina (71 vs. 68 %, p = 0,472) ni en las dosis pautadas. No hubo modificación en las pérdidas proteicas peritoneales (5,8 ± 1,9 vs. 6,0 ± 2,2 g/24 h, p = 0,731) ni en los niveles de albúmina sérica (3,7 ± 1,1 vs. 3,7 ± 1,2 g/dl, p = 0,697). Conclusiones: El uso de paricalcitol oral reduce de forma sustancial y segura los niveles de PTH en pacientes en DP. Su uso se asocia a un descenso de la proteinuria no relacionado con el descenso del filtrado glomerular ni con cambios en la medicación que pudiera modificarla. No encontramos una modificación en la cuantificación de las pérdidas proteicas peritoneales.
Introduction: Paricalcitol, a selective activator of Vitamin D receptors, is successfully used as a treatment of hyperparathyroidism secondary to chronic kidney disease (CKD). In addition, it has been proposed for reducing proteinuria in patients with CKD. Nonetheless, little is known about its effect on peritoneal protein loss in patients on peritoneal dialysis (PD). Objectives: To analyse the efficiency of oral paricalcitol in secondary hyperparathyroidism control in PD patients and to verify its effect on urinary and peritoneal effluent protein loss. Material and method: Prospective study with a 12-month follow-up on a cohort of PD patients. Invention consisted of the introduction of paricalcitol for the treatment of secondary hyperparathyroidism. Paricalcitol was dosed according to parathyroid hormone (PTH): 1mg/day for patients with PTH < 500pg/ml, and 2mg/day for those with higher PTH levels. Epidemiological, clinical and analytical data were analysed. Results: 38 patients (56 ± 19 years, 55% women, 16% diabetics, technique time (14 ± 10 months) were included in the study. Thirty-three of them received 1mg/day of paricalcitol; the rest received 2mg/day. The use of paricalcitol was associated with a PTH decrease of 30.7 ± 6.8% (P<.001) after 12 months of treatment with no changes in calcium (8.82 ± 0.96 vs. 9.02 ± 0.91; P = .153) and phosphate levels (4.78 ± 0.63 vs. 4.93 ± 0.77; P = .693). Patients did not modify treatment concurrent with phosphate binders over the study period, nor did they change the cinacalcet dosage. However, fewer patients needed it by the end of the study. The PTH baseline levels were independent indicators of its decrease (b = 0.689, P = .018), and the rest of the analysed parameters were not affected. Over the study period there was a proteinuria decrease (0.79 ± 0.41 vs. 0.64 ± 0.36g/day, P = .034) with no changes in renal function (7.2 ± 1.1 vs. 6.3 ± 0.9ml/min, P =.104). Similarly, no differences were found in in the percentages of patients taking renin-angiotensin system inhibitors (71 vs. 68 %, P = .472) or the doses needed. There was no significant change in peritoneal protein loss (5.8 ± 1.9 vs. 6.0 ± 2.2g/24h, P = .731) nor in serum albumin levels (3.7 ± 1.1 vs. 3.7 ± 1.2g/dl, P = .697). Conclusions: The use of oral paricalcitol reduces PTH levels safely and substantially in patients on PD. Their use is associated with a proteinuria decrease and is not linked to a decrease of glomerular filtration rate or changes in the medication that could modify it. We have found no modification in the amount of peritoneal protein loss.
INTRODUCTION
Secondary hyperparathyroidism (SPTH) is practically universal in patients with chronic kidney disease (CKD) on dialysis1 and is associated with an inferior survival rate.2 As CKD progresses, the rate of synthesis of calcitriol and serum calcium (Ca) levels deteriorate, with increases in phosphate (P), fibroblastic growth factor 23 (FGF-23), and parathyroid hormone (PTH) levels, this being the biochemical signature indicative of SPTH.3 These changes also produce increased rates of vascular calcification and bone disease.2
The treatment of SPTH was traditionally based on controlling hyperphosphatemia through diet restriction and the use of intestinal phosphate binders, controlling hypocalcaemia through the administration of calcium salts and calcitriol, and controlling PTH levels by administering calcitriol. At the beginning of the XXI century, selective vitamin D receptor activators and calcimimetics were also introduced.4,5
The characteristics of SPTH in peritoneal dialysis (PD) do not differ much from that of patients on haemodialysis, although there is typically a better P balance due to superior peritoneal clearance4 as well as a greater risk of adynamic bone disease.7
Renin-angiotensin-aldosterone system (RAAS) inhibitors are used with great success to control blood pressure. In addition, the use of these drugs is associated with reduced proteinuria due to improved vasodilatation of the efferent glomerular arteriole8 and improved capacity to reduce inflammation, oxidative stress, and vascular smooth muscle fibre remodelling.9,10 In this context, RAAS inhibitors play an important role in the treatment of the majority of kidney diseases.
Peritoneal protein losses in patients on PD are a good marker for endothelial dysfunction and an independent predictor for mortality in patients on PD.11,12 The hypothesis has been set forth that the use of RAAS inhibitors may be beneficial to patients on PD because of the capacity of these drugs to reduce peritoneal protein losses through mechanisms that remain unclear, but that are probably related to improved endothelial function.13,14 However, a recent study showed no such association.15
Paricalcitol, a selective vitamin D receptor activator, forms part of the treatment for SPTH as previously mentioned. It has been shown to be capable of reducing PTH levels with minimal changes produced in calcaemia and phosphataemia in patients on haemodialysis and on PD.16 In addition, experimental studies have shown that paricalcitol has an inhibitory effect on the renin-angiotensin-aldosterone system due to its capacity to reduce the expression of renin and angiotensinogen.17
The objective of our study was to analyse the efficacy of paricalcitol on the control of hyperparathyroidism in a large cohort of patients on PD, also examining its possible effects on proteinuria and peritoneal protein losses. To this end, we designed a study evaluating the evolution of PTH as the primary study variable. We analysed variations in calcaemia and phosphataemia, intake of other medications related to bone mineral metabolism and the RAAS, proteinuria, and peritoneal protein losses as secondary variables.
PATIENTS AND METHOD
Patient selection
We evaluated all patients in the PD programme at the Hospital Universitario Central de Asturias, both on continuous and automated techniques.
We considered the following inclusion criteria: 1) patients of both sexes and older than 18 years of age, 2) clinically stable, defined as an absence of complications requiring hospitalisation during the previous 8 weeks, 3) on the PD programme for at least 3 months, 4) no known reaction or eventual intolerance to study medications, and 5) diagnosis of SPTH, defined as a PTH value greater than 300pg/ml.18 We also excluded those patients with Ca values greater than 9.5mg/dl or P greater than 5mg/dl, or with poor adherence to treatment.
Patients who participated in the study signed an informed consent before being included.
Procedures
The intervention consisted of administering oral paricalcitol during 12 months. Initial doses were determined based on baseline PTH concentrations, as described in the literature.19 We chose a daily administration regimen, such that patients with PTH levels <500pg/ml received 1mg per day, and those with PTH values above 500pg/ml received 2mg per day. Later dose variations were based on the progression of PTH and Ca values. If the decrease in PTH was less than 15%, the dose was doubled; if the decrease was 15%-60%, the dose was maintained, and if the decrease was >60%, the dose was halved. On the other hand, if calcaemia surpassed 10.2mg/dl, the dose was halved, and if greater than 10.5mg/dl, paricalcitol was suspended. Patients who previously received calcitriol underwent a clearance period 4 weeks prior to the study in order to avoid any interference from prior treatments with the study results. Patients who for any reason did not finish the entire study period were analysed with the intention to treat.
Biochemical measurements
General laboratory analyses, including serum levels of total Ca and P, were performed using an auto-analyser following standard hospital laboratory procedures. Ca values are reported for this study in the form of total Ca, without correcting for serum albumin or protein levels. PTH was measured by electrochemiluminescence (EQL Elecsys® PTH, Roche), and results were corrected by a coefficient of 0.97 in order to express values as IRMA NICHOLS, as per the K/DOQI guidelines.20 Renal function was determined using creatinine clearance values collected in 24-hour urine samples.
Timeline
Prior to commencing the study, we performed general laboratory and physical assessments. We also registered all medicines prescribed to the patient, with special emphasis on drugs commonly used for the control of bone mineral metabolism and RAAS inhibitors. We also registered on a monthly basis all possible incidences and adverse effects that might be related to the study medication. At the start of the study and after 1, 3, 6, and 12 months, we determined serum levels of PTH, proteinuria, and peritoneal protein losses, and any changes to medication regimens were registered.
Statistical analysis
Continuous variables were expressed as mean and standard deviation when following a normal distribution, and as median and interquartile range when not normal; we previously applied the Kolmogorov-Smirnov test to determine whether values followed a normal distribution. Categorical variables were expressed as a percentage. In order to analyse the evolution of study parameters during the study period, we used a linear general model with repeated measures. Percentage changes were compared using Student’s t-tests or Mann-Whitney U-tests as appropriate. For univariate correlations, we used Pearson’s r coefficient. In order to evaluate what variables acted as predictors for patient response to treatment, we applied a linear regression model. We used SPSS® statistical software version 15.0 for Windows (SPSS Inc, Chicago, IL) for all analyses.
RESULTS
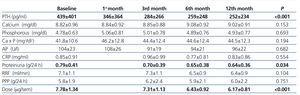
Of all the patients evaluated in our study, 38 complied with the inclusion and exclusion criteria and were finally included in the study. Table 1 summarises the baseline characteristics of the study sample. Initial dosages were as follows: 33 patients received 1mg/day of paricalcitol and the other 5 received 2mg/day. Thirteen patients had previously taken oral calcitriol, which was suspended for the administration of paricalcitol; the remaining 25 patients were considered naïve cases. The mean follow-up period was 10.8±0.9 months. Two patients died and another two received kidney transplants during the study, causing early exit. No patients abandoned the study due to adverse effects associated with taking paricalcitol; one patient required associated treatment with a proton pump inhibitor due to gastric problems.
Effectiveness of treatment
The use of oral paricalcitol was associated with decreased PTH levels (Figure 1). In terms of percentages, the greatest decrease was produced in the first 4 weeks of treatment (22.1%±2.4%; P<.001), followed by continued reductions at a lower rate, with a mean decrease after 12 months of 3.07%±6.8%. We did not observe significant differences in serum Ca or P levels throughout the study period, and no episodes of hypercalcaemia or hyperphosphataemia requiring suspension or reduction of medication doses were recorded (Table 2). We did not observe differences in terms of PTH decreases when comparing patients who had previously received oral calcitriol to those who had not (29.6%±2.5% vs. 30.8% vs. 3.2% 12 months after treatment). The linear regression analysis showed that baseline PTH values were the only predictor for patient response in the form of decreased PTH levels (b=0.689 [95% confidence interval –CI- =0.067-0.233; P=.018).
The efficacy in reducing PTH allowed for reducing the dosage of paricalcitol, which went from a mean 8.03mg/week at the beginning of the study to 5.94mg/week after 6 months and 5.32mg/week after 12 months.
As regards other treatments implicated in the control of parameters related to bone and mineral metabolism, there was only a slight change in the percentage of patients who received calcium- (30% vs. 28%) and non-calcium-based binders (lanthanum: 32% vs. 29%; sevelamer: 38% vs. 39%) between the start and end of the study. We did observe a slight decrease in the dose of Ca administered (1664±346mg/day vs. 1571±333mg/day) and an increase in the dosage of lanthanum (1575±325mg/day vs. 1875±425mg/day) and sevelamer (2222±245mg/day vs. 2455±238mg/day). The use of cinacalcet was reduced from 21% to 16% of patients, with a change in the mean maintenance dose from 30mg/day to 28mg/day. No changes were observed in terms of Ca concentrations in dialysate over the course of the follow-up period.
Evolution of renal function and proteinuria
During the one year of follow-up in our study patients, we observed a mean 19% decrease in proteinuria (0.79±0.41g/24 hours vs. 0.64±0.36g/24 hours; P=.034) and no changes in renal function (7.1±1.1ml/min vs. 6.3±0.9ml/min; P=.104) (Table 2). There were also no changes in terms of dosages for RAAS inhibitors or the percentage of patients prescribed these drugs (71% vs. 68%; P=.472), which may have influenced the decrease in proteinuria. We observed no differences in the changes in proteinuria rates when separating patients into those who had previously received RAAS inhibitors and those who had not (18.83 vs. 19.62; P=.622). In our analysis of the independent predictive variables for this decrease, we observed a correlation between decreases in proteinuria and PTH (b=0.454 [95% CI: 0.326-0.823]; P=.007), with no observable influence from variation in renal function.
Evolution of peritoneal protein losses
Over the course of the follow-up period, we observed no changes in peritoneal protein losses (5.8±1.9g/day vs. 6.0±2.2g/day; P=.731) in peritoneal effluent, nor variation in serum albumin levels (3.7±1.1g/dl vs. 3.7±1.2g/dl; P=.697). The lack of differences remained constant even when analysing separately patients who had and had not received RAAS inhibitors.
DISCUSSION
Our study results confirm the efficacy of paricalcitol for treating SPTH in patients on PD. In addition, we have shown the capacity of this medication to reduce proteinuria, even in patients on renal replacement therapy, although it was not capable of reducing peritoneal protein losses.
Ross16 described for the first time the effect of oral paricalcitol on the control of hyperparathyroidism in a cohort of 26 patients (and another 62 on haemodialysis) who were followed for 26 weeks. They demonstrated a decrease of approximately 30% in PTH levels after only 3 weeks of treatment, followed by a slight decrease throughout the study. No changes in Ca or P levels were observed, nor in the administration of phosphate binders. This study stands out due to the elevated doses of paricalcitol administered. Recently, Coronel21 communicated an even greater decrease (approximately 60% after one month of treatment and 70% after 3 months), with similar doses to those prescribed in our study. Our study represents the largest sample of patients included as yet in an analysis of this type and with a long follow-up. We appear to have clearly shown the effects of paricalcitol on controlling PTH, with a decrease greater than 30%. One limitation of the activation of vitamin D receptors for controlling hyperparathyroidism is the increase in serum Ca and P levels produced by the increase in intestinal absorption of these ions mediated by cytosolic transit in the intestinal cells following synthesis of calbindins and Ca transporter proteins. Paricalcitol has been shown to have a lower capacity for promoting the expression of these proteins.22 In our study (as in the aforementioned studies involving patients on PD), no patients were forced to suspend medication during the study due to episodes of hypercalcaemia.
Proteinuria is an important independent progression factor for the deterioration of glomerular filtration rates.23 In addition, we know that treatments capable of reducing proteinuria have a nephroprotective effect, slowing the progression of kidney damage.24 In patients on PD, maintaining residual renal function not only contributes decisively to achieving adequate clearance of molecules,25 it also appears to influence technique and patient survival.26,27 With this in mind, any strategy aimed at preserving residual renal function would be extremely beneficial to these patients. RAAS inhibitors are commonly used drugs in the treatment of renal patients, not only because of their ability to control blood pressure, but also due to coadjuvant effects such as reducing proteinuria and nephroprotection.8-10,24 Fishbane28 and Zeeuw29 demonstrated that paricalcitol has an anti-proteinuria effect in patients with different stages of CKD, both in diabetics and non-diabetics. In a population on PD,21 administering paricalcitol was associated with a decrease in proteinuria of approximately 15%. In our study, we were able to show how administering paricalcitol was associated with a decrease in proteinuria of approximately 20% 6 months after starting treatment, with no notable changes afterwards until the end of the study. This decrease could serve as an explanation for the maintenance of residual renal function, which did not change significantly over the course of the study. One possible explanation for the anti-proteinuria effect of paricalcitol is that it is believed to have an inhibitory effect on the renin-angiotensin-aldosterone system. Deb17 proved that administering paricalcitol in a cohort of rat subjects that were receiving losartan induced a reduction in the expression of renin and angiotensinogen, concluding that the selective activator of vitamin D receptors also had an inhibitory effect on this system, acting on the higher levels of the enzymatic cascade. Based on this probable assumption, other beneficial effects could be expected from the use of paricalcitol, such as reduced left ventricular hypertrophy30 and atherogenic processes.31
Controversy exists surrounding the role of renin-angiotensin-aldosterone system inhibitors in reducing peritoneal protein losses in patients on PD. Currently, we believe that endothelial dysfunction plays an essential role in initiating the atherosclerotic process,32 both in healthy subjects33 and in patients with hypertension34 or diabetes.35 Given that the endothelium regulates vascular permeability, studies have proposed that protein loss in the urine might be due to a damaged glomerular endothelium.36 A similar process was described to explain peritoneal protein loss.11 Interesting associations have been made between peritoneal protein loss and increased risk of peripheral vascular disease,11 cardiovascular events, and higher mortality rates.37 Renin-angiotensin-aldosterone system inhibitors have been shown to be capable of improving endothelial dysfunction, thus reducing microalbuminuria and cardiovascular mortality/morbidity.38 As regards the capacity of this treatment for reducing peritoneal protein losses, irbesartan13 and candesartan14 have been shown to be capable of doing so in small studies. However, Zhuo15 found no such capacity upon analysing 156 patients treated with these inhibitors, although this may have been influenced by the low doses administered. In our study, we evaluated the capacity of paricalcitol to reduce peritoneal protein losses. Despite the anti-inflammatory39 and inhibitory effects on the renin-angiotensin-aldosterone system17 shown for paricalcitol, we observed no differences in protein losses. Our results are not in agreement with those recently published by Coronel,40 who analysed peritoneal protein losses in 23 patients on PD and under treatment with paricalcitol, observing a decrease of approximately 15%. Our results may have been influenced by the fact that the doses administered in our experiment were not high (as was the case in the studies by Zhou and Coronel), the study populations were quite different (our patients were younger and the percentage of diabetics was significantly lower, with less time on PD programmes), the study was not specifically directed towards analysing this aspect, or the fact that patients were for the most part already receiving some other type of renin-angiotensin-aldosterone inhibitor that could have already saturated the reducing effect on peritoneal protein losses.
To conclude, the use of paricalcitol in patients on PD is safe and effective for controlling SPTH, since it does not produce hypercalcaemia, hyperphosphataemia, or adverse effects that might necessitate abandoning treatment. We also achieved reductions in proteinuria that were not related to changes in renal function or the use of drugs that might modify this parameter, which could have a beneficial effect on residual renal function. Future prospective and controlled studies could examine whether this decrease in proteinuria could have some influence on residual renal function or cardiovascular risk, and specially designed studies with larger sample sizes could aid in understanding the true effect of the use of paricalcitol on peritoneal protein losses.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Evolution of parathyroid hormone values (expressed as median and interquartile range)
Table 1. Baseline characteristics of the study population
Table 2. Evolution of laboratory parameters and administered dose of paricalcitol