Diabetic Kidney Disease (DKD) is the most common cause of end-stage chronic kidney disease (CKD), conditioning these patients to a worse renal prognosis and higher cardiovascular mortality and/or requirement for renal replacement therapy. The use of novel information and communication technologies (ICTs) focused on the field of health, may facilitates a better quality of life and disease control in these patients. Our objective is to evaluate the effect of monitoring DKD patients using NORA-app.
Material and methodsProspective feasibility/validation study of NORA-app in patients with DKD stage G3bA3 or higher, followed in outpatient clinics of a tertiary care hospital. NORA-app is an application for smartphones designed to control risk factors, share educational medical information, communicate via chat with health professionals, increase treatment compliance (Morisky-Green), and collect patient reported outcomes such as anxiety and depression using HADs scale. Clinical-laboratory variables were collected at 3 months and compared to control patients who declined using NORA-app.
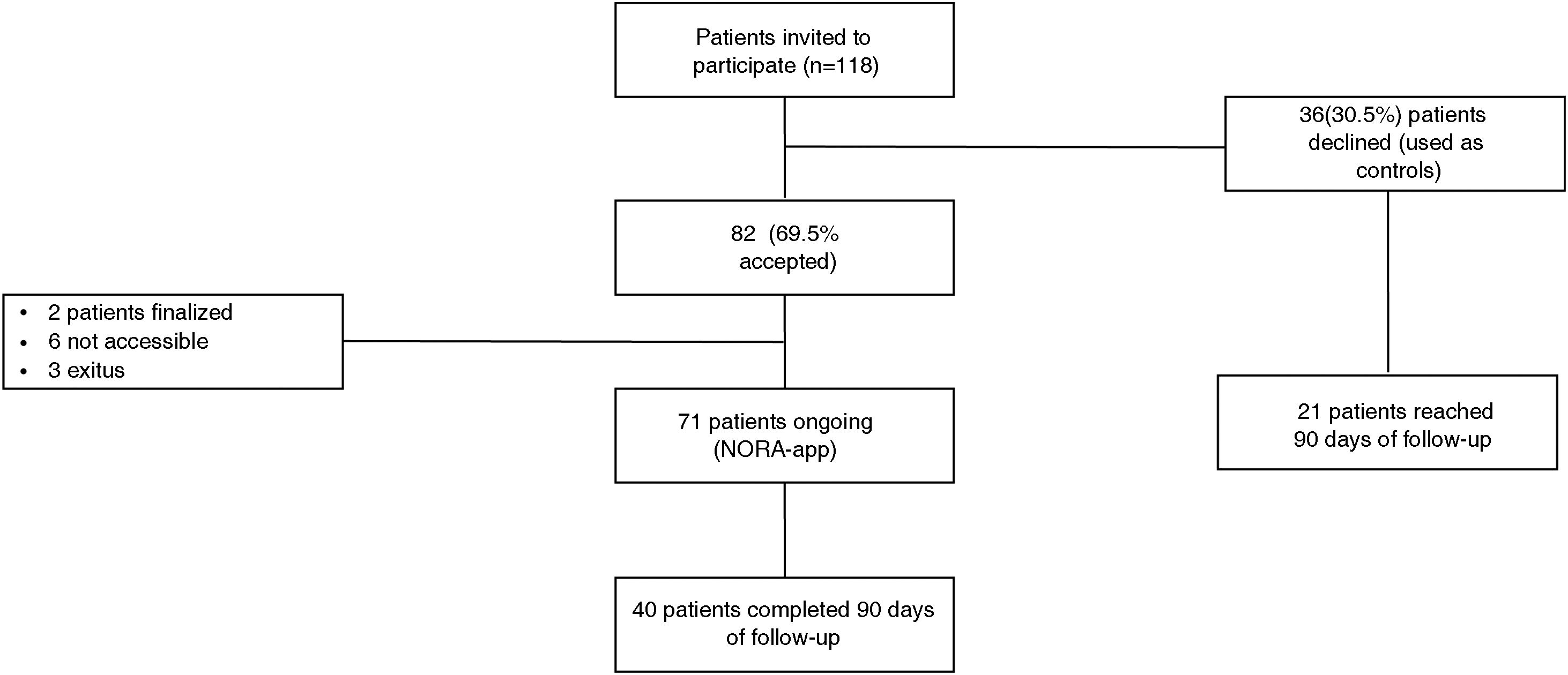
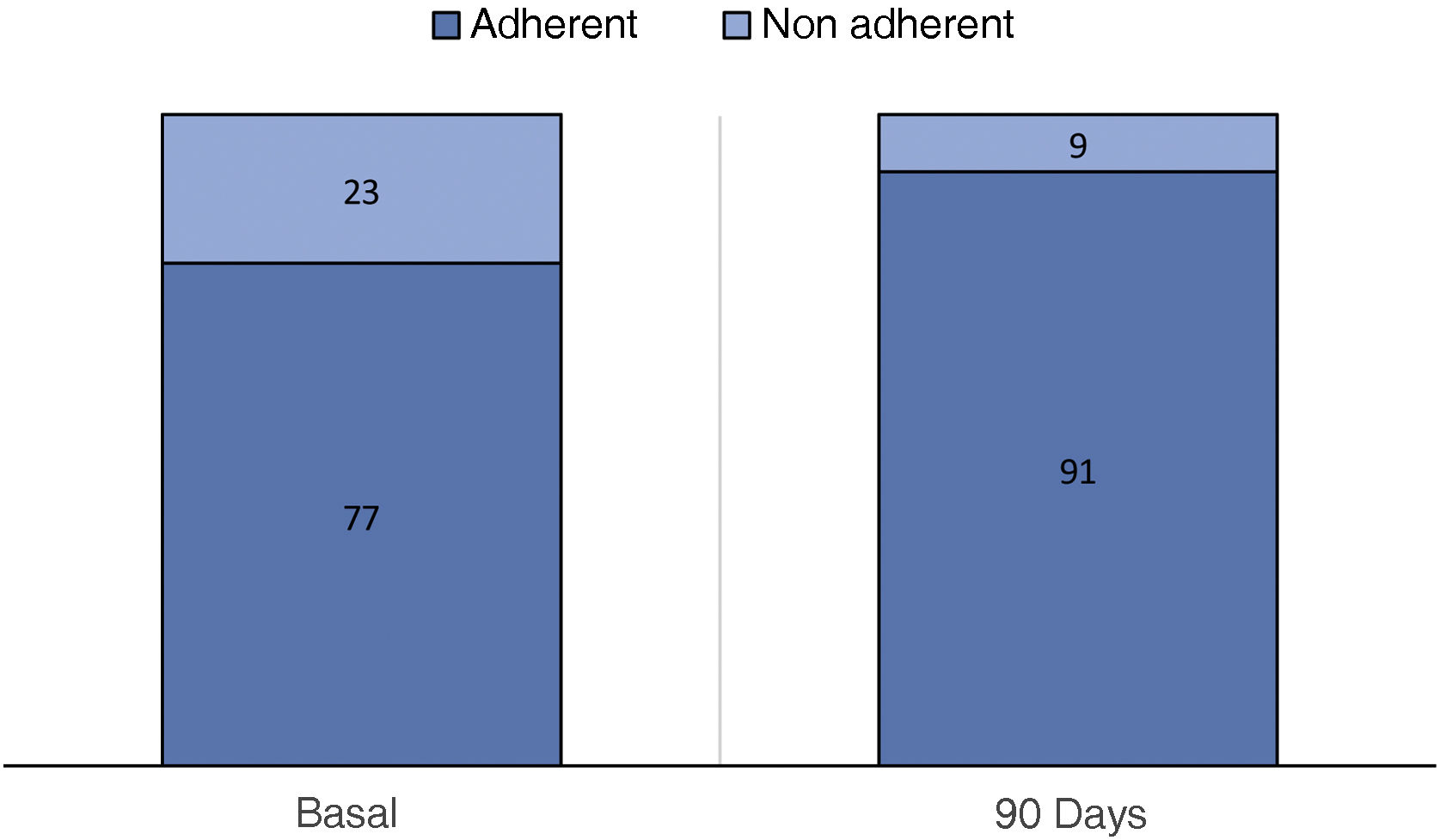
ResultsFrom 01/01/2021 to 03/03/2022 the use of NORA-app was offered to 118 patients, 82 accepted and 36 declined (controls). After a mean follow-up period of 6,04 months and at the time of data extraction 71 (86.6%) NORA-app patients remain active users, 2 have completed the follow-up at one year and 9 are inactive (3 due to death and 6 due to non-locatable). There were no differences in baseline characteristics including Creatinine [2.1 (1.6−2.4) vs. 1.9 (1.5−2.5)] mg/dL and alb/creat [962 (475–1784) vs. 1036 (560–2183)] mg/gr between Nora and control patients respectively. The therapeutic compliance rate in the NORA-app group was 77%, improving at 90 days to 91%. Patients in the NORA-group showed significantly lower levels of alb/creat than controls (768(411–1971) mg/g Vs 2039 (974–3214) p = 0.047) at 90-day follow-up.
ConclusionsIn patients with DKD the use of NORA-app was maintained in the long-term, leading to high levels of treatment compliance, and achieving a better disease control. Our study suggests that the generalized use of ICTs may help in the personalized monitoring of these patients to delay the progression of kidney disease.
La Enfermedad Renal Diabética (ERD) es la causa más común de enfermedad renal crónica (ERC) terminal, condicionando a estos pacientes a un peor pronóstico renal y mayor mortalidad cardiovascular y/o requerimiento de terapia renal sustitutiva. El uso de novedosas tecnologías de la información y la comunicación (TIC) enfocadas al ámbito de la salud, puede facilitar una mejor calidad de vida y control de la enfermedad en estos pacientes. Nuestro objetivo es evaluar el efecto de monitorización de pacientes con ERD utilizando la aplicación NORA (NORA-app).
Materiales y métodosEstudio prospectivo de viabilidad/validación de la aplicación NORA-app en pacientes con ERD en estadio G3bA3 o superior, seguidos en consultas externas de un hospital de atención terciaria. NORA-app es una aplicación para teléfonos inteligentes diseñada para controlar los factores de riesgo, compartir información médica educativa, comunicarse vía chat con profesionales de la salud, aumentar el cumplimiento del tratamiento (Morisky-Green) así como evaluación de ansiedad y depresión utilizando la escala Hospitalaria de Ansiedad y Depresión (HAD). Las variables clínico-analíticas se recogieron al inicio y a los 3 meses, comparándose con los pacientes que desistieron del uso de NORA-app (grupo control).
ResultadosDesde el 01/01/2021 al 03/03/2022 se ofreció el uso de NORA-app a 118 pacientes, 82 aceptaron y 36 rechazaron (controles). Después de un período medio de seguimiento de 6,04 meses, 71 (86,6 %) pacientes de NORA-app siguen siendo usuarios activos, 2 han completado el seguimiento al año y 9 están inactivos (3 por fallecimiento y 6 por no localizables). No hubo diferencias en las características basales, incluida la creatinina [2,1 (1,6−2,4) frente a 1,9 (1,5−2,5)] mg/dL y la alb/creat [962 (475–1784) frente a 1036 (560–2183)] mg/gr entre los pacientes NORA-app y grupo control, respectivamente. La tasa de cumplimiento terapéutico en el grupo NORA-app fue del 77%, mejorando a los 90 días al 91%. Los pacientes del grupo de NORA-app mostraron niveles significativamente más bajos de alb/creat que los controles (768 (411–1971) mg/g vs. 2039 (974–3214); p = 0,047) a los 90 días de seguimiento.
ConclusionesEn pacientes con ERD que mantuvieron el uso de NORA-app a largo plazo, llevo a presentar altos niveles de cumplimiento del tratamiento, logrando un mejor control de la enfermedad. Nuestro estudio sugiere que el uso generalizado de las TIC puede ayudar en el seguimiento personalizado de estos pacientes para retrasar la progresión de la enfermedad renal.
The prevalence of chronic kidney disease (CKD) is increasing considerably worldwide,1 becoming one of the fastest growing pathologies as a cause of death.2,3 CKD is associated with an increased risk of cardiovascular morbidity, premature mortality and decreased health-related quality of life.4 The main cause of CKD is diabetic kidney disease (DKD), conditioning these patients to a poorer renal prognosis and higher cardiovascular mortality.5–7 In addition, there has been a substantial increase in the number of people with end-stage CKD (stage 5) requiring renal replacement therapy such as dialysis or transplantation, so that the management of CKD currently represents a major challenge for governments and health financial costs.8
Prevention and control of cardiovascular risk factors are essential in the management of CKD. During the last few years, follow-up studies on the control of cardiovascular risk factors have been carried out according to the KDIGO5 guidelines, with the aim of modifying lifestyle and creating healthy habits to reduce the risk of progression of kidney disease. In this sense, the LOOK-AHEAD study evaluated the effect of a pattern of intensive lifestyle modification aimed at weight reduction in overweight diabetic patients, compared to patients who followed standard treatment, patients assigned to the intensive follow-up program presented greater weight reduction, better glycosylated hemoglobin records and better control of various cardiovascular risk factors, as well as a significant reduction in alb/creat, also highlighting the importance of the results achieved with active monitoring reducing the burden of vascular risk associated with diabetes and considering these key therapeutic alternatives in the management of the diabetic patient.9 In addition, the classic studies of the STENO group have shown that intensive treatment of cardiovascular risk factors (HTN, smoking, glycosylated hemoglobin, etc.) slow down the progression of chronic kidney disease.10 Currently, strategies have been developed aimed at improving therapeutic compliance and healthy lifestyle modification in chronic patients, since there is little point to design and develop new drugs aimed at improving disease control if, by contrast, there is systematic non-compliance with medical guidelines and advice.
Studies have shown an increase in the control of specific cardiovascular risk factors (obesity, diabetes, hypertension), thanks to information and communication technologies (ICT), i.e., telemedicine.11 In this regard, the Stroke Unit of Vall d'Hebron University Hospital has developed an application for smartphones called NORA-app (formerly known as Farmalarm), which allows the control of medical treatment by means of medication alarms, recording of the biometric profile (weight, height) and control of cardiovascular risk factors (recording of blood pressure, capillary glucose levels, oxygen saturation, weight and daily steps). In addition, it allows to send health education material, surveys (adherence to treatment, anxiety/depression, health status), visits calendar, generation of reports, module of frequently asked questions, bidirectional communication via chat and video call, and contact information of the Unit. NORA-app has been validated during the last five years by the Stroke Unit of the Vall d'Hebron University Hospital, demonstrating an improved control of cardiovascular risk factors in patients who had suffered a stroke, increasing the degree of knowledge about the disease.12 Our objective is to adapt and evaluate the effect of monitoring in patients with DKD using NORA-app, focusing on the control of risk factors for progression of renal disease considered as composite major adverse renal event (MARE).13
Material and methodsWe conducted a prospective feasibility and validation study of the NORA-app application in patients over 18 years of age with DKD stage G3bA3 or higher (eGFR CKD-EPI<45 mL/min/m2 and alb/creat >300 mg/g), followed in outpatient clinics of a tertiary hospital and who have a mobile device (the participant or family member) that allows the use of applications such as WhatsApp. Patients with DKD stage G3bA2 or lower, as well as patients in stage G5 (eGFR < 15 mL/min/m2) with an expected early requirement (less than one year) of renal replacement therapy were excluded. Patients with moderate-severe cognitive impairment, psychiatric pathology, language barrier, severe sensory deficits (hypoacusis/lack of vision) and/or inability to collaborate in the study (if necessary, no social/family support) were also excluded.
Exploratory study variables include demographics, comorbidities, laboratory tests (every three months determination of serum creatinine, estimated glomerular filtration rate [CKD-EPI], alb/creat, glycosylated hemoglobin, hemoglobin, ferritin, transferrin saturation index [TSI], sodium, potassium, calcium, phosphorus, parathyroid hormone [PTH], calcidiol, total cholesterol, HDL cholesterol, and LDL cholesterol, and scales such as treatment adherence (Morisky-Green),14 signs and symptoms, depression and anxiety (HADS),15 health status (PROMIS).16 The scales for assessing patient status were sent at different periods: baseline at seven days, 90 days and one-year of follow-up.
The Morisky-Green scale evaluates adherence to pharmacological treatment by means of four items that ask about how the patient complies with the medication prescribed by the physician (Appendix 1). It is considered altered when one or more of the answers is positive, indicating poor compliance by the patient in taking the medication.14 The signs and symptoms scale refers to the presence of nausea/vomiting, edema and respiratory distress (Appendix 2). The Hospital Anxiety and Depression (HAD) scale is composed of 14 items that are divided into two groups HAD-anxiety and HAD-depression (Annex 3). The scores obtained as absolute numbers have a range from 0 to 21, with the highest scores being considered unfavorable scores. From 0 to 7 are the limit of normality, from 7 to 10 "abnormal" and greater than 10 indicates alteration in anxiety and depression.15 The state of health is self-assessed using the Patient-Reported Outcomes Measurement Information System 10 (PROMIS-10) scale, which assesses the general state of health based on 10 items (Appendix 4): physical health, pain, fatigue, emotional state, social relations, general perception of the state of health. The results obtained are grouped into two subgroups "physical health" and "mental health", and considering absolute numbers, the range is four as a minimum, and 20 as the maximum value. Therefore, the higher the score, the better the self perception of his or her state of health.16 This study was approved by the Ethics Committee for Research with Medicines and Research Projects Commission of the Vall d'Hebron University Hospital (PR(AG)608/2021).
ResultsFrom January 1, 2021 to March 3, 2022, 118 patients were offered the use of NORA-app, of which 82 accepted to participate in the study (NORA) and 36 did not participate (control) because they were included in ongoing clinical trials (20 patients) or because they did not have a smartphone (16 patients). After a mean follow-up period of 6.04 months, 71 (86.6%) patients are still active users (27 through a caregiver); two patients have completed one year of follow-up at and nine are inactive, (three because of death and six because they are not accessible) (Fig. 1).
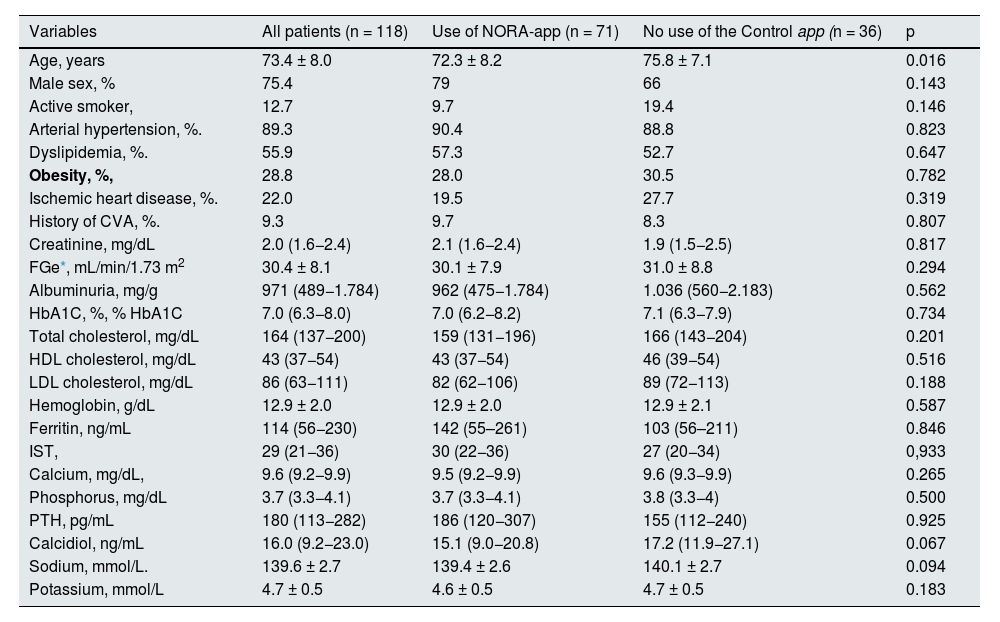
The baseline demographic and laboratory variables are shown in Table 1. The mean age of the patients was 73.4 ± 8 years, mostly male (75.4%), mean serum creatinine of 2.0 (1.6−2.4) mg/dL, with eGFR 30.4 ± 8.1 mL/min/1.73m2 (CKD-EPI), alb/creat was 971 (489−1,784) mg/g and glycosylated hemoglobin levels were 7.0 (6.3−8.0) %. The mean age of patients in the NORA-app group was 72.3 ± 8.2 years vs. 75.8 ± 7.1 years in the control group, being statistically significant (p = 0.016). There were no significant differences between the two groups in terms of comorbidities or baseline analytical values, including serum creatinine (2.1 [1.6−2.4] vs. 1.9 [1.5−2.5]) mg/dL and alb/creat (962 [475−1,784] vs. 1,036 [560−2,183]) mg/g.
Baseline clinical and laboratory characteristics.
| Variables | All patients (n = 118) | Use of NORA-app (n = 71) | No use of the Control app (n = 36) | p |
|---|---|---|---|---|
| Age, years | 73.4 ± 8.0 | 72.3 ± 8.2 | 75.8 ± 7.1 | 0.016 |
| Male sex, % | 75.4 | 79 | 66 | 0.143 |
| Active smoker, | 12.7 | 9.7 | 19.4 | 0.146 |
| Arterial hypertension, %. | 89.3 | 90.4 | 88.8 | 0.823 |
| Dyslipidemia, %. | 55.9 | 57.3 | 52.7 | 0.647 |
| Obesity, %, | 28.8 | 28.0 | 30.5 | 0.782 |
| Ischemic heart disease, %. | 22.0 | 19.5 | 27.7 | 0.319 |
| History of CVA, %. | 9.3 | 9.7 | 8.3 | 0.807 |
| Creatinine, mg/dL | 2.0 (1.6−2.4) | 2.1 (1.6−2.4) | 1.9 (1.5−2.5) | 0.817 |
| FGe*, mL/min/1.73 m2 | 30.4 ± 8.1 | 30.1 ± 7.9 | 31.0 ± 8.8 | 0.294 |
| Albuminuria, mg/g | 971 (489−1.784) | 962 (475−1.784) | 1.036 (560−2.183) | 0.562 |
| HbA1C, %, % HbA1C | 7.0 (6.3−8.0) | 7.0 (6.2−8.2) | 7.1 (6.3−7.9) | 0.734 |
| Total cholesterol, mg/dL | 164 (137−200) | 159 (131−196) | 166 (143−204) | 0.201 |
| HDL cholesterol, mg/dL | 43 (37−54) | 43 (37−54) | 46 (39−54) | 0.516 |
| LDL cholesterol, mg/dL | 86 (63−111) | 82 (62−106) | 89 (72−113) | 0.188 |
| Hemoglobin, g/dL | 12.9 ± 2.0 | 12.9 ± 2.0 | 12.9 ± 2.1 | 0.587 |
| Ferritin, ng/mL | 114 (56−230) | 142 (55–261) | 103 (56–211) | 0.846 |
| IST, | 29 (21−36) | 30 (22−36) | 27 (20−34) | 0,933 |
| Calcium, mg/dL, | 9.6 (9.2−9.9) | 9.5 (9.2−9.9) | 9.6 (9.3−9.9) | 0.265 |
| Phosphorus, mg/dL | 3.7 (3.3−4.1) | 3.7 (3.3−4.1) | 3.8 (3.3−4) | 0.500 |
| PTH, pg/mL | 180 (113−282) | 186 (120−307) | 155 (112−240) | 0.925 |
| Calcidiol, ng/mL | 16.0 (9.2−23.0) | 15.1 (9.0−20.8) | 17.2 (11.9−27.1) | 0.067 |
| Sodium, mmol/L. | 139.6 ± 2.7 | 139.4 ± 2.6 | 140.1 ± 2.7 | 0.094 |
| Potassium, mmol/L | 4.7 ± 0.5 | 4.6 ± 0.5 | 4.7 ± 0.5 | 0.183 |
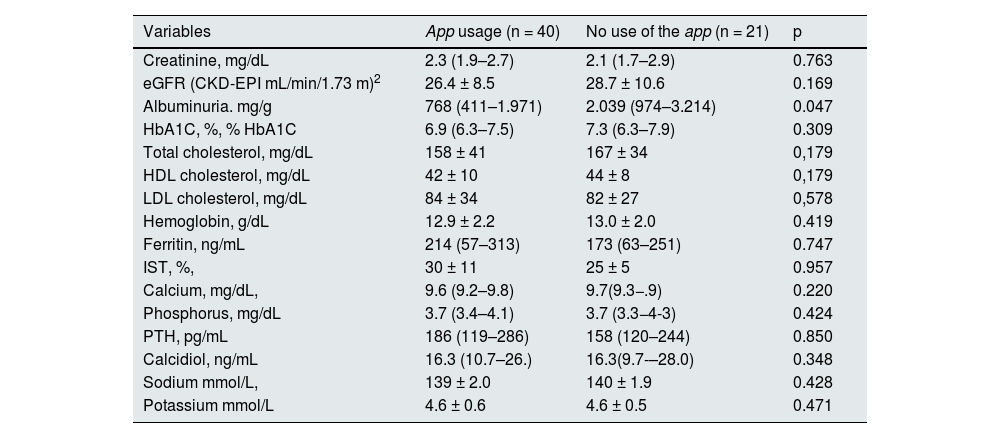
From the NORA-app group, 40 patients have reached 90 days of follow-up, while from the control group there were 21. Patients in the NORA-app group showed significantly lower alb/creat levels than controls (768 [411−1,971] mg/g vs. 2,039 [974−3,214]; p = 0.047). Serum creatinine was 2.3 (1.9–2.7) mg/dL in the NORA-app group vs. 2.1 (1.7–2.9) mg/dL in the control group, not statistically significant (p = 0.763). The eGFR was 26.4 ± 8.5 mL/min/1.73 m2 (CKD-EPI), in the NORA-app group vs. 28.7 ± 10.6 mL/min/1.73 m2 (CKD-EPI), in the control group, not being statistically significant (p = 0.169). Total cholesterol levels were 158 ± 41 mg/dL in the NORA-app group vs. 167 ± 34 mg/dL in the control group, not being statistically significant (p = 0.179) (Table 2).
Analytical variables at 90 days of follow-up.
| Variables | App usage (n = 40) | No use of the app (n = 21) | p |
|---|---|---|---|
| Creatinine, mg/dL | 2.3 (1.9–2.7) | 2.1 (1.7–2.9) | 0.763 |
| eGFR (CKD-EPI mL/min/1.73 m)2 | 26.4 ± 8.5 | 28.7 ± 10.6 | 0.169 |
| Albuminuria. mg/g | 768 (411–1.971) | 2.039 (974–3.214) | 0.047 |
| HbA1C, %, % HbA1C | 6.9 (6.3–7.5) | 7.3 (6.3–7.9) | 0.309 |
| Total cholesterol, mg/dL | 158 ± 41 | 167 ± 34 | 0,179 |
| HDL cholesterol, mg/dL | 42 ± 10 | 44 ± 8 | 0,179 |
| LDL cholesterol, mg/dL | 84 ± 34 | 82 ± 27 | 0,578 |
| Hemoglobin, g/dL | 12.9 ± 2.2 | 13.0 ± 2.0 | 0.419 |
| Ferritin, ng/mL | 214 (57–313) | 173 (63–251) | 0.747 |
| IST, %, | 30 ± 11 | 25 ± 5 | 0.957 |
| Calcium, mg/dL, | 9.6 (9.2–9.8) | 9.7(9.3−.9) | 0.220 |
| Phosphorus, mg/dL | 3.7 (3.4–4.1) | 3.7 (3.3−4-3) | 0.424 |
| PTH, pg/mL | 186 (119–286) | 158 (120–244) | 0.850 |
| Calcidiol, ng/mL | 16.3 (10.7–26.) | 16.3(9.7-–28.0) | 0.348 |
| Sodium mmol/L, | 139 ± 2.0 | 140 ± 1.9 | 0.428 |
| Potassium mmol/L | 4.6 ± 0.6 | 4.6 ± 0.5 | 0.471 |
eGFR, estimated glomerular filtration rate; CKD-EPI, Chronic Kidney Disease Epidemiology collaboration; HbA1C, glycosylated hemoglobin A1C; PTH, parathyroid hormone; TSI, transferrin saturation index.
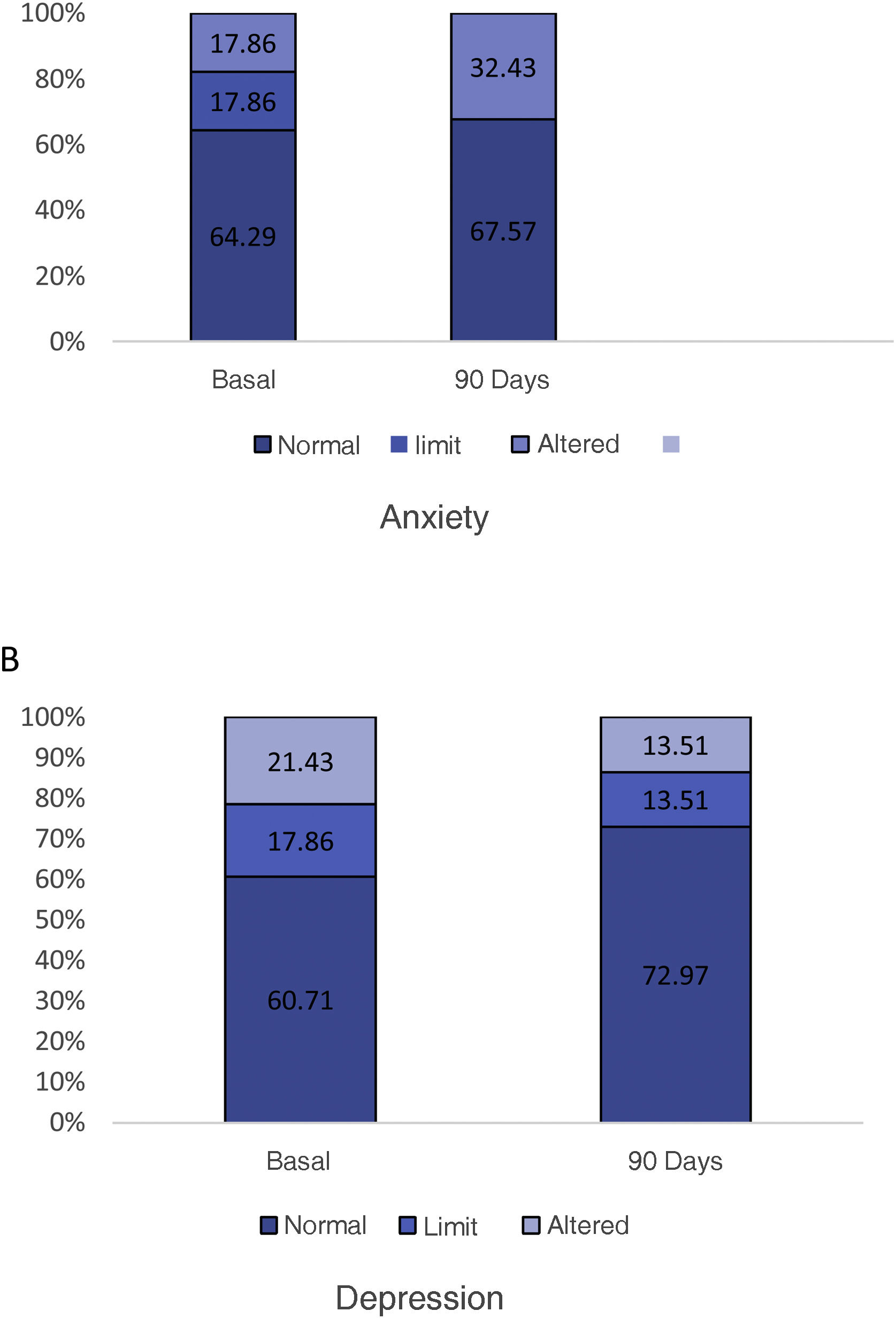
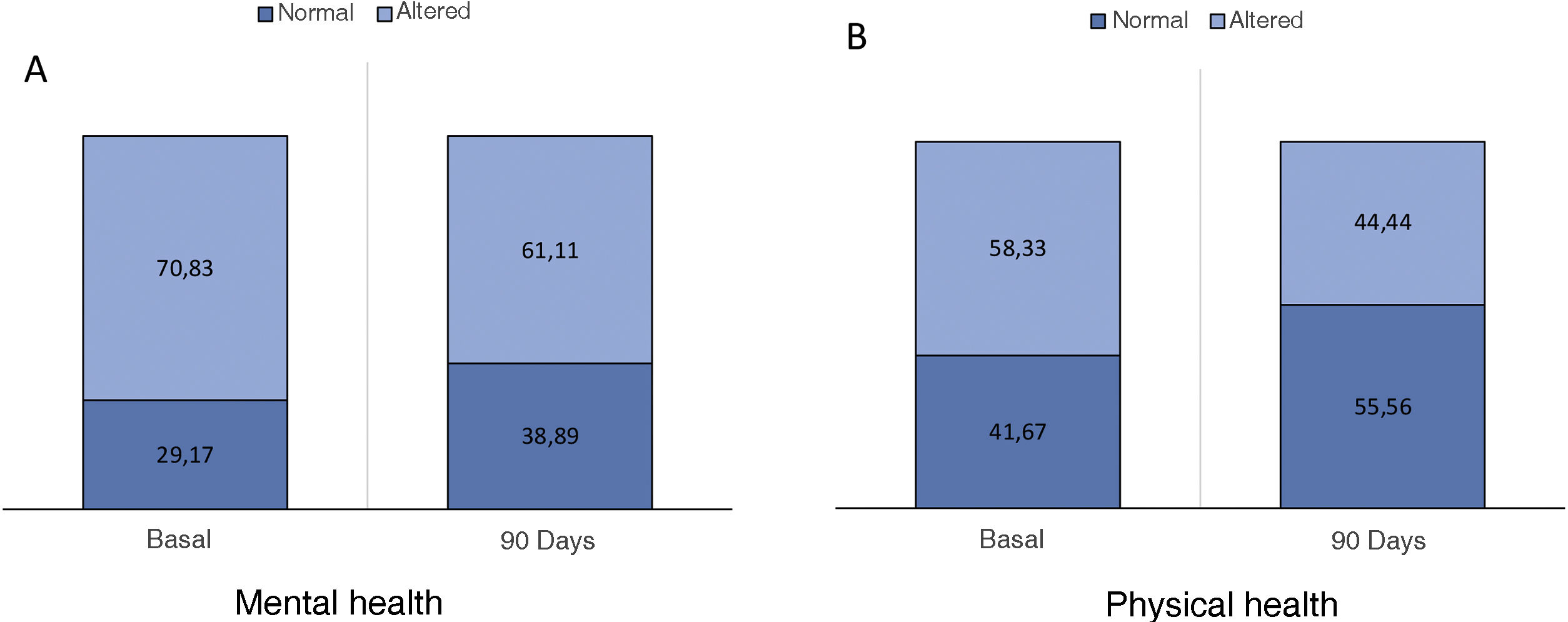
The optimal treatment compliance rate as measured by the Morisky-Green scale was 77% at baseline and 91% at 90 days (Fig. 2). Anxiety measured by the HAD scale was 35.7% (borderline 17.9%, altered 17.9%) at baseline and 32.43% at 90 days, while depression was 39.29% (borderline 17.86%, altered 21.43%) at baseline and 27.02% (borderline 13.51%, altered 13.51%) at 90 days (Fig. 3). According to the health status scale (PROMIS), altered mental health at baseline was 70.83% and 61.11% at 90 days, while altered physical health was 58.33% at baseline and 44.44% at 90 days (Fig. 4).
DiscussionThis is the first study performed on the use of NORA in patients with advanced kidney disease and diabetes. It shows that the use of a cell phone application that offers information on the management of cardiovascular risk factors provides more awareness and better control of the disease, since the rate of adherence to treatment improved from 77 to 91% in the first 90 days of follow-up. Similarly, in a different pathology such as stroke, Requena et al. demonstrated that the use of NORA-app improved patient awareness and compliance (86 vs. 69.2%, p < 0.01), together with better control of cardiovascular risk factors at 90 days of follow-up.12
To reach the maximum potential of the therapies currently available, it is necessary to ensure good compliance and awareness on the part of our patients so that they become actively involved in the management of their disease, a process currently known as health literacy.17–19 This therapeutic compliance is especially important in the case of cardiovascular diseases where recurrence rates are high.20 A digital platform that incentivizes compliance can offer added value to commonly used treatment guidelines.
It should be noted that, despite the advanced age of CKD patients, the use of mobile devices is becoming widespread among people in their sixth or seventh decade of life. It is currently not uncommon to see our patients with these devices and in the coming years it is going to be increasingly common. A systematic review conducted in 2019 showed that telemedicine was acceptably used by older adults,21 and if they perform adequate training they show significant improvements in their ability to perform tasks in their telemedicine unit.22 In our study, patients in the NORA-app group had a mean age of 72.3 ± 8.2 years, of which 44 have individual use of the app, while 27 is through caregiver, demonstrating easy management with the app thanks to the interaction between patient and healthcare professional.
It is classically known that alb/creat is a biomarker of renal disease progression,23 moreover it is an independent risk factor for cardiovascular disease, so that a higher urinary albumin excretion rate is associated with a higher incidence of morbidity, cardiovascular mortality and progression of renal disease.24–26 and therefore its control is essential to delay the progression of renal disease. Currently, there are several pharmacological options aiming to control alb/creat.27–29 Moreover, the use of telemedicine could help in the management through modification to a healthier lifestyle of CKD patients. Katz et al. conducted a virtual follow-up program of CKD patients with eGFR < 30 ml/min/m2 and alb/creat > 30 mg/g and during one year, reporting an adequate follow-up of patients with safety and efficiency.30 In our study, in patients with diabetes and CKD, who used the NORA app presented significantly lower levels of alb/creat at follow-up as compared to the control group, being statistically significant, thus favoring the delay of disease progression, while other analytical variables such as total cholesterol and glycosylated hemoglobin showed a tendency to be lower than in the control group.
Regarding the evaluation of anxiety and depression, studies have shown that CKD patients have a high risk of presenting mental illness, being more frequent in patients on renal replacement therapy,31 in addition, it has been shown that the increased inflammation and oxidative stress that is present in patients with CKD leads to the development of depression.32 Studies have shown that digital mental health interventions can be effective in improving depression, anxiety and psychological well-being.33 In our pilot study patients in the NORA-app group showed a tendency to improve anxiety and depression during the first 90 days of follow-up. Narayanan et al. evaluated an integrated telemedicine platform to assess health status through scales (PROMIS-10) in oncology patients demonstrating feasibility and satisfaction of patients, presenting less burden of symptoms and a greater concern about lifestyle.34 In this regard our work demonstrated better mental and physical health status in the first 90 days of follow-up.
The use of telemedicine has increased during the last decade to facilitate the communication between health professionals and patients, providing remote medical care that aims to respond to the needs reported by the patient and promote a coordinated care between the different health services through remote monitoring.35,36 The ICTs appear to be very useful tools in a society that is increasingly accustomed to the use of new technologies and which make it possible to offer health services to people with geographical limitations or logistical difficulties to travel easily to the health center.11,37,38 Furthermore, during the period of the COVID-19 pandemic, it was given priority to the implementation of telematic follow-up in patients with CKD in order to avoid hospital visits aiming of reducing the risk of COVID-19 infection. It is of interest to mention that terminal CKD has been identified as one of the first risk factors of mortality secondary to COVID-19.38–42
Our study is one of the few that has evaluated telemedicine in the progression of diabetic kidney disease, showing encouraging results for disease control and improving health literacy in the first 90 days of follow-up. Additionally, among the most important limitations of our study are the number of patients included, as well as the lack of information regarding sociocultural and economic level, since these are factors that could have influenced our results. In addition, since this was a pilot study, data such as blood pressure, weight, etc. was not available in the control group which would have allowed a more detailed comparison between the two groups. Randomized studies in this group of patients with diabetes mellitus and CKD could be very useful in the near future.
ConclusionsIn patients with DKD who maintained the use of NORA-app in the first 90 days, it was shown to have high level of compliance with treatment, achieving better control of the disease. The generalized use of ICT can help in the personalized follow-up of these patients, with the objective of delaying the progression of renal disease, improving quality of life and reducing health care costs.
FundingThis work has been performed thanks to a grant awarded by the "Ayudas Fundación Senefro a la investigación en Nefrología", 2021 of the Sociedad Española de Nefrología (Spanish Society of Nephrology).
Conflicts of interestM.J.S. reports speaking fees, consulting from AstraZeneca, Novo Nordisk, Esteve, Vifor, Bayer, Mundipharma, Ingelheim Lilly, Jansen, ICU Medical and Boehringer.
The other authors declare that they have no conflicts of interest.
Patients with chronic kidney disease and their involvement with the new technologies available.