Introduction: Plasmapheresis (PP) is a therapeutic apheresis technique used in the treatment of various renal and systemic diseases with varying degrees of proven clinical efficacy. Objective: To review our experience with PP at the Hospital Universitario de Canarias, focused on effectiveness and safety results in different disease groups. Material and methods: A retrospective-descriptive study of patients treated with PP from 01/01/2006 to 31/12/2009 at the hospital. We analysed medical histories and demographic data (sex, age), biochemical parameters, underlying disease, volume and type of replacement used in the PP sessions (5% human albumin and/or fresh frozen plasma), complications with the technique, delay in starting PP treatment after suspected clinical diagnosis, number of PP sessions received, patient mortality, degree of renal impairment and evolution of renal function. Results: There were 51 patients studied, aged 50±18 years, of whom 60% were male; 331 PP sessions were performed. The diseases treated were grouped as: 11 vasculitis, 15 transplant immune activation, 5 haemolytic-uraemic syndrome (HUS), 7 idiopathic or thrombotic thrombocytopaenic purpura, 2 foetal Rh immunisations, 2 haematological diseases, 4 neurological diseases, among others. Overall mortality was 19.6% (n=10): 6 cases secondary to septic shock and the rest as a result of the evolution of the underlying disease, with 1 due to haemorrhagic shock in the renal biopsy area. There were no deaths in the transplant immune activation group. In the vasculitis group, there were 3 deaths (2 secondary to septic shock). Of the 10 patients who died, 9 did so within the first three months after diagnosis. Of the 26 renal biopsies performed, the most frequent indications were: vasculitis (23%), humoral rejection (42%), humoral rejection with calcineurin-inhibitor toxicity (12%) and HUS (8%), among others. Haemodialysis (HD) was required by 24 patients at the start of clinical symptoms: 9 of the 11 patients with vasculitis, 4 of the 5 patients with HUS and 5 of the 15 patients with transplant immune activation. At the end of evolution, 14 of them remained on the HD programme: 5 of the 11 patients with vasculitis, 2 of the 15 transplant patients and 3 of the 5 HUS patients. Significantly, patients who developed end kiney disease (EKD) in the vasculitis group were older and had higher creatinine at the onset of the disease. The transplant patients were monitored for anti-HLA class I or II before and after PP; there was a mean decrease of antibody titres in all but one patient; with an average decrease of 51% to 31%. In general, the PP technique was virtually free of complications. There were only 5 (3%) mild-moderate reactions to fresh plasma (perioral tingling and urticarial reactions) requiring pre-medication with steroids, but which did not lead to discontinuation of the treatment. Conclusion: Taking into account the wide variety of diseases that can benefit from PP and the nature of some of them, publishing our experience with this therapeutic method is of great importance. By increasing the description of case series by centre, we can add survival and renal function evidence in many uncommon diseases. Our study provides useful information for clinical practice and has also led us to reflect on future strategies to optimise outcomes in our patients.
Introducción: La plasmaféresis (PF) es una técnica de aféresis terapéutica utilizada en el tratamiento de diversas enfermedades renales y sistémicas con distintos grados de eficacia clínica demostrada. Objetivo: Analizar los resultados globales de la indicación de PF en el Hospital Universitario de Canarias, enfocados a resultados de su efectividad y seguridad en diversos grupos de enfermedades. Material y métodos: Se trata de un análisis descriptivo retrospectivo de una serie de casos que analiza los resultados de la indicación de PF desde el uno de enero de 2006 hasta el 31 de diciembre de 2009 en nuestro centro. Se revisaron las historias clínicas y se recogieron datos demográficas (sexo y edad), parámetros bioquímicos, enfermedad de base, volumen y tipo de reposición utilizado en la sesión de PF (albúmina humana al 5% y/o plasma fresco congelado), complicaciones asociadas con la técnica, días transcurridos desde la sospecha clínica diagnóstica hasta el inicio de la técnica de aféresis, número de sesiones de PF recibidas, mortalidad del paciente, grado de afectación renal y evolución de la función renal. Resultados: Estudiamos a 51 pacientes, de 50 ± 18 años, el 60% eran hombres, 331 sesiones de PF. Las enfermedades tratadas se agruparon como: 11 vasculitis, 15 inmunoactivaciones del trasplante renal, cinco síndromes hemolítico urémicos, siete casos de púrpura trombótica trombocitopénica o idiopática, dos inmunizaciones Rh fetal, dos enfermedades hematológicas y cuatro casos de enfermedades neurológicas, entre otras. La mortalidad global fue del 19,6 % (n = 10); en seis de los casos, secundaria a shock séptico y en el resto como resultado de la evolución de la enfermedad de base y uno por shock hemorrágico en el área de la biopsia renal. No hubo fallecimientos en el grupo de inmunoactivación del trasplante. En el grupo de vasculitis se produjeron tres fallecimientos (dos de ellos secundarios a un shock séptico). Nueve de los 10 pacientes que fallecieron lo hicieron dentro de los tres primeros meses tras el diagnóstico. De las 26 biopsias renales realizadas, las indicaciones más frecuentes fueron: vasculitis (23%), rechazos humorales (42%), rechazo humoral más toxicidad por anticalcineurínicos (12%) y síndrome hemolítico-urémico (8%), entre otros. Veinticuatro pacientes precisaron hemodiálisis al inicio del cuadro clínico, nueve de los 11 pacientes con vasculitis, cuatro de los cinco pacientes con síndrome hemolítico-urémico y cinco de los 15 pacientes con inmunoactivación del trasplante. Al final de la evolución, 14 de ellos permanecieron en programa de hemodiálisis. Concretamente, cinco de 11 pacientes con vasculitis, dos de 15 pacientes sometidos a trasplante y tres de cinco pacientes con síndrome hemolítico-urémico. De forma significativa, los pacientes que evolucionaron hacia enfermedad renal terminal en el grupo de las vasculitis eran de mayor edad y tenían una mayor creatinina en el comienzo de la enfermedad. En los pacientes sometidos a trasplante en quienes se monitorizaron anticuerpos anti-HLA de clases I o II medidos por luminex pre y post-PF se objetivó una media de descenso del título de anticuerpos en todos excepto en un caso; el descenso medio fue del 51 al 31%. En general, la técnica de PF transcurrió prácticamente libre de complicaciones. Se constataron cinco reacciones al plasma fresco (3%) de carácter leve-moderado (hormigueo peribucal y reacciones urticariformes) que requirieron premedicación con esteroides y no supusieron la interrupción del tratamiento. Conclusión: Teniendo en cuenta la gran variedad de enfermedades que pueden beneficiarse de la PF y el carácter esporádico de algunas de ellas, la publicación de la experiencia con esta modalidad terapéutica cobra gran importancia, ya que si incrementamos la descripción de series de casos por centros, podemos ayudar a ampliar el nivel de evidencia en términos de supervivencia y función renal en múltiples patologías infrecuentes. Nuestro estudio aporta una información útil y valiosa para la práctica clínica habitual y, sin duda, nos hace reflexionar sobre estrategias futuras que optimicen el pronóstico en nuestros enfermos.
INTRODUCTION
Plasmapheresis (PP) is a therapeutic apheresis technique that removes high molecular weight substances from blood plasma (autoantibodies, immune complexes, myeloma light chains, endotoxins, cryoglobulins, lipoproteins)1-3 by convective transport through a semipermeable membrane. PP is indicated over a wide range of specialities, in many hospitals it has become a subspeciality of nephrology, because of the dominance of blood purification techniques by nephrologists. Given the sporadic nature of diseases that may benefit from PP, experience in this technique often relies on published case series or uncontrolled studies, making it difficult to gather good quality survival and renal function evidence. Hence, the overall individual experience, though generally limited, is important in this therapy.
As a result, it was our aim to analyse the overall performance of PP in the Hospital Universitario de Canarias (University Hospital of the Canary Islands), focusing on the safety and efficacy results in various disease groups.
MATERIAL AND METHODS
It was a retrospective analysis of a case series analysing the results from PP indication between 1 January 2006 and 31 December 2009 at our hospital. The analysed medical histories and demographic data (sex, age), biochemical parameters, underlying disease, volume and type of replacement used in the PP sessions (5% human albumin and/or fresh frozen plasma), complications with the technique, delay in starting PP treatment after diagnosing clinical suspicion, number of PP sessions received, patient mortality, degree of renal impairment and evolution of renal function.
Indication of plasmapheresis
The indication of PP and its application were based primarily on the guidelines prepared and updated periodically by the American Society for Apheresis (ASFA) and the American Association of Blood Banks (AABB). Briefly, according to the AABB,2,3 the indications are classified into four categories based on the clinical efficacy found in the literature1-3 and summarised as follows:
Category I. PP is indicated as first line therapy or as an adjuvant therapy to other initial treatments for certain conditions. Efficacy is based on clinical trials or a broad base of published evidence, where efficacy is proven and accepted.
Category II. It is accepted as support to other more established treatments, but not as first line treatment.
Category III. The use of therapeutic apheresis is not based on clear scientific evidence. Experience is insufficient to establish efficacy and the risk/benefit ratio is not clearly demonstrated.
Category IV. Available and contrasted studies have shown a lack of therapeutic efficacy.
Plasmapheresis protocol and technical aspects
The volume extracted in each PP session was 1 to 1.5 times the plasma volume, and the total number of sessions depended on the underlying disease and the clinical course of each patient. Patients receiving fresh frozen plasma as replacement fluid received calcium gluconate as prophylaxis of hypocalcaemia secondary to citrate.
Arteriovenous fistula was used in 22% of patients as vascular access (immune activation pre- and post- kidney transplant patients). The remaining patients had channelled high-flow, central venous catheters (1.67±0.9 catheters per patient).
The choice of replacement fluid varied among the patients. However, those with bleeding, general lack of clotting factors, thrombotic thrombocytopaenic purpura (TTP) or haemolytic-uraemic syndrome (HUS), were given fresh frozen plasma.1,4
To analyse the results, patients were grouped according to the reason for PP indication:
Group 1. Patients indicated for PP to maintain renal function: vasculitis, kidney transplant immune activation, HUS and rapidly progressive renal failure. Clinical resolution was considered complete if creatinine decreased below 2mg/dL after treatment, and the response was partial if there was renal function with creatinine greater than 2mg/dL, but without a need for dialysis, and proteinuria higher than 1.5g in 24 hours.
Group 2. Patients for whom PP was indicated due to vital risk: blood diseases, systemic lupus erythematosus (SLE), septic shock or cholangiocarcinoma.
Group 3. Patients indicated PP to improve clinical parameters: neurological diseases, TTP or idiopathic TTP (ITP), foetal Rh immunisation or transplant desensitisation.
The latter two groups were considered as partial or complete resolution, depending on the patients’ partial or complete improvement in clinical and biochemical parameters. Thus, in neurological diseases, the objective was recovery without neurological deficit or rapid improvement in symptoms that would resolve serious situations for the patient's functional autonomy. For TTP, ITP and haemolytic anaemias, the aim was recovery of haematological and clinical parameters. For hyperviscosity syndrome, the resolution of neurological symptoms was sufficient. The evolution of each patient is discussed in detail.
Statistical study
As it was a descriptive patient study, only basic descriptive statistical tests were applied, i.e. means and frequencies. The software used for the statistical analysis was SPSS 13.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
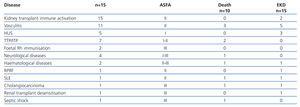
Over a period of 4 years, 51 patients received a total of 331 PP sessions. 60% were male, and the mean age was 50±18 years. The treated diseases were grouped according to initial clinical suspicion (Table 1). As can be seen, most initial diagnoses were vasculitis and immune activation in transplantation, followed by TTP/ITP and HUS.
After obtaining the final diagnoses, it can be seen that, of the 15 patients treated for immune activation renal transplantation, 14 had acute antibody-mediated rejection on graft biopsy. 50% of the patients with vasculitis had Wegener's granulomatosis or microscopic polyangiitis (MPA). The other were HIV-negative vasculitis, 1 case of SLE associated with MPA, a case of Goodpasture's syndrome, one of pulmonary-renal syndrome and 1 case of cryoglobulinaemia associated with hepatitis C. Of the 5 patients treated for HUS, 1 was due to acute humoral graft rejection, 2 secondary to tacrolimus toxicity and the other 2 were secondary to mutation in the complement factor H and malignant hypertension (HT), respectively.
Of the initial group with thrombocytopaenia, 4 cases were due to TTP (1 case associated with paraneoplastic syndrome due to gastric neoplasm) and 3 cases to ITP. Neurological diseases included 1 case of Guillain-Barré syndrome, a myasthenic crisis, 1 case of Miller-Fisher syndrome and amyotrophic lateral sclerosis; while the haematological diseases included 1 case of myeloma with associated coagulopathy and a case of Waldeström's disease.
Of the 26 renal biopsies performed, the most frequent indications were: vasculitis (23%), humoral rejection (42%), humoral rejection with calcineurin-inhibitor toxicity (12%) and HUS (8%).
The evolution of the patients by diagnostic groups is described below, with a summary in Tables 2 and 3.
Group 1
Vasculitis
1. A 50-year old man who was referred from La Palma due to rapidly progressive renal failure (RPRF), creatinine of 7.5mg/dL and positive anti-neutrophil cytoplasmic antibody (ANCA) findings. He had clinical symptoms of 1 month evolution, consisting of arthralgia, myalgia, frothy and tea-coloured urine. He was transferred to our hospital, where haemodialysis was started and a renal biopsy found pauci-immune necrotising crescentic glomerulonephritis (GN). He was treated with steroid boluses then oral prednisone at a dose of 1mg/kg/day with subsequent dose reduction and a bolus of cyclophosphamide (0.5g/m2), to continue with monthly boluses in La Palma. He also received 6 PP sessions with a total removed volume of 22L: 5L replaced with fresh frozen plasma and 17L with a stabilised solution of plasma proteins (SSPP). He received one dose of immunoglobulins at the end of the last PP session. There were no adverse reactions. His subsequent evolution was favourable with regard to systemic symptoms, with partial recovery of renal function.
2. A 71-year old man without any significant medical history. He visited the emergency department on 23 June 2008 with asthenia, anorexia, nervous fever and dark urine with creatinine of 12mg/dL, proteinuria and microhaematuria. Additional tests highlighted the findings of positive PR3 ANCA at the upper limit. A kidney biopsy was performed on 30 June 2008 which found pauci-immune extracapillary crescentic GN. He was treated with steroid boluses (500mg/day for 3 days) then oral prednisone, 4 boluses of cyclophosphamide (0.5g/m2) and 6 PP sessions (3 per day, 3 every other day), with total SSPP replacement and post-PP immunoglobulins (0.5kg/m2) in the last 4 PP sessions. The systemic symptoms improved partially, but not the renal function, and the patient remained on haemodialysis. As a complication, he suffered severe bleeding deferred one month after treatment due to a renal biopsy puncture, and died.
3. A 16-year old man diagnosed with SLE associated with vasculitis (antinuclear antibody [ANA] 1/160, negative anti-DNA, positive ANCA, anti-myeloperoxidase [anti-MPO] positive) with type IV lupus glomerulopathy diagnosed by biopsy in December 2007. He had been treated with steroids and monthly cyclophosphamide boluses. He was admitted to La Palma in April 2008 due to pulmonary haemorrhage treated at his local hospital with steroid boluses and up to 8 intravenous doses of cyclophosphamide (the last in September 2008). He was admitted to our hospital in September 2008 due to anaemia, asthenia and elevated erythrocyte sedimentation rate (ESR) after the eighth monthly bolus of cyclophosphamide, with the appearance of a diffuse alveolar infiltrate in the lung bases on the chest X-ray. Computed tomography (CT) showed diffuse bilateral pneumonitis in ground-glass opacities, probably secondary to a previous pulmonary haemorrhage. A bronchoscopy was performed, which confirmed the recent pulmonary haemorrhage (99% haemosiderophages in the bronchoalveolar lavage). Together with the rheumatology and lung disease departments, we decided to start a PP programme (6 sessions per day) with partial replacement with fresh frozen plasma due to the history of pulmonary haemorrhage. There were no adverse reactions and the system lines coagulated on 5 occasions. After the last PP session, he received a 0.25g/kg dose of immunoglobulins and a dose of rituximab. The outcome was favourable with pulmonary haemorrhage control. Renal function remained normal at all times. After the start of PP, the ANA titre decreased from 1/160 to 1/40, and the anti-MPO titre from 40 to 28.5U/L. The complement and ESR returned to normal values. Subsequently, the patient received 4 doses of rituximab, with later administration of mycophenolate and steroid maintenance, resulting in stable renal function, proteinuria of 1.8-2g/24 hours, persistent microhaematuria and normal blood pressure.
4. A 66-year-old was admitted in February 2009 to the intensive care unit (ICU) for pulmonary-renal syndrome with pulmonary haemorrhage and acute renal failure (ARF). Wegener's disease with positive PR3-ANCA titre was suspected. No renal biopsy was performed due to detecting a nodule in the lower pole of the left kidney. She was treated with steroid boluses (3g IV) and then oral prednisone at a dose of 1 mg/kg with subsequent dose reduction. Cyclophosphamide: 4 monthly boluses of 1g and initial maintenance therapy with mycophenolic acid and subsequently with azathioprine. She also received 7 PP sessions (every other day) from 8 to 20 February 2009, with no adverse reactions. The response was favourable, with improvement of systemic symptoms and progressive reduction of ANCA titres. After starting with creatinine of 10.4mg/dL and oliguria with a haemodialysis need, renal function recovered slowly and she was withdrawn from the programme 3 months later. Since June 2009, her creatinine has been 1.7mg/dL, with microalbuminuria, and negative ANCA titres since May 2009. She is taking maintenance treatment with prednisone and azathioprine.
5. An 81-year-old man admitted on 18 April 2009 due to acute on chronic renal failure and pneumonia. The underlying disease was nephroangiosclerosis. Initially, it was suspected that the deterioration of renal function was in relation to a respiratory sepsis, but the immunological study showed positive MPO-ANCA titres, which suggested a rapidly progressive glomerulonephritis (RPGN), grade III. The deterioration in respiratory status, along with anaemia, suggested the existence of a pulmonary haemorrhage, but the bronchoscopy was inconclusive. The patient was admitted to the ICU from 23 to 28 April 2009 and received steroid boluses (500mg/day, 3 days) then oral prednisone. He also received 2 cyclophosphamide boluses (0.5g/m2) and 7 daily PP sessions with total SSPP replacement. He also received 1 dose of immunoglobulin at the end of the last PP session. The patient had no adverse reactions and, although the clinical outcome was favourable with a decrease in ANCA from 320 to 130 and 30 after the PP, the renal function evolution was not, the patient remaining on haemodialysis.
6. A 62-year old woman in chronic haemodialysis since May 2008, with an underlying disease of unknown aetiology (she had never undergone a renal biopsy). She was admitted to the ICU on 4 July 2009 with clinical symptoms of pulmonary haemorrhage interpreted in the context of HIV-negative vasculitis. She received a bolus of cyclophosphamide, steroids and 8 PP sessions with SSPP replacement. The last session was held on 20 July 2009, when she received 1 dose of immunoglobulin at the end. The clinical outcome was not favourable and she died 2 months later on 6 September 2009 due to multiple complications during hospitalisation, mainly respiratory sepsis from pneumonia associated with mechanical ventilation due to Morganella morgagni and Klebsiella pneumoniae. In addition, in August, she had gastrointestinal bleeding secondary to mucosal erosions in the ileum, jejunum and second portion of the duodenum, ulcerous lesions in the jejunum and neoformation in the duodenal papilla. The latter finding, together with abrupt hyperbilirubinaemia up to 15mg/dL of direct dominance and a clinically severe, persistent thrombocytopaenia which was refractory to treatment, which initially coincided with the PP sessions and was subsequently maintained over time. However, it was decided to limit the treatment and monitoring by the palliative care unit until her death.
7. A 49-year old man with a medical history of hepatitis C liver disease, leukocytoclastic vasculitis secondary to mixed cryoglobulinaemia in the context of infection with hepatitis C (HCV). He was monitored by our department, with mid-range proteinuria of less than 1g per day and microhaematuria and normal renal function. He was admitted to the rheumatology department in December 2007 due to ulcers in the lower limbs of one week duration, weight loss and neurological abnormalities compatible with acute exacerbation of his underlying disease, for which he was receiving prednisone (1mg/kg/day). The patient underwent 9 PP sessions with SSPP replacement and treatment with rituximab for compassionate use. He received 1 dose of immunoglobulin at the end of the last PP session. There were no technical complications. A marked improvement in clinical symptoms was found, especially in the neurological symptoms and lesions in the lower limbs. A renal biopsy was performed to check renal function, resulting in membranoproliferative glomerulonephritis probably secondary to HCV and cryoglobulinaemia. His current creatinine is 0.9mg/dL with a mid-range proteinuria of 1g in 24 hours.
8. A 25-year old man with a history of nasal polyps surgery in childhood was attended to on 18 December 2008 due to RPRF. He had previously had 1-month clinical symptoms consistent with arthralgia, myalgia, sore throat, skin lesions, frothy and tea-coloured urine, nervous fever and oral aphtae. Laboratory tests suggested ARF, with creatinine of 4mg/dL and no proteinuria or oliguria. Microhaematuria was observed in the urinary sediment and the immunological study detected positive PR3-ANCA. A renal biopsy showed necrotising crescentic GN. He was diagnosed with Wegener's disease, and was treated with boluses of methylprednisolone, then oral prednisone. Cyclophosphamide was administered by bolus and then orally for 1 year. He also received 9 PP sessions between 19 December 2008 and 8 January 2009, and received 1 dose of immunoglobulin at the end of the last session. No adverse reactions were found. The systemic symptoms significantly improved. ANCA titres decreased progressively until negative values after a year. He underwent dialysis twice, and creatinine was 1.1mg/dL after a year, without proteinuria or microhaematuria. Maintenance therapy was performed with prednisone and azathioprine.
9. A 72-year old woman without a significant medical history was admitted in September 2006 due to ARF, with creatinine of 12mg/dL. She reported macroscopic haematuria, frothy urine, dyspnoea, fever and weight loss of more than one month duration. She underwent renal biopsy with a finding of extracapillary GN as well as detecting a positive result for anti-MBG antibodies. She was treated with steroid boluses, then oral prednisone at 1mg/kg/day, which was subsequently reduced. She also received cyclophosphamide: initially 4 boluses and subsequently orally. She had 10 sessions of PP with replacement of 5% human albumin, and received 1 dose of immunoglobulin at the end of the last session. There were no adverse reactions. She was asymptomatic at discharge, with antibody titres decreasing from 128U at admission to 16.3U at discharge. Renal function development was unfavourable, remaining on haemodialysis since the start without recovery.
10. A 75-year old man with no relevant personal history. He was admitted to the ICU in July 2007 with symptoms of pulmonary haemorrhage and oliguric ARF that required continuous veno-venous haemodiafiltration. Immunological studies were negative. No renal biopsy was performed initially due to the patient's clinical condition, but he received 2 PP sessions in the first 48 hours with replacement of fresh frozen plasma due to the history of pulmonary haemorrhage. Initial clinical improvement was seen in the pulmonary haemorrhage and diuresis was regained with a decrease in creatinine. Renal replacement therapy was therefore suspended, but subsequently it evolved to sepsis secondary to probable nosocomial pneumonia (without the responsible microorganism being identified), primary respiratory distress and multi-organ failure (including further deterioration of the glomerular filtration rate) that evolved unfavourably until his eventual death 10 days after admission.
11. A 79-year old man with a single kidney was admitted with ARF in April 2009, with a positive finding of PR3-ANCA. Due to the initial suspicion of RPGN-III, he underwent 3 PP sessions. However, the imaging test showed renal artery thrombosis, so he remained on chronic haemodialysis. The positive PR3-ANCA finding was not confirmed by ELISA.
In 7 of the 11 vasculitis cases (64%), antibodies were found both before and after PP, and a disappearance or decrease in antibodies was noted in 86% of cases.
Immune activation in kidney transplantation
12. A 57-year old woman with polycystic kidney disease as the underlying disease had a kidney transplant on 23 May 2008. This was a patient who had undergone a transplant in 1991 and was hypersensitised, with 95% anti-HLA class I antibodies. She received induction with polyclonal antibodies and immediately achieved renal function, but at 8 days showed an acute antibody-mediated rejection and received 7 PP sessions (3 daily and 4 every other day), then replenished with SSPP and administration of intravenous immunoglobulins (250mg/kg after each session and a final dose of 1g/kg). Since she was dependent on haemodialysis and had oligoanuria, she underwent a second biopsy which detected an IIA acute cellular rejection with positive C4d staining. Given this finding, she was again administered boluses of methylprednisolone and two doses of 700mg rituximab. She also received tacrolimus and mycophenolate immunosuppressive therapy. Creatinine at discharge was 1.6mg/dL. Levels of lymphocytotoxic antibodies remained similar to those before transplantation. One possible complication was gastrointestinal bleeding of unclear origin 1 week after the PP sessions.
13. A 43-year old man with systemic vasculitis as the underlying disease received his third kidney transplant on 21 July 2009. The two previous transplants were rejected, with an antibody rate by CDC of 54%-94%. He received induction with polyclonal antibodies. Two weeks after the transplant, renal function deteriorated, so a renal biopsy was performed which showed acute humoral rejection with positive C4d staining and the presence of positive donor-specific anti-HLA antibodies. He received 3 boluses of methylprednisolone and 8 PP sessions (3 daily and 5 every other day), and was replenished with SSPP. He also received intravenous immunoglobulin with a similar dosage regime to the previous case and 2 doses of rituximab. There were no complications related to the PP. The response was favourable, with a decrease in creatinine from 4.2 to 1.4mg/dL. He also received tacrolimus and mycophenolate immunosuppressive therapy.
14. A 38-year old woman who underwent her first kidney transplant on 28 February 2006, with chronic glomerulonephritis as the underlying disease. She was hypersensitised and had an antibody rate of 73%-90% by CDC (history of multiple abortions and previous transfusions). She received induction with polyclonal antibodies. Subsequent renal function was delayed and a renal biopsy showed an IIb-III acute rejection with negative C4d staining. Before receiving the biopsy results, since she was hypersensitised, she was administered empirically with 3 boluses of methylprednisolone and given a PP session, in addition to adjusting levels of tacrolimus and mycophenolate. She received 1 dose of immunoglobulins at the end of the PP session. The subsequent evolution was favourable and creatinine at discharge was 1.9mg/dL.
15. A 41-year old man who underwent a kidney transplant on 6 October 2008, with focal segmental hyalinosis as the underlying disease. He had previously undergone a kidney transplant in May 2005, which was lost due to thrombosis. The patient underwent nephrectomy and was not sensitised. After receiving induction with polyclonal antibodies, he developed immediate renal function with a decrease in creatinine to 1.8mg/dL. On the 7th day after the transplant, creatinine levels increased to 2.3mg/dL, with a decrease in diuresis. A renal biopsy was performed and an acute antibody-mediated rejection with positive C4d was observed, as well as positive donor-specific antibodies. He underwent four PP sessions with total SSPP replacement, with intravenous immunoglobulin and 2 doses of rituximab after the PP sessions. He had a subcapsular and abdominal haematoma as a complication. A week after treatment, the creatinine decreased to 1.9mg/dL and at discharge was 1.7mg/dL.
16. A 74-year old woman with an underlying disease of unknown aetiology underwent a transplant on 30 August 2006. A previous kidney transplant in 2003 was lost due to immediate thrombosis. She was hypersensitised, with anti-HLA antibodies at 38%-56% and received induction with polyclonal antibodies. On the 5th day after transplantation, there was an acute deterioration of renal function with creatinine rising to 3.2mg/dL. A renal biopsy showed acute antibody-mediated rejection with positive C4d and circulating positive donor-specific antibodies. She was administered 3 methylprednisolone boluses of 500mg and underwent 10 PP sessions. Replacement was mainly with fresh frozen plasma and she underwent an intravenous immunoglobulin regime similar to that of the previous cases. She had some non-specific, post-PP itchy reaction resolved with antihistamines. Renal function recovered and creatinine at discharge was 0.8mg/dL.
17. A 33-year old woman who underwent her second kidney transplant in May 2000 (her first having been lost due to hyperacute rejection). She was admitted in May 2006 due to renal function deterioration with creatinine rising from 1.6 to 2.8mg/dL. A graft biopsy showed late antibody-mediated, C4d-positive, acute rejection. She received 3 boluses of methylprednisolone and 6 PP sessions (3 on consecutive days and 3 every other day), with total volume SSPP replacement. She also received intravenous immunoglobulin as outlined in previous cases. There were no complications related to the PP, and renal function partially improved with creatinine at discharge of 2.2mg/dL.
18. A 56-year old man with mesangial IgA GN as the underlying disease who underwent his first kidney transplant on 1 June 2007. His creatinine at discharge was 2.5mg/dL and he received everolimus due to previous calcineurin-inhibitor toxicity. He was admitted in August 2007 due to glomerular filtration deterioration with a rise in creatinine to 3.9mg/dL. A renal biopsy showed borderline changes with positive C4d staining, and no observable donor-specific antibodies. The immunosuppressive treatment was changed to tacrolimus, and he was given 3 boluses of methylprednisolone and underwent 5 PP sessions with total volume SSPP replacement. He also received intravenous immunoglobulin after the PP sessions. There were no complications, and partial improvement was observed with a decrease in creatinine to 2.9mg/dL.
19. A 28-year old man with an underlying disease of the heat shock factor family. He underwent his first transplant in March 2007 and was not sensitised. Follow-up revealed poor compliance. He was admitted in November 2009 due to renal function deterioration (creatinine of 7.7 versus 1.5mg/dL). A renal biopsy showed IA acute cellular rejection with positive C4d staining and anti-class II donor-specific antibodies. He received boluses of methylprednisolone (500mg in 3 doses) and 6 PP sessions with total volume SSPP replacement, as well as immunoglobulins. He had some non-specific, post-PP itchy reaction which was resolved with antihistamines. Renal response was partial, with a decrease in creatinine to 4.2mg/dL. Subsequently, another rise in creatinine was detected, so it was decided to administer thymoglobulin (4 doses due to cytopaenias), with no response. A second biopsy was then performed, which confirmed the persistence of both acute cellular and humoral rejection. A rescue treatment with immunoglobulin was attempted, but the first dose was poorly tolerated and the treatment was discontinued. It was considered an irrecoverable graft and the patient resumed haemodialysis.
20. A 75-year old man who underwent a transplant in 1994 whose underlying disease was Alport’s syndrome. In October 2009, there was a sharp deterioration in glomerular filtration rate with an increase in creatinine from 1.3 to 1.8mg/dL. A renal biopsy showed an acute antibody-mediated rejection due to positive C4d and positive lymphocytotoxic antibodies. Only 2 PP sessions were given due to the advanced age and chronic graft dysfunction of the patient. Replacement was with SSPP and intravenous immunoglobulin was administered after the PP sessions. There were no complications or evidence of a decrease in creatinine. A negative result for lymphocytotoxic antibodies and a subsequent biopsy showed improved histology (although still with residual positive C4d). The graft remained functioning for another year until the death of the patient due to a prostate neoplasia.
21. A 37-year old man who received his third kidney transplant on 4 March 2007. The first was in 1995, which was lost due to acute cellular rejection and he underwent a nephrectomy. The second transplant lasted from 1996 to 2003, and was lost due to chronic graft nephropathy, and again he underwent a nephrectomy. He was hypersensitised, had an antibody rate by CDC of 41%-51% and received OKT3 induction. On the fourth day after transplantation, they found acute deterioration of renal function whereupon a renal biopsy showed acute rejection compatible with acute humoral rejection and positive C4d in the peritubular capillaries. He was administered boluses of methylprednisolone and intravenous immunoglobulins, and underwent 10 sessions of PP. Total volume replacement was mainly with fresh frozen plasma (due to previous surgical site bleeding requiring a transfusion of three packed red cells and intravenous desmopressin). There were no complications related to the PP. From day 21 after transplantation, diuresis began to progressively increase and renal function improved until discharge, when creatinine was 2.1mg/dL. The lymphocytotoxic antibody profile showed no change compared to the pre-transplant situation.
22. A 33-year old woman who underwent her second kidney transplant in May 2006. The first transplant (functioning from 1995 to 2005) was lost after haemorrhagic shock due to metrorrhagia. Underlying disease: ischaemic nephropathy. She was a highly hypersensitised patient with antibodies of 53%. The immediate post-transplant produced an acute antibody-mediated rejection, which interestingly was corticoid-sensitive. She was admitted in December 2006 with a graft dysfunction (creatinine 2.8 versus 1.4mg/dL) in her monitoring centre. Boluses of methylprednisolone were administered with a partial response and she was referred to our hospital for a renal biopsy and treatment. The biopsy showed a mixed cellular and humoral rejection with positive C4d staining. She received 4 PP sessions with 50% total volume replacement of fresh frozen plasma and the rest with SSPP. She also received intravenous immunoglobulins and there were no complications secondary to the PP. Renal function improved, with recovery of creatinine to 2mg/dL.
23. A 35-year old man with interstitial reflux nephropathy as the underlying disease who received his second kidney transplant on 14 October 2006. He was a sensitised patient with an anti-HLA antibody rate of 30%-38%. The first transplant was lost due to unrecovered acute tubular necrosis. This evolved to delayed renal function and the lymphocytotoxic antibody profile changed, so a renal biopsy was performed which revealed C4d-positive humoral rejection. It was decided to treat him with 8 PP sessions, with total volume replacement with mainly SSPP, and intravenous immunoglobulins. There were no complications secondary to PP and the response was favourable, with increased diuresis and creatinine decreasing from 6.8 to 2.5mg/dL. The creatinine in monitoring carried out in consultation decreased to 1.5mg/dL.
24. A 51-year old man who underwent a transplant on 3 July 2008, with an underlying disease of renal interstitial nephritis secondary to neurogenic bladder due to a spinal cord injury. This evolved to immediate renal function until the fourth day after transplantation, when creatinine increased from 2.3 to 4.3mg/dL and diuresis decreased. Steroid boluses were started and two days later a renal biopsy showed IIb acute rejection with positive C4d. PP was indicated, but only 1 session was received due to a bleeding complication with abdominal haematoma. Immunoglobulins were administered intravenously with a good response, and creatinine decreased to 1.4mg/dL.
25. A 52-year-old man who underwent a transplant on 4 July 2006 with chronic GN as the underlying disease, and an anti-HLA antibody rate of 41%-53%. There was delayed oliguric renal function during the first 72 hours, so a renal biopsy was performed with inconclusive histological findings. Lymphocytotoxic antibodies were positive, but not donor-specific. However, given the immunological background, humoral rejection was suspected and a total of 6 PP sessions were begun. Volume replacement was with SSPP and he also was administered intravenous immunoglobulins. The patient improved, with increased diuresis and decreased creatinine to 1.9mg/dL.
26. A 46-year old man who underwent his first kidney transplant in December 2006, which was lost due to vascular thrombosis on the fourth day after transplantation. The patient then underwent a nephrectomy, and a second kidney transplant in August 2009. He was a hypersensitised patient with an antibody rate of 48%-91% by CDC. The patient received induction with polyclonal antibodies, and renal function recovered immediately until the 5th day after the transplant, when renal function deteriorated with oligoanuria and HUS data, so a conversion to rapamycin was done. A subsequent biopsy showed that it was associated with antibody-mediated rejection. He received boluses of methylprednisolone, 7 PP sessions with mainly SSPP and a small amount of fresh frozen plasma and intravenous immunoglobulins, and a dose of rituximab. There was a surgical bleeding complication with haemorrhagic shock, so the PP sessions were suspended and an urgent nephrectomy performed.
Haemolytic-uraemic syndrome
27. A 64-year old woman, hypertensive and with membranoproliferative glomerulonephritis as the underlying disease. She received a cadaveric kidney transplant on 22 November 2005 from an expanded criteria donor. With 1A and 1DR compatibility, she received immunosuppression with basiliximab, tacrolimus, prednisone and mycophenolate. There was post-transplant haemorrhagic shock which required a transfusion of 3 packed red cells, 3 plasma units and 3 units of pooled platelets. She evolved with delayed renal function, requiring 3 dialysis sessions, with progressive increases in diuresis and creatinine at discharge of 1.2mg/dL. During follow-up visits, she had nephrotic range proteinuria and microhaematuria, so a renal biopsy was performed which showed transplant glomerulopathy. On 25 September 2006, there was a progressive increase in lactate dehydrogenase (LDH) observed, together with anaemia and a decrease in haptoglobin. The tacrolimus dose was reduced and prednisone increased to 1mg/kg/day. Renal biopsy showed thrombotic microangiopathy data and humoral rejection. Thus, the patient was given intravenous immunoglobulin and rituximab, without favourable clinical response. It was finally decided 20 days after admission to start a PP programme (9 sessions) with replacement using fresh frozen plasma and albumin. The PP sessions had to be suspended after the appearance of bacteraemia with Staphylococcus aureus and Pseudomonas aeruginosa. The platelets and haemoglobin however were normal, but within a month, in November 2006, the patient was re-admitted due to renal function impairment, with biopsy data of chronic graft nephropathy, and started a haemodialysis programme and underwent graft embolisation.
28. A 32-year old woman with no past medical history was admitted in February 2008 after 2 weeks of asthenia, vomiting up to 3-4 times per day, along with paroxysmal nocturnal dyspnoea and dyspnoea on minimal exertion, oligoanuria, severe hypertension and a front-orbital, pulsating headache. She arrived at the emergency department with tachypnoea, oxygen saturation of 86% and bilateral alveolar infiltrate on the chest X-ray, and so was admitted to the ICU. A fundus examination showed grade 3 hypertensive retinopathy (malignant hypertension) and transthoracic echocardiography showed a hypertrophic cardiomyopathy. Laboratory tests on admission revealed a microangiopathic haemolytic anaemia with schistocytes in peripheral blood and kidney failure. Therefore, haemodialysis and 9 PP sessions were started, with replacement with fresh frozen plasma and steroid boluses, and 1 dose of immunoglobulin at the end of the last PP session. The outcome was good with increase and normalisation of platelet count, improvement of anaemia and normalisation of LDH. A renal biopsy showed 32% ischaemic glomeruli with sclerosis, 20 arterioles with concentric luminal hyperplasia and fibrinoid necrosis foci with microthrombi. The patient did not recover renal function and remained on haemodialysis.
29. A 57-year old woman on haemodialysis for 53 months, with underlying polycystic kidney disease, who underwent a transplant on 21 April 2006 from a 54-year old donor sharing 1B, 1DR and with optimal renal biopsy. She started induction with thymoglobulin, tacrolimus, methylprednisolone and mycophenolate 5 days after the operation. Renal function was initially delayed, and required dialysis, but from the fourth day there was a progressive increase in diuresis. On the 8th day after the transplant, renal function and diuresis deteriorated, with signs of microangiopathic haemolytic anaemia (anaemia, thrombocytopaenia, decreased haptoglobin and increased LDH, up to 2250U/L). Boluses of methylprednisolone were administered (500mg in 3 doses) and 6 PP sessions with fresh frozen plasma replacement started. She received intravenous immunoglobulin. The graft biopsy revealed thrombotic microangiopathy with data indicating calcineurin-inhibitor nephrotoxicity. Immunosuppression was changed from tacrolimus to everolimus, with an improvement in renal function. Platelets and haemoglobin were normalised after PP. On day 22 after transplantation, there was a further deterioration in renal function, which coincided with the removal of the ureteral guide catheter. An ultrasound showed perigraft collection and a cystography showed urinary leakage that resolved after catheterisation and rest. At discharge, the patient had a creatinine level of 1.2mg/dL, with subsequent stabilisation at 0.9mg/dL, without microalbuminuria or haematuria.
30. A 21-year old woman with no significant history, except oral contraceptives 5 months before admission. She was referred to our hospital in October 2010 with headaches, after taking anti-inflammatories, as well as asthenia and anorexia, with analytical data indicating ARF, anaemia, thrombocytopaenia, proteinuria and microhaematuria. A renal biopsy was performed revealing signs of HUS thrombotic microangiopathy. Twelve PP sessions were conducted with fresh frozen plasma replacement (there were plasma side effects that required antihistamines and steroids). Doses of steroids, starting at 1mg/kg/day, were reduced with gradual improvement in haemoglobin, platelets and normalisation of haptoglobin. She also required transient haemodialysis, with subsequent recovery of renal function with creatinine of 1.5mg/dL at discharge. A genetic study was conducted of the HUS, which showed a heterozygous mutation in the factor H gene, a susceptibility factor to developing HUS. She was re-admitted 3 months after discharge due to renal function deterioration without evidence of haemolysis, but with anaemia and thrombocytopaenia. Daily PP treatment was begun with fresh frozen plasma replacement, but renal function recovery was incomplete (creatinine of 2.4mg/dL). A month after admission, she presented with a new suspected recurrence of HUS with anaemia, thrombocytopaenia, mild renal function impairment and persistently elevated LDH. After resuming PP sessions, an improvement in haemoglobin, platelet and haptoglobin count was again observed, as well as a decrease in LDH, but renal function did not improve, and creatinine remained at 3-3.4mg/dL, with mid-range proteinuria and persistent microhaematuria. Because of the activity data (fluctuating LDH with low C3 and haptoglobin), she remained on weekly PP until starting a chronic haemodialysis programme in February 2011. Adverse events presented after the PP sessions included itching, rash and wheal coincident with the infusion of fresh frozen plasma, which sometimes required infusion with hydrocortisone and antihistamines, as well as reducing the replacement with plasma and substituting it with SSPP.
31. A 36-year old man with focal segmental hyalinosis as the underlying disease. He had a previous graft from 1998 to 2006 which was lost due to C4d-positive chronic nephropathy. He underwent a nephrectomy in 2007. He was not sensitised and his antibody rate by CDC was 0%. He underwent his second kidney transplant in October 2008. Thirty-six hours after the transplant, renal function deteriorated and diuresis reduced with evidence of haemolytic anaemia, which was interpreted as microangiopathic, probably secondary to the administration of tacrolimus. He was converted to sirolimus and was also given 8 PP sessions with replacement with primarily fresh frozen plasma. Intravenous immunoglobulins were also administered, and there were no complications related to the technique. Subsequent evolution was favourable, with increased diuresis and normalisation of LDH, bilirubin, and platelets. There was also an improvement in renal function with a decrease in creatinine from 9.2 to 5.6mg/dL, where it stabilised. Therefore, a renal biopsy was performed 5 days after the last PP session, which showed an IIA acute rejection with weakly positive C4d staining. It was then decided to administer steroid boluses (500mg methylprednisolone in 3 doses) and rituximab 375mg/m2 (2 doses), with subsequent increased diuresis and creatinine decreasing to 1.9mg/dL.
Rapidly progressive renal failure
32. A 67-year old woman with a long-standing history of hypertension and arthritis; and chronic non-steroidal anti-inflammatory drugs (NSAIDs) use. She was admitted with ARF requiring haemodialysis. She was treated with steroid boluses due to suspicion of rapidly progressive glomerulonephritis and a renal biopsy revealed severe extracapillary glomerulonephritis with positive antibasement membrane antibodies. Therefore, oral cyclophosphamide was started with PP (7 consecutive daily sessions and 5 every other day), with replacement with fresh frozen plasma and SSPP, and a dose of immunoglobulin. There was a decrease in the antibody titre from 128 to 14.6U/L. Renal function did not improve and she remained in chronic haemodialysis.
Group 2
Haematological diseases
33. A 57-year-old man with a history of primary hypereosinophilic syndrome and Waldeström’s macroglobulinaemia was admitted to the internal medicine department in February 2009 with suspected acute myocarditis (troponin elevation with injury-free coronary catheterisation), along with neurological clinical symptoms (visual agnosia, patellar and bilateral Achilles clonus and bilateral Babinski sign). The magnetic resonance imaging (MRI) was compatible with acute ischaemic lesions in the cortical grey matter of both frontal, parietal and occipital lobes, the right thalamic nucleus and both cerebellar hemispheres. Given the history of Waldeström’s syndrome with suspected hyperviscosity syndrome, a series of 8 PP sessions were begun, with a decrease in IgM component of 1250mg/dL to 273mg/dL. Bone marrow aspiration was performed and hyperplastic marrow with dysplastic megakaryocytes and lymphoplasmacytosis with overexpression of lambda and IgM were observed. Clinical evolution was satisfactory, with progressive recovery of vision and progressive normalisation of the eosinophil count.
34. A 65-year old man with a history of hypertension, bilateral hearing loss, deep vein thrombosis in the right leg under oral anticoagulant therapy, osteonecrosis of the jaw in September 2006 and lumbar shingles in 2005. He had been diagnosed with Bence-Jones multiple myeloma in April 1999 with renal failure and bone disease. He was treated with chemotherapy and a bone marrow transplant in March 2000. He remained in complete remission with light chains and negative Bence-Jones proteinuria and normal renal function. He was admitted in December 2008 with left heart failure, complete atrioventricular block (a permanent pacemaker was needed) and renal failure. He remained on chronic haemodialysis due to blood disease progression with thrombocytopaenia up to 23 000/μL. Secondary to the paraprotein, a coagulation disorder was observed with elongation of prothrombin time, activated partial thromboplastin time (APTT), thrombin time and reptilase time with spontaneous haematomas with multiple transfusion requirements. To prevent the effect of the paraprotein, PP was begun with SSPP and fresh frozen plasma replacement (7 sessions in total). As a result, coagulation returned to normal levels. To control the monoclonal component, he was administered a course of cyclophosphamide and dexamethasone, but after a week he had plasma cell leukaemia which was treated with bortezomib, Adriamycin and dexamethasone. The outcome was not favourable, with the development of acalculous cholangitis, right lumboabdominal shingles and oropharyngeal candidiasis. The patient died in February 2009.
Systemic lupus erythematosus
35. A 21-year old woman with a history of epilepsy treated with lamotrigine, with an aortic valve bioprosthesis within native valve due to endocarditis since 2004, obesity, recurrent urinary tract infections and thrombocytopaenia of immunological origin since childhood which was being treated with steroids by a haematologist. She was admitted in May 2006 due to a respiratory failure that required mechanical ventilation for 14 days, ARF, microcytic anaemia with thrombocytopaenia, increased LDH and decreased haptoglobin. A renal biopsy was performed which indicated HUS. Additional tests detected positive lupus anticoagulant, anticardiolipin and antiphospholipid. The patient was admitted to the ICU, where she was treated with continuous veno-venous haemodiafiltration, high-dose steroids and PP, as well as with two courses of intravenous immunoglobulins. Renal function did not improve, leaving the patient at discharge in chronic haemodialysis. She was diagnosed with primary antiphospholipid syndrome and started treatment with oral anticoagulant agents. During the subsequent monitoring in haemodialysis, the patient had multiple vascular access problems, with a tendency to thrombocytopaenia, which made anticoagulation difficult, with the only vascular access being the left femoral central line, and led to multiple episodes of infections. In August 2008, she was re-admitted to the UCI due to an exacerbation of the underlying disease, with onset of pulmonary haemorrhage. She received 3 PP sessions, boluses of methylprednisolone and intravenous immunoglobulins (0.5mg/kg, 3 doses) and 2 doses of rituximab, which controlled the disease. However, in December 2008, she was again re-admitted to the UCI due to a prosthetic aortic valve endocarditis with haemodynamic instability, requiring amines and broad-spectrum antibiotics, with torpid evolution and death.
Septic shock
36. A 40-year old man with anxiety-depressive disorder who was a heavy smoker. He was admitted with impaired mental status secondary to septic shock of respiratory origin with bilateral basal pulmonary infiltrate on the chest X-ray. The lumbar puncture ruled out the existence of meningitis. He was admitted to the ICU for orotracheal intubation and administration of noradrenaline at high doses. He also had anuria and multi-organ failure. Under monitoring, he was given continuous venous haemodiafiltration and broad-spectrum antibiotics with meropenem, ciprofloxacin, linezolid and antifungal coverage with amphotericin. In the context of sepsis, he presented consumptive coagulopathy, which was treated with immunoglobulins and a PP session with fresh frozen plasma replacement. Of the 6 blood cultures extracted, there was only 1 with a growth of S. epidermidis. The growth of enterococci was observed in a throat smear. Legionella and pneumococcal antigens were negative, as well as the atypical pneumonia serology. Multiple organ failure continued until cardiac arrest occurred 15 days after admission.
Cholangiocarcinoma
37. A 75-year old man in chronic haemodialysis due to diabetic nephropathy. He had a cholangiocarcinoma in palliative treatment with biliary endoprosthesis which had to be replaced regularly. He was admitted in September 2009 due to cholangitis, which was refractory to itching drug treatment, and so received 6 PP sessions with albumin replacement. Despite the expansion of antibiotic coverage with meropenem, teicoplanin and levofloxacin, a complication of the cholangitis was haemodynamic deterioration and consumptive coagulopathy secondary to sepsis, which required multiple blood product transfusions. Blood cultures revealed the growth of Corynebacterium species and negative coagulase staphylococcus, with Enterococcus faecalis growing in the urine culture, all sensitive to the antibiotic coverage applied. Hydrocortisone treatment was added, but haemodynamic deterioration and coagulopathy secondary to sepsis continued with clinical deterioration and death.
Group 3
Neurological diseases
38. A 43-year-old man, smoker, diagnosed with multifocal motor neuropathy in October 2005. He was initially treated with intravenous immunoglobulin without clinical response, so a PP programme was started (a total of 12 sessions with albumin replacement) and monthly intravenous cyclophosphamide (1.5g). Evolution was not favourable: he was not able to walk and was dependent for daily life activities. Six months after treatment, the patient was admitted to the pulmonology department due to severe dyspnoea and significant difficulty for the removal of secretions. Non-invasive positive pressure ventilation was started with poor tolerance, and he died on 26 November 2006. The diagnosis was amyotrophic lateral sclerosis.
39. A 27-year-old woman diagnosed with myasthenia gravis in 1995, with thymectomy in 1998 and under treatment with azathioprine, was admitted in October 2007 due to the worsening symptoms of her disease (progressive generalised asthenia, diplopia, palpebral ptosis, dysphonia and dysphagia). Nine sessions of PP were begun with albumin replacement and intravenous immunoglobulin at a dose of 5g/kg/day for 5 days with conversion to cyclosporine, which reversed the symptoms. One month after discharge, the patient was admitted again for cyclosporine neurotoxicity, so she was given oral prednisone alone. She had cytomegalovirus hepatitis and was treated with intravenous ganciclovir and oral valganciclovir later. During follow-up, the patient has needed periodic admissions for maintenance of intravenous immunoglobulin infusion.
40. A 54-year-old man with a history of type 2 diabetes mellitus (T2DM), dyslipidaemia, ischaemic heart disease who had undergone surgery for recurrent superficial bladder cancer. He was admitted in May 2008 due to weakness in the upper and lower limbs with proximal predominance, without clinical sensitivity, with flaccid tetraplegia appearing after 24 hours. A neoplastic, tumoral or paraneoplastic cause was ruled out, along with autoimmune or infectious disease and he was diagnosed with Guillain-Barré syndrome. He received 6 PP sessions with albumin replacement and showed clear clinical improvement. He received doses of immunoglobulins after the PP sessions.
41. A 20-year old male, allergic to penicillin, was admitted in December 2008 due to neurological deterioration with somnolence, ataxia (walking), decreased tendon reflexes in the upper limbs, bilateral diplopia, weakness of swallowing, chewing and phonatory facial muscles. Imaging and lumbar puncture tests were normal. Neurophysiological studies were performed with no sensory-motor response in the limbs, absence of voluntary movements and acute denervation activity. His face had voluntary movement motor response indicative of demyelinating polyradiculopathy with cranial nerve involvement. A ganglioside antibody test was performed which was negative. Suspecting Miller-Fisher syndrome, 4 PP sessions were begun with total replacement with albumin. Partial clinical improvement was seen. He received an immunoglobulin dose after the PP sessions. He presented a fever peak with cultures of urine, cerebrospinal fluid (CSF), blood and bronchial secretion negative. The patient requested a transfer to his home country for follow-up.
Thrombotic thrombocytopaenic purpura
42. A 29-year old woman was admitted to the haematology department in August 2006 due to severe anaemia and thrombocytopaenia and was diagnosed with TTP. She was treated with methylprednisolone, intravenous immunoglobulins and 7 sessions of PP with fresh frozen plasma replacement. The thrombocytopaenia improved significantly with an increase in platelets from 7000 to 165 000/μL. Haemoglobin was normal and peripheral blood schistocytes disappeared. Treatment with oral steroids continued until November 2006. She relapsed in August 2008 and received a new cycle of steroids until October 2008, but thrombocytopenia continued until finally a splenectomy was performed in January 2009, with subsequent normalisation of platelet counts.
43. A 41-year-old woman, obese, smoker, with a history of discoid lupus. She was admitted in April 2007 with general malaise with anaemia of 6.3g/dL for haemoglobin, schistocytes in peripheral blood and thrombocytopaenia of 10 000/μL. On admission, she was treated with steroids at a dose of 1mg/kg/day, intravenous immunoglobulins and received 12 PP sessions with fresh frozen plasma replacement. Haemoglobin levels and platelet counts were normalised after the PP and steroids were gradually decreased until they were stopped.
44. A 75-year-old man, former smoker with insulin-dependent diabetes mellitus of more than 20 years standing was admitted to the ICU in June 2008 with symptoms of malaise, anorexia and asthenia. His medical history revealed a lesion in the orbital area of the left eye with a biopsy compatible with sclerotic adnexal tumour. The chest and abdomen CT scan showed no tumorous lesions. Laboratory tests on admission showed haemolytic anaemia evidence with thrombocytopaenia of 92 000/μL. The peripheral blood smear showed schistocytosis and polychromatophilia. Suspecting TTP, 2 PP sessions with fresh frozen plasma replacement were performed. Upper gastrointestinal endoscopy was performed due to the patient presenting significant melaena during admission and an infiltrating ulcerative adenocarcinoma in the body and antrum was found, AMO showing non-haematological cell nests indicative of metastasis. Given the extent of the disease, the PP sessions were suspended and the patient died 5 days after admission.
45. A 34-year old woman with HELLP syndrome (haemolysis, elevated liver enzymes and low platelet count) in a previous pregnancy, headaches and kidney stones. In June 2003, she was admitted due to microangiopathic haemolytic anaemia and thrombocytopaenia during pregnancy. She did not respond to steroids or immunoglobulin, with HELLP syndrome that required discontinuation of the pregnancy. In May 2006, she presented with thrombocytopaenia, which responded to steroid treatment which was stopped in June 2006. In March 2009, she had haemorrhagic diathesis with 94 000/μL platelets and haemolytic anaemia. She received steroids at a dose of 1mg/kg/day, intravenous immunoglobulin and 6 sessions of PP with fresh frozen plasma replacement, leading to normalisation of the platelet count and haemoglobin levels. She had a self-limiting episode of generalised skin reaction after plasma infusion, which was reversed with steroids. In May 2009, she had a relapse and received several PP sessions. Subsequently, there were further declines in July and October 2009, and she received a total of 19 PP sessions and 4 doses of rituximab. In November 2009, a splenectomy was performed, and in December 2009 she again underwent PP sessions, and treatment with steroids and vincristine. In February 2010, there was a new outbreak of thrombocytopaenia requiring admission and the start of weekly PP sessions. In March 2010, she was treated with cyclosporine A for 6 months with plasmapheresis every 10 days (with the last session on 31 March 2010). Cyclosporine was discontinued in November 2010 with an improvement in platelet count (>200 000/μL), and no evidence of haemolysis 4 months after stopping cyclosporin A treatment.
Idiopathic thrombocytopaenic purpura
46. A 68-year old man diagnosed with ITP in April 2006 was admitted to the haematology department on 24 May 2006 for severe thrombocytopaenia. Initially, he received immunoglobulin, rituximab and PP sessions (with the first of a total of 6 sessions on 9 June 2006). Since there was no significant increase in platelet count (<15 000/μL), a splenectomy was performed on 15 June 2006, with subsequent normalisation of platelets and suspension of steroids. The patient currently has a normal platelet count.
47. A 77-year old man, diabetic, with ischaemic coronary disease and severe aortic stenosis underwent a double aortocoronary by-pass and biological aortic valve replacement in June 2006. He was admitted to the haematology department in June 2008 for examination and treatment of thrombocytopaenia and haemolytic anaemia, and was diagnosed with Evans syndrome. He received dexamethasone and anti-D gamma-globulins, but the thrombocytopaenia of 6000/μL continued. Therefore, PP sessions were begun, with a good response and the platelet count rose from 13 000 to 139 000/μL. He was treated with prednisone at doses of 30mg initially with subsequent progressive decreases to 2.5mg, achieving a stable platelets count of around 80 000/μL.
48. A 65-year old man, diagnosed with ITP in June 2007, received a course of steroids and anti-D immunoglobulins with no response. In August 2007, outpatient intravenous immunoglobulins were prescribed and steroids were increased. The response was moderate, with increased platelets to 119 000/μL. In the control carried out in September 2007, he presented with widespread ecchymosis and thrombocytopaenia, and so began a new cycle of intravenous immunoglobulins, steroids and anti-D immunoglobulins, which were ineffective. He also received a dose of rituximab and 6 PP sessions with fresh frozen plasma replacement between September and October, with no response. A cycle of rituximab, vincristine and cyclophosphamide and another dose of immunoglobulins were administered, but unfortunately with no response. Given the surgical risk, it was decided to partially embolise the splenic artery, and a progressive increase in platelets count was achieved (up to 49 000/μL). An attempt at surgical splenectomy was made, but was suspended due to heavy bleeding during surgery. Finally, he received a cycle of treatment with danazol and azathioprine, but the platelets counts was still around 9000/μL. He was admitted to the ICU for febrile illness with respiratory failure. A chest CT showed bronchoalveolar parenchymal consolidation in the upper and the middle lobes. The results of blood cultures, catheter tip culture, bacillus sputum smears and galactomannan tests were negative. A fibroscopy was not performed due to thrombocytopaenia. He received antibiotic treatment with meropenem, ceftazidime, metronidazole, amikacin, petamidine, caspofungin and voriconazole. The outcome was not favourable, with multiple organ failure and death in November 2007.
Foetal Rh desensitisation
49. A 31-year old woman with subclinical hypothyroidism in the third pregnancy with two previous normal deliveries. At week 22, anti-kell antibodies were detected at a titre of 1/256, which increased in week 24 to 1/1024. She received 19 PP sessions with albumin replacement with a progressive decrease in the antibody titre. At 32.5 weeks gestation, it was decided to induce foetal maturation and a Caesarean section was successfully performed.
50. A 37-year old woman, with a third pregnancy, was admitted for foetal Rh isoimmunisation (with an antibody titre of 1/256). A foetal ultrasound revealed ascites and pericardial effusion. Two PP sessions were conducted with albumin replacement. Percutaneous umbilical blood sampling was subsequently performed with a transfusion of 30mL of blood and atosiban treatment was administered to inhibit uterine contractions. Six days after admission, the death of the foetus was noted, so labour was induced.
Desensitisation of a hypersensitised patient for a kidney transplant
51. A 50-year old woman, with an underlying renal disease of unknown aetiology had spent 4 years on haemodialysis. The maximum and current antibody rate against a wide variety of anti-HLA-I antigens was 85%-95% for both CDC and Luminex, making kidney transplantation highly improbable. The patient had received multiple transfusions, with no previous pregnancies. She had undergone a kidney transplant in 2004 which was lost at 4 months due to acute vascular rejection and vascular thrombosis; with a nephrectomy being performed that same year. Before her second transplant, the patient had received desensitisation treatment consisting of 2 PP sessions and intravenous immunoglobulins (2g/kg) per month from December 2006 until March 2007 (8 PP sessions in total). Initially, there was no clear decrease of the antibody, so in July 2007 she received 2 doses of rituximab, 3 PP sessions and 3 monthly doses of intravenous immunoglobulin (2g/kg) until December 2007. Although there was no significant decrease in the level of antibodies after treatment, some anti-HLA specificities had gone, so a thorough report was conducted of class I antigens permissible for a kidney transplant. The patient received a cadaveric renal graft from a 34-year old donor in September 2008, and received an immunosuppressive treatment protocol consisting of tacrolimus, mycophenolate mofetil, methylprednisolone and anti-thymocyte globulins, 2 doses of rituximab and PP sessions on days 3, 5 and 7 after transplantation, intravenous immunoglobulin infusion at doses of 0.5g/kg after each session and then a reinforcement of 1g/kg on days 10, 11 and 30 after transplantation. Initially, there was immediate renal function and creatinine reached of 0.7mg/dL at discharge. She suffered no acute rejection immediately after transplantation and no immediate side effects from the medication. BK virus PCR at discharge and follow-up was negative, as well as the cytomegalovirus PCR and IgM serology for HSV-1, HSV-2, varicella zoster and Epstein-Barr virus (EBV). Renal function measured by creatinine levels at 3, 6 and 9 months was excellent. The CD19+ count of lymphocyte B populations during follow-up was 0.3±0.02%. In July 2009, renal function deteriorated with elevation of creatinine to 2.3mg/dL, in the context of recent introduction of angiotensin receptor antagonists (ARB) and slightly elevated levels of tacrolimus. The biopsy showed no evidence of acute rejection, the C4d staining in peritubular capillaries was negative and BK virus nephropathy was ruled out. Tubular and vascular calcineurin-inhibitor toxicity was observed, with moderate-severe hyaline deposits in two or more arterioles. Renal function improved partially up to a creatinine of 1.6mg/dL after optimising the calcineurin levels.
Evolution
Overall mortality was 19.6% (n=10), and it was secondary to septic shock in 6 cases, due to haemorrhagic shock in the renal biopsy area in 1 patient, and as a result of the evolution of the underlying disease in the others. There were no deaths in the immune activation transplant group. In the vasculitis group, there were 3 deaths (2 secondary to septic shock). Nine of the 10 patients who died did so within the first three months after diagnosis.
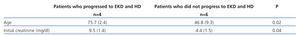
A total of 24 patients required haemodialysis at the start of clinical symptoms: 9 of the 11 patients with vasculitis, 4 of the 5 patients with HUS and 5 of the 15 immune activation transplantation patients. At the end of evolution, 14 of them remained on haemodialysis: 5 of the 11 patients with vasculitis, 2 of the 15 patients who underwent transplantation and 3 of the 5 patients with HUS (Table 1). Cases such as that of the hypersensitised patient on haemodialysis and that with SLE associated with MPA were already on haemodialysis. Significantly, patients who developed EKD in the vasculitis group were older and had higher creatinine at the onset of the disease. The 62-year old patient who was already on haemodialysis was not of course included (Table 4).
In 25 cases with evidence of haemolytic anaemia, haptoglobin consumption was found in 9 of them (36%), and was corrected in 2 of 3 cases after treatment with PP. Of the 29 patients where a blood smear was requested, schistocytes were detected in 9 (31%), which disappeared after treatment with PP in all cases.
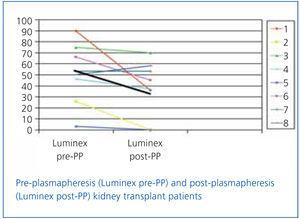
In transplant patients who were monitored for anti-HLA class I or II antibodies by Luminex before and after PP, a decrease in antibody titre was found in all but 1 patient, with the average decrease being from 51% to 31% (Figure 1).
Relevant complications
The PP technique was virtually free of complications. There were 5 (3%) mild-moderate reactions to fresh plasma (perioral tingling and urticarial reactions) which required pre-medication with steroids and did not lead to discontinuation of the treatment. There was only one proven catheter infection. In 20% of patients, there was extracorporeal circuit coagulation, which was more frequent in obese patients (90 versus 68kg, P=.005) and those who underwent a greater volume replacement (25L versus 16L, P=.01).
DISCUSSION
The indication of apheresis technology in general, and PP in particular, is the subject of ongoing debate. The lack of a large series or controlled studies leaves a wide margin of uncertainty for its indications and results. Therefore, most centres continue to use PP on an individual basis within a non-experimental, clinical setting, and as salvage therapy in most cases. Clinical organisations using PP as a first-line therapy (criterion I of the ASFA recommendations)1-3 are an exception.
In our hospital, indications for the use of PP were established in accordance with the recommendations of the American Association of Blood Banks and the American Society for Apheresis1-3 (Table 1). Indeed, patients with Guillain-Barré syndrome, TTP-HUS, vasculitis or immune activation transplantation, among others, were treated with this technique according to the largest scientific evidence previously reported in these institutions.5-10,13,16-20 In other cases, treatment was more empirical in the absence of scientific knowledge to support it.11,12,14,15, 21
To our knowledge, there have been no controlled prospective studies to assess predictors of overall survival in patients treated with PP, probably because of the complexity of such an analysis, especially due to the heterogeneity and complexity of diseases eligible for this technique. In our study, overall mortality was 19.6%, mainly caused by infection, followed by the evolution of the underlying disease. Mortality in vasculitis cases (27%) in our series was similar to that described in the literature and confirms the benefits of PP in these diseases.7-8,22,23 For vasculitis, 2 of the 3 deaths were caused by infections, and it is possible that the degree of systemic disease and immunosuppression administered to these patients contributed to these mortality results. In fact, it has been suggested that for these patients, increased age, a time delay in starting PP, an inadequate dose of PP and start with renal failure and a need for dialysis are independent risk factors associated with a worse prognosis.22-24 Therefore, proper and early use of this technique may improve results in the short to medium term. Furthermore, the presence of ARF can bring about a higher risk of serious infections and bleeding complications that can obscure the prognosis.
The use of immunoglobulins after PP sessions is not widespread. Future randomised studies that compare groups receiving intravenous immunoglobulins after PP treatment may clarify whether its use is beneficial in patients undergoing aggressive immunosuppressive treatments, such as patients with systemic vasculitis. The infusion of immunoglobulins not only provides a defence, but also produces a number of effects on the immune system to induce the favourable development of many illnesses towards their resolution via multiple mechanisms, including regulation of autoantibodies and cytokines via anti-cytokine or anti-idiotype antibodies in the mixture of immunoglobulins, inhibition of the binding of the complement fractions to the target cells, inhibition of the proliferation of T and B cells, blockade of Fc receptors on macrophages and blocking anti-membrane antibodies of Schwann cells and myelin, among others.25
There were no deaths in patients with HUS in our series, although there were only 5 cases. For this particular disease, the mortality rate reached 90% before the use of PP, which was reduced to 20% after the introduction of PP in the treatment.8 Pathogens responsible for most diseases treated with PP are intravascular primarily. The effectiveness of a PP session for a substance depends on its degree of distribution between the intravascular and extravascular space, as well as on the time taken for levels of the substance to recover after a PP session.1,3 Regarding the intensity of plasma exchange, analysis of the published series for the treatment of HUS indicates that the replacement volume should be about 1.5 times the patient's plasma volume (plasma volume infused between 50 and 60mL/kg) and to be performed daily until remission. In most patients, the response (as measured by increase in platelet and haemoglobin count, the disappearance of neurological symptoms and normalisation of LDH and haptoglobin levels)7 is noticeable within the first 5 days of treatment. Therefore, the absence of such a response in a week would be an indicator of treatment failure. However, an improvement in renal function does not usually appear until after several weeks of treatment.6,7 Resolution rates range between 50% and 80%, and these uneven results suggest considerable variability in the efficacy of treatment and show that a high proportion of patients do not respond to PP. Such variability in response may be due to disparities in the intensity with which the plasma exchange is applied, the speed at which it begins, the type of disease (idiopathic HUS, due to genetic or atypical alteration), or the patient features in each series. In this respect, recent studies have found that a delay in the start of PP, the presence of stupor or coma and a higher degree of renal failure are poor prognosis factors.7,8
The evolution of renal function varied according to the underlying disease involved. Some 47% of patients required haemodialysis at the onset of the disease, mainly those in the vasculitis, HUS and kidney transplantation groups. In the meta-analysis by Walters Giles et al,23 PP provides a significant benefit to patients with renal involvement due to vasculitis, reducing the number of patients requiring dialysis at 12 months. The MEPEX study26 shows that in patients with vasculitis and severe renal disease, PP improves the prognosis of renal function and survival. In fact, guidelines available to us advise PP as first-line treatment for these patients. In our study, at the end of evolution, more than half of the patients were withdrawn from haemodialysis, with 45% of patients with vasculitis, 60% of HUS patients and 13% of transplant patients remaining on haemodialysis. Significant factors associated with progression to EKD in patients with vasculitis were creatinine at the onset of the disease and patient age.
Currently, there are few options for a kidney transplant in hypersensitised patients if they are not subjected to prior desensitisation treatment or powerful induction therapy. High-dose intravenous immunoglobulins have reduced the rate of circulating antibodies, but despite their effectiveness, many patients only respond partially and its effect is variable among individuals.27 Other researchers have shown that PP may decrease circulating antibodies.28 However, after the end of the sessions, there is a significant rebound in the titre, and it is therefore now considered more of a complementary technique to the use of immunoglobulins for reducing the antibody rate. Similarly, rituximab has also shown a beneficial effect in combination with immunoglobulins and PP in reducing anti-HLA antibodies and in the treatment of antibody-mediated rejection.27,29-31 In any case, the strategy of combining these measures may represent the best therapeutic choice for these patients, both as a desensitising treatment and when detecting an acute antibody-mediated rejection. It remains to be seen whether these good results will be maintained over the long term without infectious or tumoral side effects. Well-designed longitudinal studies will clarify these issues.
In the absence of conclusive scientific evidence, our study provides useful and valuable information for normal clinical practice and certainly makes us reflect on future strategies for optimising outcomes in our patients. It must be remembered that this is an expensive technique that is not free from side effects, which provides encouraging survival expectations (especially in desperate cases), but risk-benefit ratio and cost-effectiveness are still unknown. In fact, the leading cause of mortality was infection, followed by the evolution of the underlying disease. In other words, the first may be a likely complication of the disease or the adverse influence of the technique, and the second the lack of response to it. It is not known if a mortality of 19% is excessive, but the causes of death are a clear message of warning. Resolving this was beyond the scope of our analysis.
In summary, taking into consideration the wide variety of sporadic diseases that can benefit from PP, publishing the experience with this therapeutic modality is of great importance, as increasing case series publications by centre can help expand the level of evidence in terms of survival and renal function in many uncommon diseases.
Figure 1. Evolution of percentage class I and II anti-HLA antibodies measured by Luminex in kidney transplant patients
Table 1. Classification of patients by disease groups according to initial diagnostic suspicion. Plasmapheresis indication criteria and patient evolution
Table 2. Baseline characteristics and summary of plasmapheresis treatment by diagnostic groups
Table 3. Evolution of patients by diagnostic groups
Table 4. Comparison of patients who progressed to EKD and those who did not in the vasculitis group