Long-term consequences associated with kidney donation are controversial. Pre- and post-donation glomerular filtration rates (GFRs) are determinants of renal and cardiovascular risk weighting. In Latin America, there is limited experience in evaluating kidney function using GFR measurement techniques in kidney donors. The MDRD 4-variable and CKD-EPI equations are considered reasonable options. The objective of this study was to evaluate the performance of the MDRD and CKD-EPI equations in post-nephrectomy GFR dynamics in kidney donors.
Materials and methodsA prospective cohort study with GFR measurement and estimation in 189 kidney donors who underwent nephrectomy between 2007 and 2016 at the Hospital Privado Universitario de Córdoba [Private University Hospital of Córdoba] in Córdoba, Argentina. GFRs were evaluated before and after nephrectomy by iothalamate clearance determined by HPLC and by the MDRD and CKD-EPI equations for estimating GFR. Two groups were formed for this study: Group 1 (n=107), with an evaluation time subsequent to GFR stabilization (3 months) of up to 5 years, and Group 2 (n=82), with an evaluation time of 5–10 years following donation. Measured GFR (mGFR) was assessed by iothalamate clearance determined by HPLC.
ResultsRenal compensation values were 61.9% (52.0%–71.1%) and 75.6% (64.9%–84.4%) for Group 1 (n=107) and Group 2 (n=82), respectively. MDRD underestimated the GFR in 3.2% (90ml/min/1.73m2) and 38.6% (60ml/min/1.73m2) compared to the mGFR, and CKD-EPI underestimated the GFR in 2.6% (90ml/min/1.73m2) and 13.8% (60ml/min/1.73m2). Diagnostic performance was evaluated with a ROC curve (mGFR<60ml/min/1.73m2) for MDRD (ABC=0.66; CI: 0.59–0.73; sensitivity: 98.7%; specificity: 63.3%) and for CKD-EPI (ABC=0.79 CI: 0.73–0.85; sensitivity: 96.9%; specificity: 76.4%. Estimated GFR (eGFR) showed poor performance for estimating the glomerular filtration rate in the post-nephrectomy follow-up of donors over 50 years of age.
ConclusionsEquations for estimating GFRs showed poor performance for long-term follow-up of post-nephrectomy GFRs. Measuring GFRs to determine kidney function is recommended in the screening and follow-up of some donors under the current selection criteria.
Las consecuencias a largo plazo asociadas con la donación renal resultan controvertidas. La tasa de filtración glomerular (TFG) pre y posdonación resulta determinante en la ponderación del riesgo renal y cardiovascular. En Latinoamérica, existe escasa experiencia sobre la evaluación de la función renal por técnicas de medición del filtrado glomerular en donantes renales. Las ecuaciones MDRD y la CKD-EPI son consideradas alternativas razonables. El objetivo del trabajo fue evaluar el rendimiento de las ecuaciones MDRD y CKD-EPI en la dinámica del filtrado glomerular posnefrectomía en donantes renales.
Materiales y métodosEstudio prospectivo de cohorte con medición (mTFG) y estimación de la tasa de filtrado glomerular (eTFG) en 189 donantes renales con nefrectomía entre 2007 y 2016 en el Hospital Privado Universitario de Córdoba, Argentina. Las TFG se evaluaron, previo y posterior a la nefrectomía, mediante el aclaramiento de iotalamato determinado por cromatografía líquida de alta eficacia y por las ecuaciones para estimación de TFG: MDRD y CKD-EPI. Se constituyeron 2 grupos de estudio: grupo 1 (n=107) con un tiempo de evaluación posterior a la estabilización de la TFG posdonación (3 meses) hasta los 5 años y grupo 2 (n=82) con un tiempo entre 5 y 10 años posdonación.
ResultadosEl valor de compensación renal fue del 61,9% (52,0-71,1%) y 75,6% (64,9-84,4%) para los grupos 1 (n=107) y 2 (n=82), respectivamente. La ecuación MDRD subestimó la TFG en el 3,2% (90ml/min/1,73m2) y el 38,6% (60ml/min/1,73m2) respecto a la mTFG y la CKD-EPI subestimó en un 2,6% (90ml/min/1,73m2) y un 13,8% (60ml/min/1,73m2). Se evaluó el rendimiento diagnóstico con curva ROC (mTFG<60ml/min/1,73m2) para MDRD (ABC=0,66, IC: 0,59-0,73), sensibilidad: 98,7% y especificidad: 63,3%, y para CKD-EPI (ABC=0,79, IC: 0,73-0,85), sensibilidad: 96,9% y especificidad: 76,4%. Las eTFG mostraron un bajo desempeño para estimar el filtrado en el seguimiento posnefrectomía de los donantes mayores de 50 años.
ConclusionesLas ecuaciones de estimación de la TFG muestran un bajo desempeño para el seguimiento a largo plazo del filtrado posnefrectomía y la medición del filtrado sería recomendable en la selección como en el seguimiento de ciertos donantes bajo los criterios actuales de selección.
The long-term consequences of kidney donation in terms of morbidity and mortality are a matter of debate.1,2 While certain studies have ruled out a significant risk of chronic kidney disease or death compared to the general population,3,4 others have reported an increase in renal and cardiovascular risk.5,6
Due to organ scarcity, the screening criteria for potential donors have become more flexible in most transplant programmes, leading to a higher proportion of marginal candidates with a growing number of comorbidities.7,8 This could lead to accelerated loss of kidney function post-donation.
Although the absolute risk of terminal kidney disease post-donation has historically been low (0.31%–0.47%), comorbidities in marginal donors such as albuminuria, hypertension and obesity9,10 could shift this paradigm.
Therefore, precise assessment of kidney function pre-donation and follow-up thereof post-nephrectomy are essential for identifying increased-risk donors in order to implement prevention and follow-up strategies.
Measured glomerular filtration rate (mGFR), determined using an exogenous marker, is considered the best method for measuring kidney function.11 However, the complexity and costs of this technique limit its availability at most transplant centres.12
Estimated GFR (eGFR) equations, including the modification of diet in renal disease (MDRD) equation13 and the chronic kidney disease epidemiology collaboration (CKD-EPI) equation,14 are considered reasonable alternatives.15
However, these equations were designed and validated in populations with chronic kidney disease and therefore underestimate GFR in the highest range.16,17
Creatinine clearance may also be useful, but it generally shows a great deal of variability.18
The limitations of the MDRD formula include poor correlation with mGFR with values exceeding 60ml/min/1.73m2. The CKD-EPI formula was validated based on a cohort that, unlike the MDRD formula, included not only patients with reduced kidney function but also individuals with normal kidney function. Thus, it offers better correlation to mGFR in healthy subjects.19,20
In Latin America, there is limited experience in assessment of kidney function by techniques for measuring GFR in kidney donors as an acceptance criterion, or in follow-up of GFR post-nephrectomy.
The objective of this study was to assess the long-term performance of the MDRD and CKD-EPI equations in kidney donors in order to examine post-nephrectomy GFR dynamics.
Materials and methodsStudy designA prospective cohort study was conducted with repeated GFR measurements and estimates in 189 kidney donors, all over 18 years of age, who underwent nephrectomy between 2007 and 2016 at Hospital Privado Universitario de Córdoba [Córdoba University Private Hospital] in Córdoba, Argentina. All donors with at least one GFR measurement pre-donation and who underwent another GFR measurement following nephrectomy (both measurements performed using iothalamate clearance) in 2017 and 2018 were enrolled. All participants signed an informed consent form for this study.
The following kidney donors were excluded: those allergic to iodine or to the contrast used (iothalamate meglumine); those with confirmed evidence of a urinary tract infection or systemic infection of any origin at the time when the study was being conducted; those on treatment with diuretics, non-steroidal anti-inflammatory drugs or trimethoprim/sulfamethoxazole; and those with any decompensated cardiovascular disease, any of the above-mentioned infectious diseases or dehydration which affected their GFR at the time when the clearance was being determined.
GFR measurement was performed with iothalamate determined by HPLC and the corresponding value was used as an acceptance criterion for donation. Another post-donation measurement was done at different times in relation to the pre-donation measurement. The study was approved by the institutional ethics committee (IEC). All procedures were performed in accordance with the Declaration of Helsinki and the Declaration of Istanbul. Height, weight and body mass index (BMI) data were collected at all visits.
Kidney function measurementIothalamate clearance measurementAn iothalamate clearance test was performed on donors who fulfilled the conditions set out in the protocol. The determination was made under fasting conditions, without taking any drugs.
First, the bladder was emptied and the urine was discarded. Next, 1ml of iothalamate meglumine (Conray® [Mallinckrodt Inc. Blanchardstown, Dublin, Ireland] 60%) was administered subcutaneously. Following an equilibration period of 60min, the first blood sample was taken. The donor remained at rest and maintained oral hydration with 150ml of water every 15min until they emptied their bladder again. Two hours after administration of iothalamate, the entire volume of urine was collected and the second blood sample was taken.
The glomerular filtration rate was determined using non-radiolabelled iothalamate renal clearance determined by high-performance liquid chromatography. The instrument used was a Gilson® HPLC system (Middleton, WI, United States) with a Model 189 UV/visible detector. The column used was a Phenomenex® column (Torrance, CA, United States) (C18, 20×4.5mm). Plasma samples were taken with lithium heparin as an anticoagulant, and urine samples were collected in sterile bottles. Clearance was calculated as U×V/P, where U was the concentration of iothalamate determined in urine, P was the concentration of iothalamate determined in plasma and V was the volume (ml) adjusted for time and BMI.11,21
Calculation of the estimated glomerular filtration rateThe abbreviated MDRD equation (MDRD-4),13 with creatinine standardised by isotope dilution mass spectrometry (ID-MS), was used. The CKD-EPI equation was used with differentiation by gender and stratification by creatinine level.14 Creatinine concentration in serum was determined using a compensated kinetic Jaffe technique traceable to the ID-MS reference method in MODULAR P® (Roche, Mannheim, Germany) and COBAS 6000® autoanalysers (Roche, Mannheim, Germany). The reagents and calibrators used were from Roche, the internal quality controls used were from RANDOX® ACUSERA (Randox Laboratories Ltd. County Antrim, United Kingdom) and the external control used was from RIQAS (United Kingdom).
Statistical analysisContinuous variables were expressed in terms of mean and 95% confidence interval (CI) or in terms of median and interquartile range (p25–p75), depending on the type of distribution. Qualitative variables were expressed in terms of percentage and 95% CI.
The Kolmogorov–Smirnov test was used to determine the normality of the quantitative variables. For intergroup comparisons of independent quantitative variables, the following were used as non-parametric tests: the Kruskal–Wallis test for more than two samples and the Wilcoxon test for analysis of two related samples. To evaluate agreement between two systems of measurement, a Bland–Altman analysis was performed. Receiver operating characteristic (ROC) curves were constructed from which different diagnostic parameters were obtained, such as the area under the curve (AUC) for each eGFR for the detection of an mGFR lower than 60ml/min/1.73m2. The CIs were computed at the 95% level. The level of significance was 95% (p<0.05).
Kidney compensation was defined as the percentage GFR achieved by the remaining kidney compared to the baseline GFR determined prior to nephrectomy.
The percentage was calculated of donors whose post-nephrectomy GFR, estimated using the MDRD and CKD-EPI equations, underestimated the filtration rate when compared with the GFR measured by iothalamate clearance. The values studied were 60 and 90ml/min/1.73m2.
To conduct the statistical analysis, the statistical software package IBM SPSS® Statistics version 19 (Armonk, New York, United States) was used.
ResultsThe cohort’s demographic characteristics, anthropometric characteristics and kidney function pre-donation are shown in Table 1. GFR stabilisation dynamics post-nephrectomy were studied in a subgroup of donors. GFR stabilisation was seen as of three months post-nephrectomy (results not shown).
Demographic characteristics and parameters of kidney function in donors pre-donation.
| Variable | Pre-donation |
|---|---|
| Number of donors | 189 |
| Age (years) | 42 (33–53) |
| Female sex, n (%) | 115 (60.3) |
| Height (cm) | 167 (158–172) |
| Weight (kg) | 74.0 (63–83) |
| BMI (kg/m2) | 26.4 (24.1–30.0) |
| Creatinine (mg/dl)a | 0.74 (0.72–0.77) |
| mGFR (ml/min/1.73m2)a | 114.9 (112.2–117.6) |
| CKD-EPI (ml/min/1.73m2)a | 109.0 (106.8–111.1) |
| MDRD (ml/min/1.73m2) | 101.4 (86.6–114.8) |
Quantitative variables were expressed in terms of median p(25,75).
BMI: body mass index; CKD-EPI: chronic kidney disease epidemiology collaboration; MDRD: modification of diet in renal disease; mGFR: measured glomerular filtration rate.
Table 2 shows demographic and kidney function values. Two groups were established considering post-donation GFR evaluation time. The evaluation time for group 1 (n=107) was between three months (period observed for post-donation GFR stabilisation) and five years. The evaluation time for group 2 (n=82) was between five years and 10 years post-donation. A statistically significant difference was seen in all pre- and post-donation parameters, both in group 1 and in group 2, except in height and weight. The groups studied showed no differences in long-term versus short-term changes in creatinine (group up to 5 years and more than 5 years, p=0.47). Kidney compensation for the two groups was 61.9% (52.0%–71.1%) for group 1 and 75.6% (64.9%–84.4%) for group 2.
Demographic characteristics and parameters of kidney function by post-nephrectomy follow-up time.
| Variable | Group 1 pre-donation | Group 2 pre-donation | Group 1 post-donation (<5 years) | Group 1 p value | Group 2 post-donation (>5 years) | Group 2 p value | Group 1 versus Group 2 p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of donors | 107 | 82 | 107 | 82 | |||||||
| Age (years) | 43 | (41–45) | 42 | (39–45) | 47 | (45–49) | <0.02 | 50 | (47–53) | <0.0002 | 0.09 |
| Female sex, n (%) | 73 | (68) | 42 | (51) | 73 | (68) | 42 | (51) | |||
| Height (cm) | 164 | (163–166) | 167 | (165–169) | 164 | (163–165) | 0.98 | 166 | (164–168) | 0.54 | 0.12 |
| Weight (kg) | 74 | (71–77) | 74 | (70–78) | 78 | (75–81) | 0.06 | 77 | (73–81) | 0.26 | 0.59 |
| BMI (kg/m2) | 27.5 | (26.7–28.3) | 26.6 | (25.4–27.8) | 28.7 | (27.8–29.6) | <0.05 | 29.5 | (28.3–30.7) | <0.001 | 0.28 |
| Creatinine (mg/dl) | 0.76 | (0.73–0.79) | 0.71 | (0.68–0.74) | 1.14 | (1.12–1.16) | <0.03 | 1.13 | (1.11–1.15) | <0.0001 | 0.47 |
| mGFR (ml/min/1.73m2) | 111.3 | (108.1–114.5) | 120.0 | (116.0–124.0) | 70.6 | (69.3–71.9) | <0.0001 | 77.7 | (75.9–79.5) | <0.0001 | <0.001 |
| GFR difference (ml/min/1.73m2) | 40.7 | (39.7–41.7) | 38.0 | (36.7–39.3) | 0.11 | ||||||
| CKD-EPI (ml/min/1.73m2) | 107.1 | (103.9–110.3) | 112.3 | (109.3–115.3) | 73.6 | (71.9–75.3) | <0.0001 | 74.0 | (71.4–76.6) | <0.0001 | 0.78 |
| GFR difference (ml/min/1.73m2) | 33.5 | (32.5–34.5) | <0.0001 | ||||||||
| MDRD (ml/min/1.73m2) | 97.4 | (93.9–101.0) | 110.2 | (105.3–115.1) | 52.2 | (51.1–53.3) | <0.0001 | 52.6 | (51.0–54.2) | <0.0001 | 0.67 |
| GFR difference (ml/min/1.73m2) | 45.2 | (42.8–47.6) | 57.0 | (53.6–60.4) | <0.0001 | ||||||
| Proportion of compensation (95% CI) | 0.62 | (52.0–71.1) | 0.760 | (0.65–0.84) | |||||||
Quantitative variables were expressed in terms of mean (95% confidence interval [CI]).
BMI: body mass index; CKD-EPI: chronic kidney disease epidemiology collaboration; MDRD: modification of diet in renal disease; mGFR: measured glomerular filtration rate.
The post-donation groups were compared to one another, and a statistically significant difference was seen in mGFR alone (p<0.001).
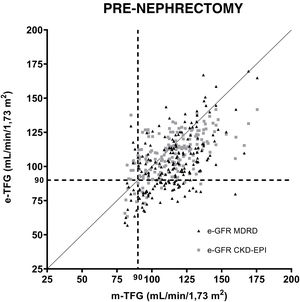
Fig. 1 shows the correlations between the GFRs estimated using the MDRD and CKD-EPI equations in relation to the GFRs measured by iothalamate clearance prior to nephrectomy. A value of 90ml/min/1.73m2 was established as a reference. Both formulas were subjected to regression analysis. The resulting equation for MDRD was y=0.6608 X+26.52, r: 0.53 p<0.001. The same for CKD-EPI was y=0.4702 X+54.69, r: 0.58 p<0.001.
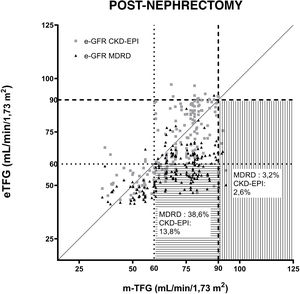
Fig. 2 shows the GFR estimated using the MDRD and CKD-EPI equations compared to the GFR measured by iothalamate clearance post-nephrectomy. Two GFR values were established and considered references: 60ml/min/1.73m2 and 90ml/min/1.73m2.
Correlation of equations for estimating GFR (MDRD and CKD-EPI) after uninephrectomy. The questions are compared to iothalamate clearance. A GFR value of 90ml/min/1.73m2 is indicated with a dashed line (----). A GFR value of 60ml/min/1.73m2 is indicated with a dotted line (….). The horizontal lines mark the area where underestimated GFRs are found in relation to 60ml/min/1.73m2. The vertical lines mark the underestimated GFRs in relation to 90ml/min/1.73m2.
The percentage was determined of donors whose post-nephrectomy GFR determined using each equation underestimated its value when compared to the reference mGFRs of 60 and 90ml/min/1.73m2. The MDRD equation underestimated GFRs for 3.2% and 38.6% of donors compared to mGFRs greater than 90 and 60ml/min/1.73m2, while the CKD-EPI equation showed that 2.6% and 13.8% of the eGFRs underestimated when evaluating mGFRs greater than 90 and 60ml/min/1.73m2, respectively.
Diagnostic performance was determined using the area under the ROC curve for each eGFR in the post-nephrectomy donor assessment: MDRD (AUC=0.66, CI: 0.59–0.73), sensitivity 98.7%, specificity 63.3% and CKD-EPI (AUC=0.79, CI: 0.73–0.85), sensitivity 96.9% and specificity 76.4%, considering a GFR of 60ml/min/1.73m2 as a reference.
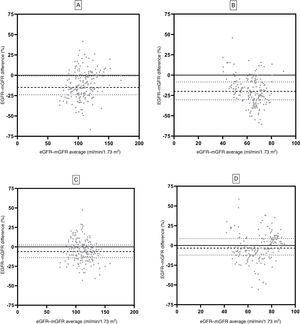
Fig. 3 shows the agreement of the eGFR pre-and post-donation using the Bland–Altman test. The MDRD equation showed a pre-donation bias of –15.0 (CI: –24.0; –0.2) ml/min/1.73m2 and a post-donation bias of –20.5 (–30.4; –08.5)ml/min/1.73m2. The CKD-EPI equation showed a bias of –6.1 (–14.0; 2.4)ml/min/1.73m2 and –3.4 (–12.1; 8.6)ml/min/1.73m2 in pre- and post-nephrectomy GFR.
Bland–Altman analysis using both GFR equations. MDRD pre-donation (A) and MDRD post-donation (B) are shown. CKD-EPI pre-donation (C) and CKD-EPI post-donation (D) are also shown. mGFR was determined using iothalamate clearance. Spaced dashed lines (- - -) indicate median, and dotted lines (….) correspond to 25th and 75th percentiles of bias.
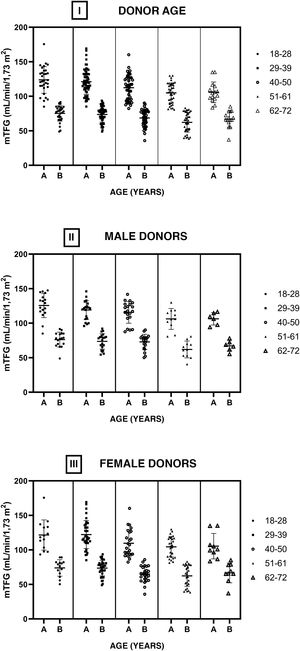
Fig. 4 shows the GFRs of the donors pre- and post-nephrectomy by age group (I). No statistically significant differences were found between intragroup GFRs (before and after nephrectomy) or between the different age groups comparing the pre-nephrectomy GFRs and the post-nephrectomy GFRs. The cohort was divided into men (II) and women (III), and the structure for analysis used for the full cohort was applied to these divisions. No statistically significant differences were found between the GFRs under the same conditions in which the complete sample was compared.
DiscussionPrior studies that have compared the MDRD equation and the CKD-EPI equation with GFR measurement methods for assessing kidney function in donor screening have yielded different results.22 The equations revealed underestimations with respect to mGFR and showed poor precision. The performance of the equations involved high percentages of potential donors who would be rejected without measurement of glomerular filtration rate using direct techniques. Similarly, it was found that some donors might be erroneously accepted with the use of the equations.21,23 Therefore, estimation of GFR with MDRD and CKD-EPI in candidates for kidney donation results in both the rejection of suitable candidates with a falsely low GFR and the acceptance of unsuitable candidates with a supposedly high GFR.
It is considered advisable to use a measured method, since approximation (correlation and percentage of error) of the eGFR by equations to the mGFR is limited.24 Despite its importance, there is no consensus on the minimum GFR allowed for a prospective donor to undergo nephrectomy. This exposes the need for more comprehensive evaluation of GFR in kidney donor screening.
This study evaluated the behaviour of kidney function and the performance of different methods for measurement and estimation thereof in a cohort of kidney donors. The compensation values seen post-nephrectomy ranged from 62% to 76% between three months and 10 years post-nephrectomy.
Several studies have analysed recovery of kidney function post-donation. All of them have linked an increase in kidney volume to GFR compensation.25–27 Different reports have given a detailed account of the process through which the remaining kidney increases its GFR following donation.25 In a live kidney donor, kidney flow is increased immediately after nephrectomy such that, despite the loss of half the donor’s functional kidney mass, a GFR corresponding to 70% of the donor’s prior kidney function is achieved. Some studies have evaluated pre-existing factors associated with recovery of kidney function and have agreed that a higher baseline GFR is predictive of better kidney function a year after donation.9,28,29
The performance of the equations for estimating GFR post-nephrectomy following the stabilisation thereof was evaluated. Both the MDRD equation and the CKD-EPI equation underestimated GFR post-donation, particularly for GFRs of 60–90ml/min/1.73m2.
Several studies have assessed these equations as tools for donor screening, but few studies have evaluated them in a post-donation context.
This was the scenario in which we found the greatest underestimation effect, particularly with MDRD, which erroneously classified 38.6% of donors post-nephrectomy with GFRs exceeding 60ml/min/1.73m2. The area under the ROC curve for the MDRD equation, for an mGFR <60ml/min/1.73m2, was a low value (0.66) due to its poor diagnostic specificity, which could cause incorrect evaluation of GFRs in kidney donors post-nephrectomy.
This study reported biases on the part of the MDRD and the CKD-EPI, in line with other published studies,19,22,30 finding an increase in the bias of the MDRD equation when it was used post-nephrectomy (–19.5ml/min/1.73m2).
We evaluated the distribution of estimated and measured GFR values considering donor age group. Both equations applied to different age groups showed a lack of agreement with measured values, both in the evaluation before donation and in the evaluation after it.
The CKD-EPI equation showed a distribution with greater agreement compared to the MDRD in relation to mGFR values. However, underestimation was seen across all age groups. The underestimation and high variability of the equations, where wide ranges of estimated values compared to measured values were seen for the same values, call into question their applicability.
This takes on great importance when precise determination of GFR is required in the care of kidney donors, as well as in their screening, particularly in those with expanded criteria.
The possibility of detecting modest changes in GFR is essential for identifying donors at risk and thus for being able to promptly implement strategies for prevention and personalised monitoring. Most transplant programmes assess serum creatinine levels or estimate GFR with the equations that we assessed in this study for long-term follow-up of their donors, all for reasons of accessibility, despite the limitations previously reported in the general population and confirmed by our study when applied to donors.18,19,31
The KDIGO guidelines affirm the need to determine GFR, but do not establish the most suitable methodology to be used to do so.32 The results of our series suggest that, in donors under 40 years of age with a family history of chronic kidney disease, albuminuria or blood pressure at the upper limit of normal, a strategy of measurement rather than estimation with traditional formulas would be recommended.
In conclusion, post-donation GFR and GFR stabilisation post-nephrectomy achieves an average level of compensation of 70% of the initial GFR. Equations for estimating GFR show poor performance for long-term follow-up of GFR post-nephrectomy. It would be advisable to measure GFR in the screening and follow-up of certain donors under the current criteria for screening and acceptability for donation.
FundingThis study received no specific funding from public, private or non-profit organisations.
Conflicts of interestNone.
Please cite this article as: Luján P, Chiurchiu C, Capra R, de Arteaga J, de la Fuente J, Douthat W. Medición y estimación del filtrado glomerular posdonación renal. Nefrologia. 2021;41:191–199.