Glomerulonephritis (GN) is one of the main causes of chronic terminal kidney disease; however, few studies assess its prognosis in dialysis. We analyze the survival and characteristics of patients on peritoneal dialysis (PD) with primary GN (PGN), and compare their results with other kidney patients.
MethodsThis prospective observational study took place from 1995 to 2014. We included all incident patients who were initiated on the technique in the Levante registry. Data were transferred to an anonymized database in Access. Statistical analysis was performed using SPSS software (version 19.0).
ResultsThe study included 2243 patients, with GN representing the main cause of primary kidney disease (21,5%). IgA nephropathy was the most frequent histologically confirmed form of PGN. Compared with the rest of the sample, patients with PGN were more often men (65% vs 58%, p = .004), and they were on average younger (48 years vs 55 years, p < .001). They also had fewer comorbidities and a higher rate of inclusion on the waitlist for a kidney transplant (87 vs 63%, p < .001). Patients with PGN also had more transplants (48,9%, p < .001), and this was the most frequent reason for stopping PD; beyond that, their peritonitis mean rate was lower (0,34 vs 0,45 episodes/patient-year, p < .001). Technique survival was 90,6% at one year, 71,7% at 3 years, and 59,0% at 5 years (median 76,8 months); there were no differences between groups. Survival was 94,9% at one year, 80,1% at 3 years, and 63,7% at 5 years (median 90,7 months). Patients with PGN showed better mean survival than patients with other kidney pathologies (153,5 months [95% IC: 137,0–169,9] vs 110,3 months [95% CI: 100,8–119,7], p < .001). In the multivariable analysis, the main negative risk factor influencing technique survival was a higher peritoneal transport (p = .018). Factors with a negative influence on mortality were being older (p < .001) and having any comorbidity, mainly diabetes and liver disease (p < .001). By contrast, protective survival factors were inclusion on the transplant waitlist and a higher baseline residual renal function (p = .001).
ConclusionsPD has several advantages as a first dialytic treatment, and our results suggest that it is an excellent technique to manage patients with PGN while they await a kidney transplant.
Las glomerulonefritis (GN) constituyen una de las principales causas de enfermedad renal crónica estadio 5 en diálisis, sin embargo, pocos estudios se centran en su pronóstico en diálisis. Analizamos la supervivencia y características de los pacientes con GN primaria (GNP) en diálisis peritoneal (DP) y comparamos sus resultados con otros enfermos.
MétodosEstudio observacional con recogida de datos de manera prospectiva durante 2 décadas (1995–2014). Incluimos a todos los pacientes del registro Levante que iniciaron DP como primera técnica dialítica. Los datos se transfirieron a una base de datos anonimizada en Access. El análisis estadístico se realizó mediante el programa SPSS (versión 19.0).
ResultadosEl estudio incluyó a 2.243 pacientes, siendo las GN la principal causa de enfermedad renal primaria (21,5%). La nefropatía IgA fue la GNP con confirmación histológica más frecuente. Comparados con el resto de la muestra, los pacientes con GNP fueron en mayor proporción varones (65 vs. 58%, p = 0,004), con menor edad (48 vs. 55 años, p < 0,001), menos comorbilidad y mayor tasa de inclusión en lista de espera de trasplante renal (87 vs. 63%, p < 0,001). Asimismo, los pacientes con GNP se trasplantaron más (48,9%, p < 0,001) y este fue su motivo más frecuente de salida de DP; además de presentar menor tasa global de peritonitis (0,34 vs. 0,45 episodios/paciente-año, p < 0,001). La supervivencia técnica fue del 90,6% al año, del 71,7% a los 3 años y del 59% a los 5 años (mediana 76,8 meses), sin diferencias entre grupos. La supervivencia de los pacientes fue del 94,9% al año, del 80,1% a los 3 años y del 63,7% a los 5 años (mediana 90,7 meses). Los enfermos con GNP presentaron mejor supervivencia media que el resto de patologías (153,5 meses [IC 95%: 137–169,9) vs. 110,3 meses [IC 95%: 100,8–119,7], p < 0,001). En el multivariante, se relacionó de manera negativa con la supervivencia técnica tener mayor transporte peritoneal (p = 0,018), y con la supervivencia del paciente tener mayor edad (p < 0,001) y alguna comorbilidad, especialmente diabetes y hepatopatía (p < 0,001). Por el contrario, la inclusión en lista de espera y la función renal residual (p < 0,001) favorecieron ambas supervivencias.
ConclusionesA la vista de nuestros resultados y teniendo en cuenta las ventajas de la DP como primer tratamiento dialítico, consideramos que esta terapia es una excelente técnica para los enfermos con GNP mientras esperan un trasplante renal.
Glomerulonephritis (GN) is a heterogeneous group of diseases with high morbidity and mortality1 and also high cost. It primarily affects young patients and is a major cause of chronic kidney disease stage 5 on dialysis (CKD5D) in various international registries.1 In Spain, it is the fourth largest cause of CKD5D in incident patients and the primary cause in prevalent patients (REER [Registro Español de Enfermos Renales (Spanish Registry of Renal Patients)] 2013).2
Peritoneal dialysis (PD) outcomes have improved in recent years, with a survival rate that could be even higher than for haemodialysis (HD) during the first years that the technique is used and especially in young patients with low comorbidity.3–8 At the same time, PD offers other additional advantages such as not requiring vascular access, allowing greater patient autonomy and free time and reducing the economic cost for the system.9–11
Most published studies on primary GN (PGN) focus on its prevalence and clinical manifestations. There is a lack of series that analyse its prognosis, and there is disparity in the results with limitations such as the number of patients, short follow-up time and/or lack of clinical data.12–18
The main objective of this study was to analyse patient survival and the PD technique, over a 20-year period (1995–2014), in incident patients on PD with primary GN in a Spanish multicentre registry. We compared the results with those recorded in patients with other primary renal diseases (PRD), identifying the main risk factors associated with patient survival and technique. The demographic characteristics, technical aspects and complications that arose are also described.
Materials and methodsThis is a multicentre observational study. Data collection was carried out prospectively over a 20-year period (1995–2014) in the Levante Registry, made up of several public hospitals in the south east of Spain (provinces of Albacete, Alicante, Castellón, Cuenca, Murcia and Valencia), an area which is the home to 7.1 million inhabitants.
The study included a total of 2243 patients who started PD as the first dialysis technique. Patients who had undergone HD, kidney transplantation (KT) and from other centres were excluded; patients receiving treatment for cardiorenal syndrome were also excluded.
The variables studied were: age at onset of PD, gender, cause of CKD, cardiovascular (CV) risk factors and comorbidity according to the Charlson index (ACCI), inclusion on the waiting list for KT, PD modality (manual or automated), peritoneal adequacy and transport data (D/P Cr4), mean duration of use of the technique, peritonitis, status at the end of follow-up (transfer to HD, transplantation, death or ongoing PD) and cause of death, if applicable.
The final event for patient survival analysis was death (discharges for kidney transplant (KT), recovery of renal function or transfer to HD were considered lost to follow-up). The discontinuation of the technique was the transfer to HD.
Statistical analysisThe data were transferred to an anonymised database in Access for processing, and the statistical analysis was performed using the IBM SPSS Statistics software, version 19.0.
The sample obtained was described according to the nature of the variables, mean and standard deviation for continuous variables and frequencies for categorical variables.
In the bivariate analysis, Pearson’s Chi square and its standardised residuals were used to compare proportions, or Fisher’s exact test as required by the sample size. The means between two groups were compared using the Student’s t-test or the Mann–Whitney U test, while for several groups it was used variance analysis with Bonferroni multiple comparison tests, and the Pearson correlation coefficient was used to correlate quantitative variables.
The peritoneal adequacy, nutrition and kinetics variables, at baseline and the final parameters, as well as the mean annual loss, if applicable, were modelled by linear regression. The log-rank test was used for survival analysis. Regarding peritonitis, it was analyzed the presence of some form of peritonitis (logistic regression) and the time until the first episode of peritonitis (Cox regression). Finally, for definitive exclusions (death, transfer to HD, KT or improvement in residual renal function [RRF]), was analyzed by multivariate analysis using Cox regression.
ResultsThe total number of incident patients on PD between 1 January 1995 and 31 December 2014 was 2243 patients. A 60% (n: 1345) were men, and the mean age of the sample was 53.72 years (SD: 15.81).
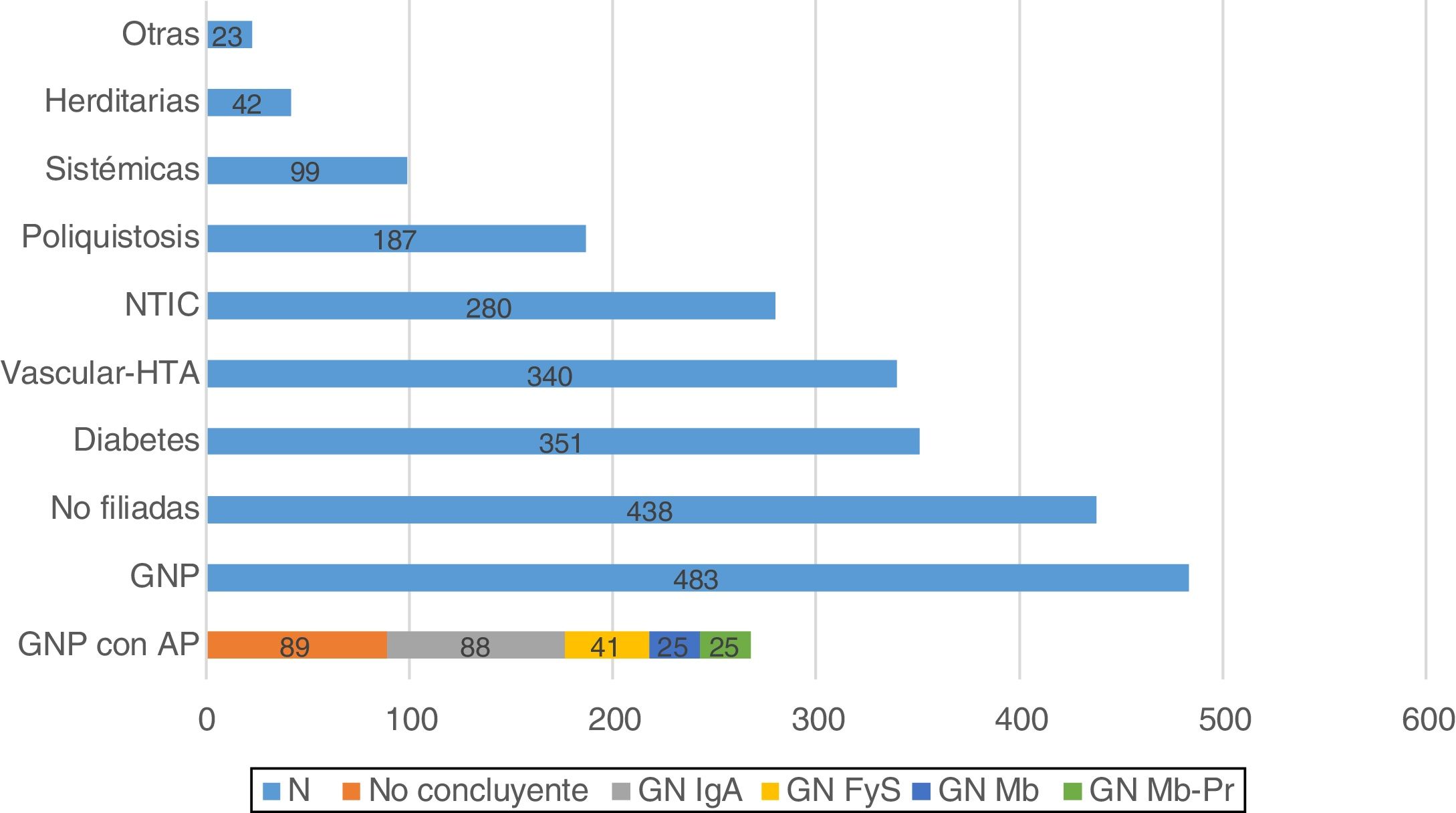
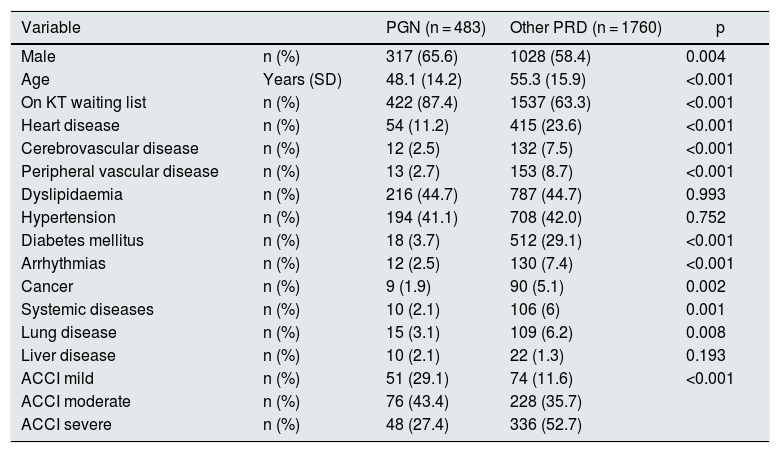
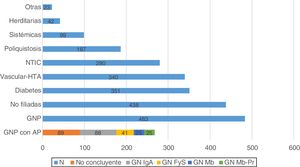
The main cause of primary renal disease (PRD) was PGN (n: 483; 21.5%), followed by nephropathies of unknown aetiology (n: 438; 19.5%), diabetic nephropathy (n: 351; 15.7%) and vascular-hypertensive nephropathy (n: 340; 15.2%) (Fig. 1).
Histology is available in 268 of the patients classified as having PGN (55.5%). Of the biopsies performed, 89 gave inconclusive results (18.4%), and of the remaining 179 (37.1%), IgA nephropathy was the most common GN (n: 88; 18.2%) (Fig. 1).
Among patients with PGN there were more males (65.6% vs 58.4%; p = 0.004), younger (48.1 years vs 55.3 years; p < 0.001), with less comorbidity and with a higher proportion on the waiting list for KT (87.4% vs 63.3%; p < 0.001) as compared to patients with other PRD (Table 1).
Risk factors and comorbidity in PGN vs the other diseases.
| Variable | PGN (n = 483) | Other PRD (n = 1760) | p | |
|---|---|---|---|---|
| Male | n (%) | 317 (65.6) | 1028 (58.4) | 0.004 |
| Age | Years (SD) | 48.1 (14.2) | 55.3 (15.9) | <0.001 |
| On KT waiting list | n (%) | 422 (87.4) | 1537 (63.3) | <0.001 |
| Heart disease | n (%) | 54 (11.2) | 415 (23.6) | <0.001 |
| Cerebrovascular disease | n (%) | 12 (2.5) | 132 (7.5) | <0.001 |
| Peripheral vascular disease | n (%) | 13 (2.7) | 153 (8.7) | <0.001 |
| Dyslipidaemia | n (%) | 216 (44.7) | 787 (44.7) | 0.993 |
| Hypertension | n (%) | 194 (41.1) | 708 (42.0) | 0.752 |
| Diabetes mellitus | n (%) | 18 (3.7) | 512 (29.1) | <0.001 |
| Arrhythmias | n (%) | 12 (2.5) | 130 (7.4) | <0.001 |
| Cancer | n (%) | 9 (1.9) | 90 (5.1) | 0.002 |
| Systemic diseases | n (%) | 10 (2.1) | 106 (6) | 0.001 |
| Lung disease | n (%) | 15 (3.1) | 109 (6.2) | 0.008 |
| Liver disease | n (%) | 10 (2.1) | 22 (1.3) | 0.193 |
| ACCI mild | n (%) | 51 (29.1) | 74 (11.6) | <0.001 |
| ACCI moderate | n (%) | 76 (43.4) | 228 (35.7) | |
| ACCI severe | n (%) | 48 (27.4) | 336 (52.7) |
ACCI: age-adjusted Charlson comorbidity index; KT: kidney transplant; PGN: primary glomerulonephritis; PRD: primary renal disease; SD: standard deviation.
Regarding the technique, patients with PGN had a similar percentage of cycler use (23.7% vs 22.5%; p = 0.02) and used less icodextrin (19.5% vs 24%; p < 0.001) than patients with other PRD.
Patients with PGN had an excellent dose of dialysis, with no differences when compared with other patients (mean total Kt/V of 2.40 vs 2.43). They had higher initial nPCR (1.11 vs 1.05) and this decreased less over time (p < 0.001), with a rate annual loss of renal residual function (RRF) similar to other nephropathies (1.5 vs 1.8 ml/min).
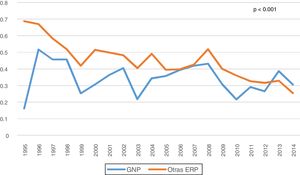
There were no differences in peritoneal transport according to baseline D/P Cr4 for PGN versus the other PRD, with an overall mean value of 0.68 (SD: 0.13) vs 0.67 (SD: 0.12), respectively. However, it was observed an increase in transport over time, more pronounced in patients with GN than in the rest of the patients (0.71 vs 0.68 at year five; p = 0.029).
At the end of the study period, 467 patients (20.8%) were still in the programme, 755 (33.7%) had received transplants, 536 (23.9%) had been transferred to HD, 394 (17.6%) had died and 50 (2.2%) had recovered RRF. In 41 patients were lost to follow-up (1.8%).
The time spent on PD for PGN was 28.5 months (SD: 30.07), with no differences in comparison with the other diseases.
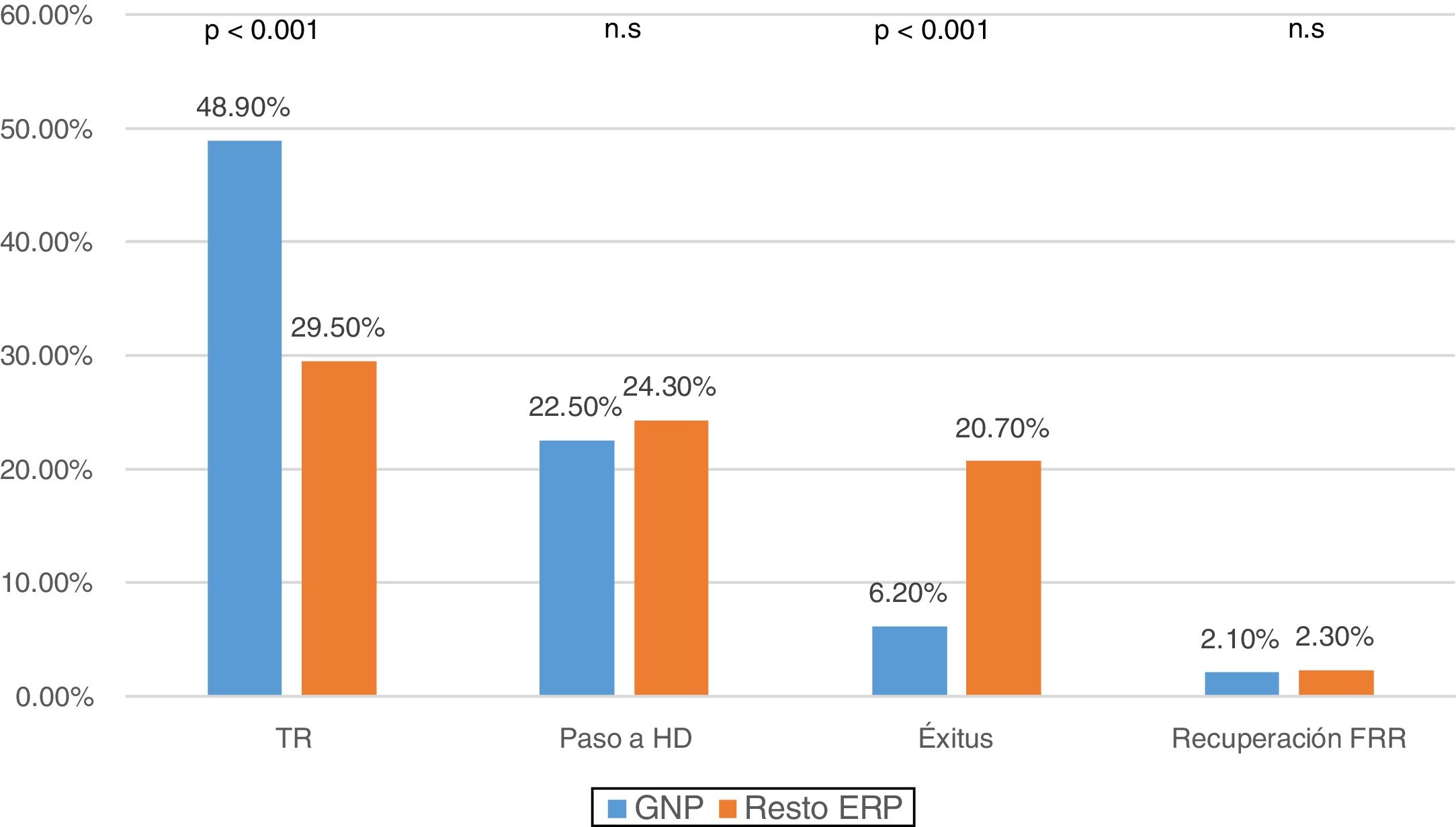
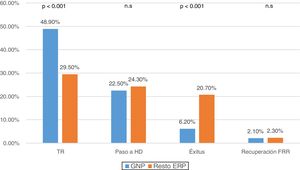
When comparing reasons for discharge according to the PRD, it was observed that in PGN patients 48.9% (n: 236) were transplanted and 6.2% (n: 30) died, compared to the 29.5% (n: 519) transplanted and of 20.7% (n: 364) mortality in the other diseases (p < 0.001) (Fig. 2).
The main cause of mortality in our study was cardiovascular (CV) (37.3%), followed by infections (26.9%). However, in patients with PGN, the primary cause of mortality was infection (36.7%), followed by CV (30%) and then cancer (13.3%), but the differences were not significant.
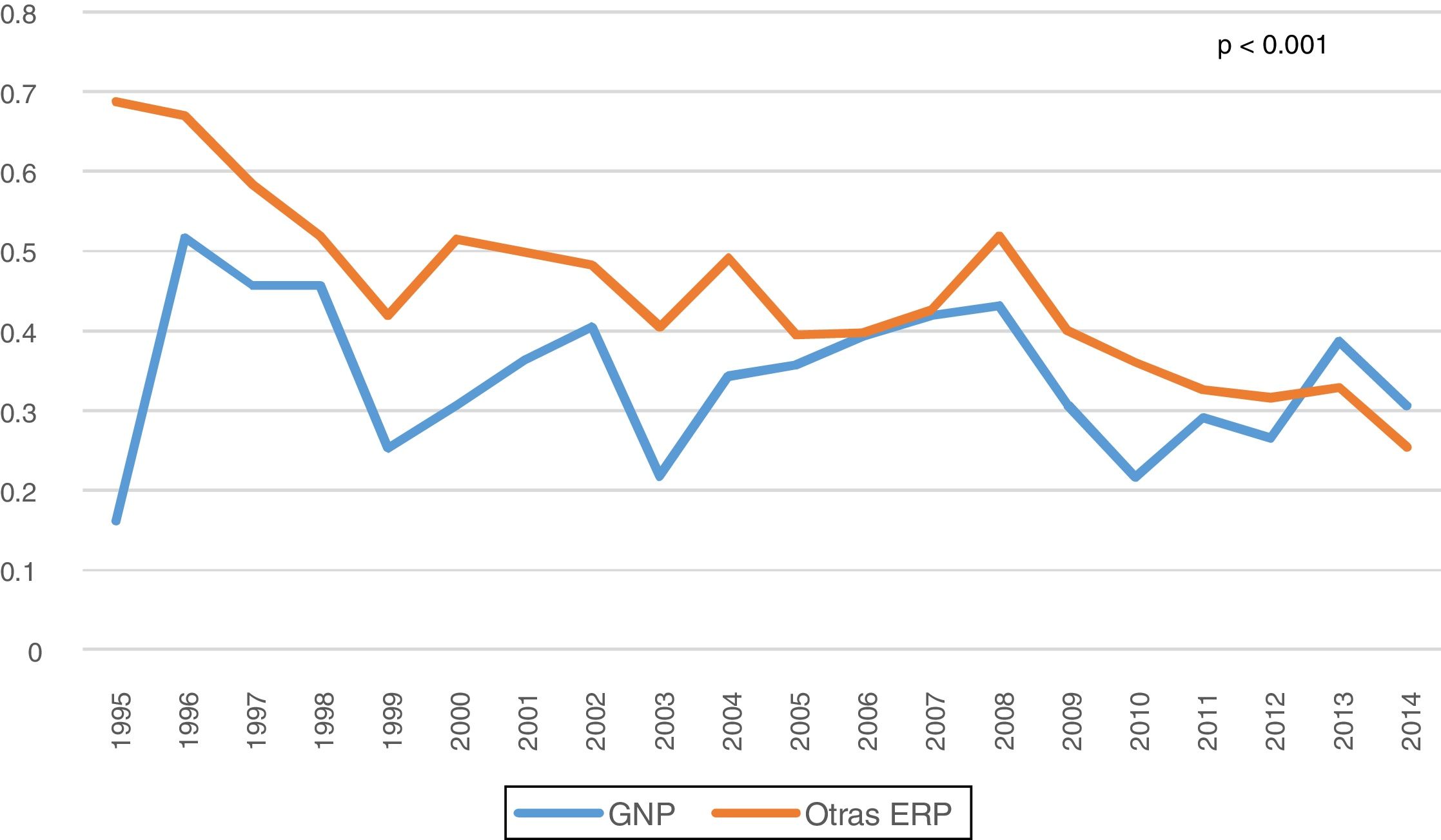
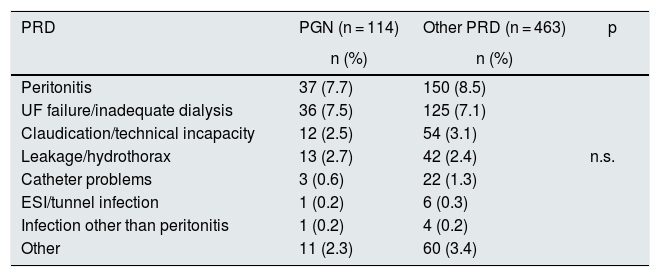
Among all the complications of the technique, peritonitis was specifically reviewed. A decrease in the overall rate of peritonitis was observed throughout the years of the study, and it was lower in PGN patients than in others (0.34 vs 0.45 episodes/patient-year, p < 0.001) (Fig. 3). Most episodes of peritonitis progressed favourably, with a recovery rate of almost 80%. However, peritonitis was the most frequent cause of switching to HD (n: 187; 34.9%), followed by failure of ultrafiltration and/or inadequate dialysis (n: 161; 30%). No differences were found in the transfer to HD between the different PRD (Table 2).
Causes of definitive discharge with transfer to HD or loss to follow-up according to PRD.
| PRD | PGN (n = 114) | Other PRD (n = 463) | p |
|---|---|---|---|
| n (%) | n (%) | ||
| Peritonitis | 37 (7.7) | 150 (8.5) | |
| UF failure/inadequate dialysis | 36 (7.5) | 125 (7.1) | |
| Claudication/technical incapacity | 12 (2.5) | 54 (3.1) | |
| Leakage/hydrothorax | 13 (2.7) | 42 (2.4) | n.s. |
| Catheter problems | 3 (0.6) | 22 (1.3) | |
| ESI/tunnel infection | 1 (0.2) | 6 (0.3) | |
| Infection other than peritonitis | 1 (0.2) | 4 (0.2) | |
| Other | 11 (2.3) | 60 (3.4) |
ESI: exit site infection; HD: haemodialysis; PGN: primary glomerulonephritis; PRD: primary renal disease; UF: ultrafiltration.
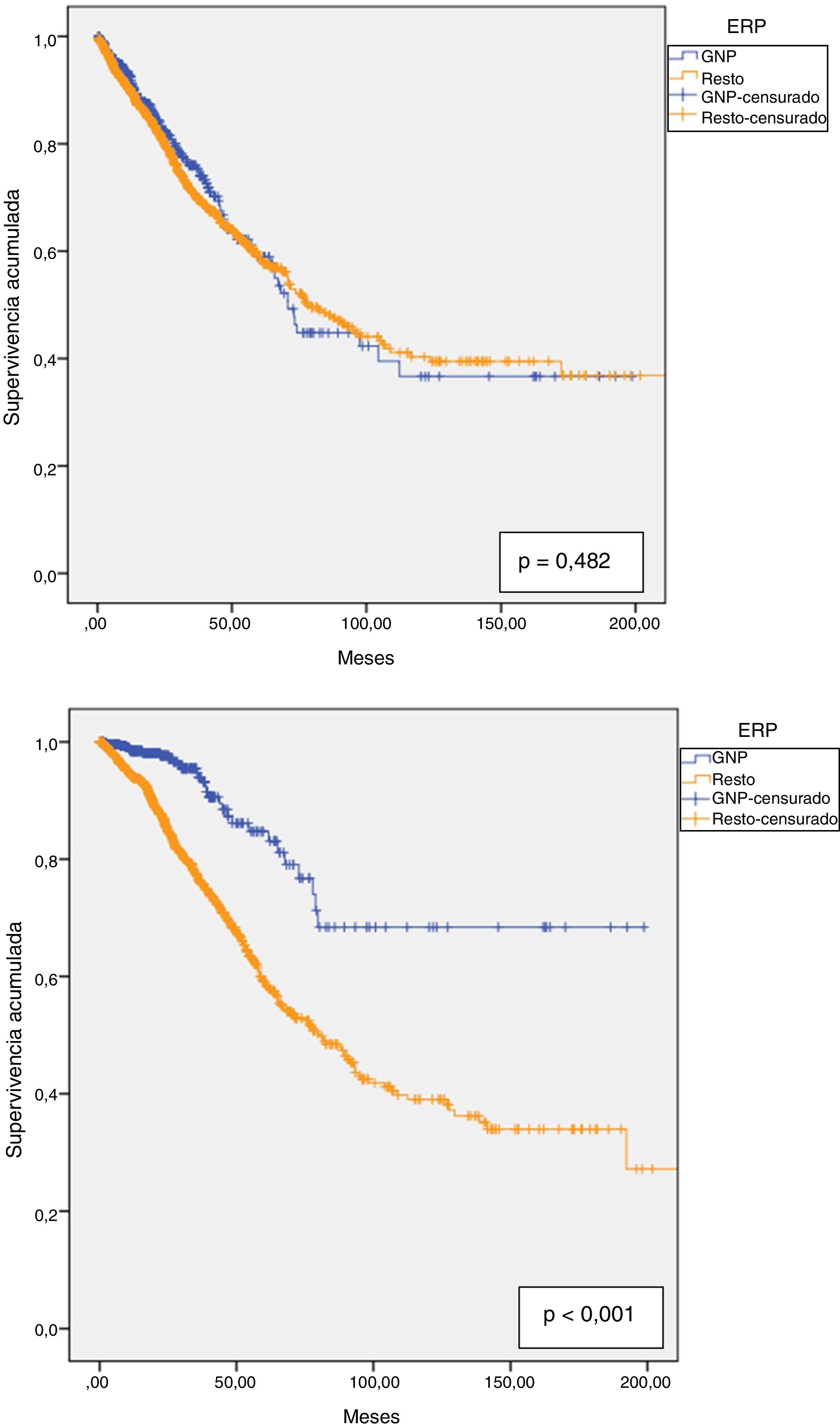
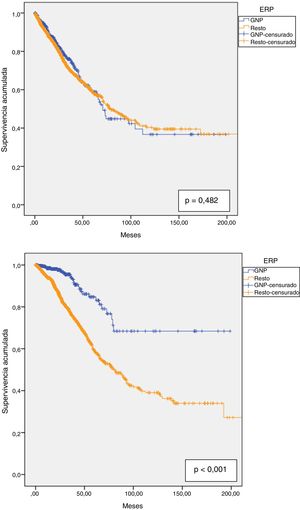
Technique survival was 90.6% at one year, 81.2% at two years, 71.7% at three years, 64.4% at four years and 59% at five years, with a median survival of 76.8 months (95% CI: 66.4–87.2). Technique survival PGN patients was not different than in the rest of patients; 92.6% at one year; 82.8% at two years; 76% at three years; 64% at four years and 59% at five years, with a median of 70.8 months (95% CI: 64.0–77.5) in PGN patients vs 78 months (95% CI: 66.9–89.2) in the rest (Fig. 4a).
In the multivariate analysis, the main risk factor for survival with the technique was the last highest value of D/P Cr4 (p = 0.018). Protective factors were being included in the waiting list for kidney transplant (p = 0.029) and a higher baseline RRF (p = 0.001) were (Table 3).
Transfer to HD: Cox regression.
| Variable | Exp (B) | 95% CI | p |
|---|---|---|---|
| D/P Cr4> | 2.78 | 1.19−6.47 | 0.018 |
| Baseline RRF> | 0.94 | 0.91−0.98 | 0.001 |
| On KT list | 0.77 | 0.61−0.97 | 0.029 |
CI: confidence interval; D/P Cr4: 4-h dialysate-to-plasma ratio of creatinine; HD: haemodialysis; KT: kidney transplant; RRF: residual renal function.
Patient survival at one year was 94.9%, at two years 88.1%, at three years 80.1%, at four years 72% and at five years 63.7%. The estimated median was 90.7 months (95% CI: 76.9–104.6). For PGN patients, the respective survival at one through to five was 98.5%; 97.7%; 94%; 87.3% and 83.1%. These patients had greater overall survival than with other diseases (153.5 months (95% CI: 137.0–169.9) vs 110.3 months (95% CI: 100.8–119.7), p < 0.001). (Fig. 4b).
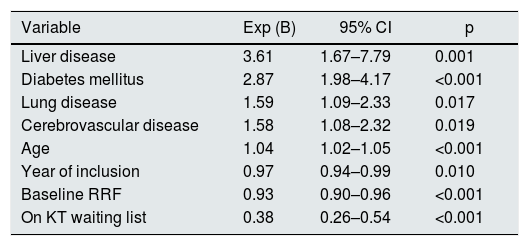
In the multivariate analysis, the following variables were found to have a negative influence on patient survival: being older (p < 0.001), diabetes mellitus (DM) (p < 0.001), liver disease (p = 0.001), lung disease (p = 0.017) and cerebrovascular disease (p = 0.019). In contrast, mortality was lower in patients who were on the waiting list for KT (p < 0.001), with a better RRF both, at the beginning of the technique (p < 0.001) and in the most recent years of the study (p = 0.010) (Table 4). In fact, survival between the two decades of study was lower for the first, with a median of 81.7 months (95% CI: 67.7–95.7) than in the second, 93.3 months (p < 0.001).
Patient overall survival: Cox regression.
| Variable | Exp (B) | 95% CI | p |
|---|---|---|---|
| Liver disease | 3.61 | 1.67–7.79 | 0.001 |
| Diabetes mellitus | 2.87 | 1.98–4.17 | <0.001 |
| Lung disease | 1.59 | 1.09–2.33 | 0.017 |
| Cerebrovascular disease | 1.58 | 1.08–2.32 | 0.019 |
| Age | 1.04 | 1.02–1.05 | <0.001 |
| Year of inclusion | 0.97 | 0.94–0.99 | 0.010 |
| Baseline RRF | 0.93 | 0.90–0.96 | <0.001 |
| On KT waiting list | 0.38 | 0.26–0.54 | <0.001 |
CI: confidence interval; KT: kidney transplant; RRF: residual renal function.
Most studies that concern PGN focus on epidemiology, clinical manifestations and immunosuppressive treatment. There are few publications that analyse its prognosis and progression to CKD5D. Moreover, some authors group GN into a single entity, regardless of whether they are primary or secondary to another systemic disease, even though they are known to differ both in their manifestation and their progression.12,13,19
PGN was found to be the most frequent aetiology of PRD in the Registry of the Levante region (21.5%). However, in the 2014 report of the ERA-EDTA (European Renal Association — European Dialysis and Transplant Association), diabetic nephropathy was the leading cause of CKD (19%), followed by GN and nephropathies of unknown aetiology, with 17% each. The data presented by the 2012 USRD (US Renal Data System) report21 show DM to be the leading cause of PRD in dialysis, followed by hypertension, while GN is the third most common cause of CKD5D. Our data contrast with those reported by other large series in which DM is the most common PRD and the leading cause of incidents in CKD5D.2,20–22 This fact could be explained by a lower indication for PD in older diabetics due to a potentially worse survival rate reported by other authors.4,5
The published annual incidence for PGN suggests that IgA nephropathy is the most common, followed by membranous GN, minimal change disease, focal and segmental GN and, lastly, membranoproliferative GN.15 The differences in comparison with this study, with low representation of membranous GN and an absence of minimal change disease, are due to the different prognosis of each of the GN, with the probability of progressing to CKD5D varying between 16% and 39% depending on the series and follow-up time.16–18
In our series, we observed a decrease in the rate of peritonitis from the beginning of the study (0.59 peritonitis episodes/patient-year) until its completion (0.26 peritonitis episodes/patient-year). The rates of peritonitis from our study are lower than those suggested by the ISPD (International Society for Peritoneal Dialysis) Guidelines (0.5 peritonitis episodes/patient-year)23, with an overall mean of 0.41 peritonitis episodes/patient-year, which was even lower in PGN with 0.34 peritonitis episodes/patient-year, probably in relation to their younger age and lower comorbidity. There are significant discrepancies between different countries. The rates of peritonitis vary between 0.06–1.66 peritonitis episodes/patient-year.24 However, there is a progressive drop in the rate of peritonitis, especially in peritonitis caused by Gram-positive bacteria, which is due to improvements in PD and the greater accumulated experience.
The most common reasons for discontinuing the PD technique are classically distributed into three thirds, as described by other authors: patient death, kidney transplant and failure of the technique.25–27 Our registry does not follow with this three-thirds rule, especially in patients with PGN, given that due to their characteristics, practically half left the programme after receiving a KT, and the mortality rate was well below the average. It should be noted that Spain ranks as the country with the highest rate of deceased-donor KT, with an overall donation rate that is increasing and that reached 70.8 per million inhabitants in 2018.28
Regarding the transfer to HD, the literature shows wide variations with percentages ranging from 9% to 32%.22,25,26,29–33 Our results are about average, with no differences in terms of PRD, and the most common reasons for technique failure (peritonitis, ultrafiltration failure and inadequate dialysis) were consistent with those of other registries.29–31,34,35
Our study identified a marked decrease in mortality over the two decades, which has also been observed in the entire country.25,29 Not only did patients with PGN have a lower percentage of deaths, but the causes of death also differed, with infections being the leading cause and cancer the third most common. Zhang et al.19 analysed the characteristics of 179 patients who died in their PD programme between 2006 and 2011. In this series, GN was the cause of PRD in 24.6% of patients, and they had a lower risk of mortality than other kidney diseases (DM, vasculitis, myeloma and amyloidosis), and the main cause of death was CV (34%), followed by infections (20%).
There were no observable differences in technique survival between our group and the rest of the patients, with figures that were comparable with other registries.25 Protective factors include having a higher baseline RRF, with all the benefits that are already known, and which include better adequacy and volume control, as well as better survival and quality of life.36–38
The overall survival of the patients in our registry is average compared to that reported by other studies,25,26,29–31,33,35,39 although it should be borne in mind that we only included incident patients. Survival was even better for patients with PGN, as can be deduced from the results of the multivariate analysis. As in other publications, the variables with a negative impact on survival were being older, suffering from DM and having some form of comorbidity (especially liver, pulmonary and cerebrovascular disease, variables that are consistent with those identified in the series by Wang et al.40). In contrast, mortality was lower in patients who were on the waiting list for kidney transplant (younger, with fewer comorbidities and better nutritional status) and with better RRF when they started to use the technique. We do not have laboratory variables such as proteinuria, albumin or haemoglobin, nevertheless nPCR and peritoneal transport were not identified as prognostic factors in our series.
One of the limitations of our study is the lack of certain data related to GN (prescribed immunosuppressive treatment and duration, time elapsed from diagnosis to inclusion in the PD programme, histological findings, etc.) that could influence the prognosis. It is important to mention the absence of histological confirmation in a significant percentage of patients classified as having PGN, although we believe that this is a correct representation similar to that of other series, and the clinical profile, age and associated comorbidity are similar to those of patients with biopsy. Another limitation of our study is the grouping of the different subtypes of PGN into a single entity when performing the analysis, despite the fact that several studies have shown that each of the subtypes of PGN are well-differentiated nephropathies in terms of their presentation and prognosis.12,13,19
The strengths of our study are the number of patients included, from a multicentre registry, as well as the long follow-up period, which offers us an overview of the evolution of PD as a dialysis technique in our setting. Another strength is the analysis of PGN as an independent group and a well-differentiated entity from GN secondary to a systemic disease, which is known to have its own specific characteristics and a different prognosis.
From the results obtained in our series, it can be concluded that patients with PGN represent the most common PRD in PD in our setting, are younger and have fewer comorbidities, have a higher inclusion rate on the waiting list for KT, better mean patient survival and similar technique survival to other diseases. We emphasise the importance of preserving RRF, which was favourably related to both patient and technique survival. We consider that patients with PGN are an ideal group to potentially benefit from PD and its advantages, like other PRD, while they wait for a KT.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz Cuevas M, Limón Ramírez R, Pérez Contreras FJ, Grupo Levante de Diálisis Peritoneal. Diálisis peritoneal en pacientes incidentes con glomerulonefritis primaria. Resultados de un registro multicéntrico durante 20 años de seguimiento. Nefrologia. 2021;41:53–61.