Background: Peritoneal dialysis (PD) seems to be a good option to initiate renal replacement therapy (RRT), but patients with graft failure choose PD less frequently than incident patients (de novo). Objective: To describe patient movements between PD and kidney transplantation (TX) and risk factors for failure of the PD technique. Method: Multicentre observational study of patients starting PD between 2003 and 2009 with follow-up until January 2010. Survival analysis based on switching from PD to HD as an event using Kaplan-Meier (KM) and forward, stepwise Cox proportional hazards models. Hazard ratio and 95% confidence intervals (HR [CI]) are shown. Main variable: Switch from PD to HD. Two-group comparison: PD post transplant (post-TX) patients (76) compared to pure incident PD (de novo-PD) patients (830). Patients: 906 PD patients from 19 public hospitals with a mean age of 54.8 years (64.9% male); main ESRD aetiology: glomerulonephritis (25.4%), diabetes (16.7%), vascular-ischaemic (10.7%), interstitial (13.6%) and polycystic (11.2%). Comorbidity conditions: Charlson Index 5.1 (SD 2.4); 21.6% diabetes mellitus (DM), 24.0% cardiovascular (CV) events. Results: Mean follow-up period on PD: 1.85 years (95% CI [1.68-2.02 years]). KM estimation for switching to HD due to PD failure was 5.46 years [4.42-6.50 years]. At the end of follow-up, 88 patients had died, 154 had been transferred to HD and 306 had received a graft (annual rate for patients on waiting list: 0.49 TX per year on PD). The best Cox multivariate model for switching from PD to HD includes: post-TX (HR: 1.63 [1.01-2.63]), DM (HR: 1.69 [1.19-2.40]) and age (1.01 [1.00-1.02]) per year. Post-TX patients were younger (43.8 years vs 55.3 years) and with less comorbidity conditions than de novo-PD patients (DM 18.4% vs 21.9%; CV 15.8% vs 24.7%). However post-TX patients had worse clinical evolution with a rapid decline of renal function (¿-3.88 vs –1.8ml/min per year); a higher admission rate (0.9 vs 0.62 per year) but similar peritonitis rate (0.45 vs 0.53 episodes per year). They also needed to be transferred to HD more frequently (28.9% vs 15.8%; P<.006) and needed more time to TX (4.8 years vs 1.7 years, Kaplan-Meier). Consequently, time spent on PD was higher in the post-TX group (2.8 vs 1.8 year). Limitations: Observational study with absence of a standard protocol to switch PD-HD. Conclusion: PD seems to be a good first choice technique due to low mortality and high TX ratio in our area. A previous graft failure is associated with a higher rate of PD-failure but time spent on PD is enough to consider this technique as a good option.
Introducción: Los pacientes trasplantados eligen diálisis peritoneal (DP) en menor proporción que los incidentes. Objetivo: Describir la supervivencia en técnica de los postrasplantados (post-TX) y estudiar los factores predictores de transferencia a hemodiálisis (HD). Método: Estudio observacional, multicéntrico de incidentes (2003-2010). Variable principal: Paso a HD. Comparación post-TX frente a DP-de novo. Pacientes: 906 pacientes (54,8 años, 64,9% hombres) de 19 centros, con seguimiento hasta siete años, un 8,4% receptores de trasplante. Etiologías: glomerulonefritis 25,4%, nefropatía diabética 16,7%, vascular 10,7%, intersticial 13,6%, poliquistosis renal 11,2%. Comorbilidad: índice de Charlson 5,1 (desviación estándar, DE = 2,4), el 21,6% con diabetes mellitus (DM), el 24,0% con evento cardiovascular previo. El 71,6% inician en diálisis peritoneal continua ambulatoria (DPCA) y el resto en automática. Al mes de inicio, la función renal (FR) es 7,3 (DE = 3,8) ml/min, Kt/V 2,6 (DE = 0,7), y el ClCr 96,3 (DE = 35,3) l/semana x 1,72 m2. Resultados: El mantenimiento en técnica estimado por KM es de 1,85 años, con un intervalo de confianza (IC) al 95% de [1,68-2,02] para la salida por cualquier causa y de 5,46 años [4,42-6,50] para transferencia a HD. Durante el seguimiento fueron sometidos a trasplante 306 pacientes (0,49 trasplantes por año en lista) y pasaron a HD 154. El mejor modelo de regresión de Cox para paso a HD incluye: DM con Hazard ratio (HR) 1,69 [1,19-2,40], trasplante previo: 1,63 [1,01-2,63] y edad 1,01 [1,00-1,02]. Los post-TX son más jóvenes (43,8 frente a 55,3 años) y con menos comorbilidad (DM 18,4 frente al 21,9%; CV 15,8 frente al 24,7%). Sin embargo, presentan menos FR al inicio, 5,10 frente a 7,46 ml/min, y mayor pérdida de FR. Los pacientes post-TX pasan a HD en mayor proporción (28,9 frente al 15,8%; p <0,006). Limitaciones: Estudio observacional, ausencia de protocolo común para paso a HD. Conclusión: La DP parece ser una buena técnica inicial por su baja mortalidad y alta tasa de trasplantes. Aunque el riesgo de paso a HD es mayor en los post-TX, el tiempo que pasan en DP es suficiente para considerarla como una buena opción de diálisis.
INTRODUCTION
In spite of advancements made in recent decades in immunosuppressant therapy, 4% of transplant patients every year develop graft failure and must re-start dialysis treatment.1,2 Due to the increase in transplant patients, this situation is increasingly becoming more common.
Returning to dialysis following graft failure is a difficult situation for both patients and nephrologists, which contributes to postponing this decision. As such, these patients tend to be in a worse clinical situation, leading to a greater early mortality rate than in those that are starting dialysis de novo.3 This is primarily due to cardiovascular complications (CV) and infections.4 The factors that increase the risk of mortality in these patients are: advanced age, female sex, diabetes, heart failure, hypoalbuminaemia, and greater level of anaemia.4-7 A set of specific risk factors also exists, such as the time previously spent on dialysis, prolonged immunosuppressant therapy, and faster loss of residual renal function.8,9
The optimal type of renal replacement therapy (RRT) for patients re-starting dialysis due to graft failure is still unclear.10 Studies published on this subject have several limitations: they include few patients, are not multicentre, do not properly distribute patient groups, and frequently do not register relevant clinical data. No specific studies have been performed in our field of medicine, and the reality in each country can be very different. Currently, there is no evidence indicating that the type of dialysis treatment has any effect on patient survival when returning to dialysis after kidney graft failure.11 For these patients, peritoneal dialysis (PD) may be just as valid as haemodialysis (HD) and should be considered as an option for maintaining patient autonomy. However, the reality is that the majority of patients that re-start RRT after transplant choose HD.11
This study analyses the evolution of patients that start PD after kidney graft failure in 19 hospitals in central region of Spain and seeks to provide insight into the current situation of this controversial issue.
PATIENTS AND METHOD
Ours was an observational, multicentre study with consecutive systematic sampling of patients from the various health care departments from the hospitals in the Peritoneal Dialysis Centre Group (GCDP, for its initials in Spanish) and with a follow-up time of up to 7 years. This group is made up of 19 public hospitals in the central region of Spain, which in total attend to 8.8 million inhabitants.
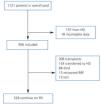
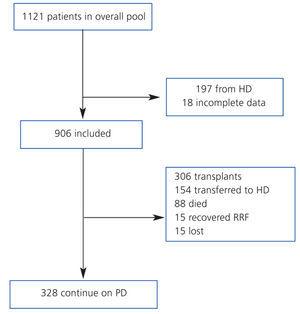
We compiled a registry of all post-transplant patients that start PD (post-TX group) and patients that start PD for the first time (de novo-PD group) during a seven-year period (from January 2003 to January 2010). Patients from HD were not included. Patients were monitored until withdrawal from this particular type of dialysis, death, or the end of the study, and we registered only 1.7% of patients lost to follow-up (Figure 1). At the start of the study, we collected demographic parameters, causes of nephropathy, comorbidity, patient origin, and reason for choosing the type of dialysis used (free choice or under medical order due to contraindications for HD). We used the Charlson index for estimating comorbidity, which was previously validated for PD.12 At the start of the study, and every six months thereafter, clinical data were recorded for objectives, efficacy, residual renal function, peritoneal transport, level of anaemia, and blood pressure (BP). Cases of peritonitis and hospitalisation were also recorded. Patient withdrawals from the dialysis programme were classified into: recovery of renal function, death, transfer to HD, transplant, and loss to follow-up and/or patient transfer.
Each hospital maintains its own database that is identical to all others and is designed specifically for multi-purpose data collection. They are integrated into a central database every year. A statistics expert sifts through the data for quality control based on ranges and logic routines. Patients gave their informed consent upon being included in this database. We managed the data and performed statistical analyses using SPSS software version 11.0. Numerical variables were analysed using mean and standard deviation (SD) and categorical variables have been presented as frequencies. All rates (mortality, hospitalisations, peritonitis) refer to the real time spent on the technique by each patient.
Patients were classified into two groups: post-TX and de novo-PD. We compared results in categorical variables between groups using chi-square tests and quantitative variables using Student’s t-tests, or Mann-Whitney U-tests when the variable did not follow a normal distribution. We also applied a Kaplan-Meier survival analysis (KM), calculating the corresponding log-rank values. We summarised survival data using medians and 95% confidence intervals (CI). If the 50th percentile of the distribution was not reached, the mean is shown. For each survival analysis, different types of events were taken into account; thus, the three types of analysis include: death as an event for patient survival, transfer to HD as an event for maintaining dialysis technique, and the aggregate of death, transfer to HD, transplant, and recovery of renal function (RF) allows us to estimate the real time spent on the dialysis technique. For the analysis of time to transplant, we considered only those patients included on a transplant waiting list at some point during follow-up.
We used backward step-wise Cox regression models for the multivariate analysis of survival, including all variables with a P<.30 in the univariate analysis. We ensured that the variables included did not violate the assumptions of proportionality.
RESULTS
Global Description
Between January 2003 and January 2010, we included 906 patients that started treatment on PD, with a mean age of 54.8 years (SD=15.9), 64.9% of which were men. Only 3.9% were on PD due to contraindications for HD, and the others by free choice. Comorbidity was estimated using the Charlson index with age of 5.1 (SD=2.4): 21.6% had diabetes mellitus (DM) and 24% had suffered from some previous cardiovascular event (8.4% acute myocardial infarction [AMI], 6.6% heart failure [HF], 4.7% acute stroke, and 12.7% peripheral arterial disease).
The aetiologies present in chronic kidney disease (CKD) were, in decreasing order: glomerular (25.4%), diabetic nephropathy (16.7%), undetermined (14.4%), interstitial (13.6%), polycystic (11.2%), vascular (10.7%), systemic (4.2%), hereditary (2.0%), and other (1.7%). The post-TX group (coming off of a failed kidney transplant) included 76 patients (8.4%), and the others were incident patients on PD as their first RRT technique (de novo-PD group). Manual PD techniques were used in 71.6% of cases (continuous ambulatory peritoneal dialysis) and the rest received continuous cycling peritoneal dialysis. At some point during the follow-up period, 47.8% of all patients were on the waiting list for a kidney transplant.
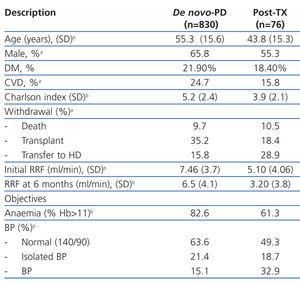
Table 1 summarises the characteristics of the two groups, highlighting the younger age of post-TX patients, with lower comorbidity and percentage of male patients. The efficacy and compliance with the previously described objectives were not different between groups, except for the lower RF at the start of treatment and the higher rate of loss of RF at six months.
Patient clinical evolution
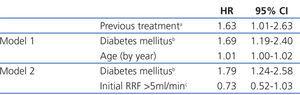
The global mean follow-up period was 1.7 years (total time at risk of 1563 patient-years). At the end of the follow-up period, 33.8% of patients received a kidney (n=306), 9.7% died (n=88), 17.0% were transferred to HD (n=154) and the rest continued with PD treatment. Only 1.7% of patients were lost to follow-up due to transfer to other hospitals. Of the patients included on the transplant waiting list, 66% received kidneys at some point during the follow-up period. The annual transplant rate is 0.49 per patient per year on the waiting list. The Kaplan-Meier estimated duration of time on the dialysis technique (median) was 1.85 years, with a 95% CI of 1.68-2.02 years for all withdrawals, 5.46 years (4.42-6.50 years) for transfer to HD, and 6.05 years (5.3-6.8 years) for mortality.
Post-TX patients had a higher rate of hospitalisations (0.90 vs 0.62 hospitalisations/year at risk; P=.006), but a similar rate of peritonitis was observed between the two groups (0.45 vs 0.53 episodes/year at risk; P=.10). However, these patients had a higher prevalence of Gram-negative infections (30.8% vs 20.1%) and a lower rate of negative laboratory cultures (10.3% vs 18.8%).
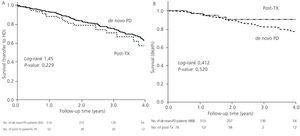
The mortality rate was similar between the two groups (post-TX: 0.05 vs de novo: 0.06 per year at risk, non significant difference) with a similar distribution of causes of death (42.0% cardiovascular, 6.8% cancer, 19.3% sepsis, 2.3% peritonitis, 4.5% refused treatment). Post-TX patients had a mean survival rate as estimated by the KM test of 6.05 years, similar to the mean survival rate in de novo-PD patients of 6.35 years, with a log-rank of 0.143 (P=.52).
The most efficient multivariate model for evaluating mortality included age, RF, previous history of CVD, and diabetes (coefficients expressed in Table 2).
Changes between renal replacement therapy techniques
35.2% of de novo-PD patients underwent their first transplant during the follow-up period, as opposed to 18.4% re-transplants in the other group (P=.006). If we only consider patients included in the transplant waiting list, 64.1% of de novo-PD patients and 32.6% of the post-TX group underwent transplants (P<.001). The KM test estimated time to transplant in patients with a previous record of transplant at 4.77 years, with a 95% CI of 2.48-7.09 years, and at 1.74 years in de novo-PD patients (1.55-1.92 years).
However, the rate of failure in dialysis technique due to transfer to HD was not significantly different (13.3 vs 9.4 per 100 patient-years at risk; P=.13). The causes for transfer to HD were similar, except for the greater percentage of patient incapacity for maintaining proper water balance (22.7% vs 6.8%; P=.016).
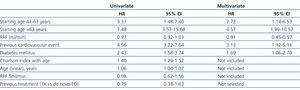
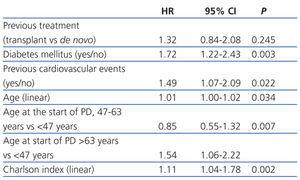
The median length of time before transfer to HD was 5.46 years, with a 95% CI of 4.40-6.52 years, compared to 4.21 years (3.42-5.00), with a HR of 1.32 and 95% CI (0.84-2.08) obtained by the Cox regression analysis (Figure 1). This negative effect of prior transplantation reached statistical significance after correcting for age and comorbidity, with an HR of 1.63 (1.02-2.60) for previously transplanted patients, and 1.12 (1.05-1.19) every 1-point increase in Charlson Index. The results from the univariate analysis of factors associated with transfer to HD are summarised in Table 3.
The most efficient multivariate analysis for evaluating the factors present at the start of PD that were associated with transfer to HD included previous transplant, DM, and age (Table 4). Working with other analytical variables (RF, efficacy, peritoneal permeability, and anaemia), we obtained a model in which baseline RF replaces age and previous treatment (Table 4).
As such, the total time spent on PD (considering withdrawal for any cause) was greater in the post-TX group (median of 2.83 [2.43-3.22] years vs 1.81 [1.64-1.97] years; log-rank: 5.44; P=.02). This difference was due to the lower rate of transplantation and the greater amount of time needed to find the second transplant.
DISCUSSION
In spite of the improved care provided to nephrological patients and advancements made in immunosuppressive treatments, an estimated 20% of patients that underwent kidney transplants must later return to dialysis due to graft failure within three years, and this value increases at a rate of 2%-4% per year. Between 4% and 10% of patients that start dialysis every year have a history of renal graft failure.13 This emergent issue within integrated RRT for CKD poses a challenge for the nephrological community.
In our field of medicine, transplants are the first cause for withdrawal from PD in incident patients, and the mean time necessary for receiving a kidney ranges around 1.7 years. As such, PD is considered to be a good choice for starting an integrated RRT programme. However, the rate of choosing PD is lower among patients with a history of graft failure. Patients coming out of a graft failure have worse evolution than de novo-PD patients, with an accelerated loss of residual RF (RRF), an increased wait time until a second transplant opportunity, increased rates of infections and hospitalisations, worse compliance with treatment objectives,14 and increased risk of failure of PD.
Several different studies have demonstrated high mortality rates in patients that re-start dialysis following graft failure, both in HD and in PD. An analysis of the USRDS registry, involving more than 170 000 patients, demonstrated a mortality rate that was 80% higher in patients that had lost a transplant than in those that remained on dialysis still awaiting their first transplant. This increased risk is concentrated in the first three months following re-initiation of dialysis, but continues to be high even after 5 years of follow-up.15 More recent studies have confirmed the increased mortality rate in post-TX patients on PD.3,15,16
What factors could explain the worse evolution of kidney graft failure patients?
The post-TX patients from our study were on average almost 12 years younger and with lower comorbidity than de novo-PD patients (lower prevalence of DM and cardiovascular events prior to PD). In spite of this, survival in this group was not higher, and their overall evolution was worse. Although mortality rates were similar between the two groups, important differences did exist in other intermediate variables of evolution, such as maintenance on PD, hospitalisation rates, infections, and compliance with treatment objectives. This seems to be influenced by the time elapsed since CKD onset, a factor that goes unnoticed in common measures of comorbidity. A recent study published in this journal compared the situation at the start of dialysis in post-TX patients with de novo patients, and the authors only observed a worse control of anaemia, which disappeared after one year of follow-up. In the study, starting HD or PD did not cause changes in prognosis.17
RRF is a key factor for maintaining patients on PD. Patients with a previous transplant have lower RRF initially, and an accelerated loss in RRF that explains the increased transfer rate to HD due to failure of maintaining a proper water balance. These data coincide with the results from the ANZ-DATA study.18 Although transfer to HD was greater in the post-TX group in our study, the time spent on PD was higher than the overall mean, and could be sufficient to maintain patients on this technique until a second transplant. We do not have access to data regarding the administration of immunosuppressant drugs in our patients, and so we cannot analyse the role of these drugs in the evolution of RRF.
Some authors report that the administration of immunosuppressant drugs, even at low doses, could favour peritoneal infection. However, in our study, the overall rate of peritonitis was similar. Studies that analyse the presence of peritonitis in patients with prior transplants are few and with varied results.19-21 In the study by Gregoor et al,21 a similar rate of peritonitis was observed, but with greater mortality due to severe infections in the group that received prednisone (mean dose of 9mg/day) than for those that did not. Other authors also observed similar rates of peritonitis between patients with graft failure and those without it.19,20 Currently, no data exist for the administration of immunosuppressant drugs in patients on PD for more than 6 months.22
What is the best technique to use in kidney graft failure patients that return to dialysis?
Few studies have compared the survival of patients with graft failures that start PD or HD. Davies23 found no differences in patient survival or technique used in 45 patients with graft failure that started dialysis (28 on PD and 17 on HD). The same results were observed by De Jonge, in a study involving 60 patients (21 that returned to PD and 39 on HD).19 Both studies had certain methodological limitations to extracting robust conclusions. In the absence of decisive evidence in favour or against, we can continue considering PD as a good option that would allow for patient independence from the treatment centre, similar to the autonomy reached with a functioning kidney transplant. However, the reality is that most patients return to dialysis on HD. According to the Madrid REMER registry, in 2008, for each incident de novo patient on PD, five started HD. However, for each patient with graft failure that started PD, 12 started on HD. According to our study, the patients that choose PD are younger and have lower comorbidity. This positive selection of patients in better condition could explain the better evolution as compared to previously published studies.3,15,16 On the other hand, PD results in general have improved in the last 10 years, as indicated by recent Spanish publications.25
The reasons for this predominance of HD over PD could be the same as in incident de novo patients. Among these, organizational aspects stand out such as the inferior implementation of PD programmes, the urgency of starting dialysis, and the absence of standardised information protocols in medical visits with patients with advanced CKD. Additionally, other aspects specific to individual situations could be important, such as patient fatigue and lack of interest in self-care. The choice of dialysis technique following graft failure may must similar criteria to those that are applied in de novo dialysis patients.10 In the absence of absolute contraindications, the patient must receive new and updated information in order to make the correct choice for which type of dialysis to receive.26
Re-transplantation as a real treatment option in graft failure patients that return to dialysis
Patients frequently return to dialysis hopeless and may even need psychological support. We know that the graft survival is lower in patients that have already undergone a transplant than in those that receive their first kidney.22 However, the survival of patients that undergo a second transplant is better than for those that remain on dialysis.22,27 Unfortunately, according to data from the USRSD,28 less than 15%-20% of patients that return to dialysis after graft failure have access to the option of a second transplant.
In the GCDP study, the main cause for withdrawal from PD in de novo-PD patients is a kidney transplant, with 2 out of 3 patients on the waiting list receiving a kidney within 18 months of starting PD. During this time period, the patient is evaluated, the necessary studies are performed for including the patient on the waiting list, and the patient must wait until a compatible organ is available. For post-TX patients on PD, the situation is more complicated, since a longer wait is expected until finding a compatible organ, which lengthens the time until a second transplant can be performed. Although the risk of transfer to HD was higher in graft failure patients, their withdrawal from PD due to re-transplantation was lower. As such, the overall time on PD in post-TX patients (more than two years) was greater than in de novo patients. This two-year period seems to be sufficient to consider that undergoing PD is a worthwhile treatment plan.
Our study did have some limitations: it was an observational study, with asymmetry in the size of the two study groups, and we did not apply a specific protocol for considering the start of PD nor the transfer to HD. However, these data are very recent and come from a systematic sampling system that includes all patients that received PD in a large area, which ensures the applicability of the results presented here.
In summary, the relationship between PD and kidney transplant can and must be bidirectional. PD is considered as a good starting technique for RRT, with transplant being the primary cause of withdrawal from a treatment programme and a reasonable wait time observed in our study. Following graft failure, we must consider PD as a very reasonable alternative to be offered to our patients. Kidney graft failure patients that return to dialysis require special attention due to the higher rate of complications, although this recommendation may be applicable for both PD and HD.
Acknowledgements:
Project co-funded by Baxter (2003-2011) Amgen (2005-2011) Fresenius (2007-2011) and Gambro (2009-2011) through the Nephrology Foundation-SOMANE Madrid. The authors have no contractual relationship and are not members of advisory boards of PD companies in Spain .
Table 1. Baseline description of the sample and parameters after 6 months
Table 2. Mortality risk according to the Cox multivariate and univariate models
Table 3. Relative risks when transferring to haemodialysis according to the Cox univariate model
Table 4. Risks when transferring to haemodialysis according to the Cox multivariate model
Figure 1. Patient flow chart
Figure 2. Kaplan-Meier survival curves