Introducción: La malnutrición ha sido descrita en los pacientes con enfermedad renal crónica, y su asociación con el riesgo cardiovascular y la mortalidad en pacientes en hemodiálisis. Recientemente se ha propuesto una nueva terminología, protein energy wasting, con nuevos criterios diagnósticos (marcadores bioquímicos y antropométricos), para identificar precozmente a los pacientes con riesgo de presentar desgaste proteico-energético (DPE) y riesgo de mortalidad. El objetivo de este estudio fue observar por primera vez en España la prevalencia, la evolución en el tiempo y el significado pronóstico del DPE en un centro de diálisis español. Pacientes y métodos: estudio observacional que incluyó a 122 pacientes prevalentes en hemodiálisis en nuestro centro. Entre enero de 2010 y octubre de 2012 se realizaron tres visitas, en las cuales se recogieron parámetros clínicos, bioquímicos, antropométricos, composición corporal mediante el uso de bioimpedancia (espectroscópica BIS) y sus características dialíticas respectivas según los criterios de la nueva definición. Se analizó la prevalencia de DPE en cada visita, la progresión de los parámetros de malnutrición y los posibles factores asociados a DPE. Tras un período de seguimiento, media de 461 días, analizamos la supervivencia. El análisis estadístico se realizó utilizando el programa R. Resultados: La prevalencia de DPE se mantuvo constante en el tiempo: 37 % en la visita basal, 40,5 % a los 12 meses y 41,1 % a los 24 meses. La introducción de la variable dinámica pérdida de masa muscular, incluida en la definición de DPE, aumentó la prevalencia a un 50 % a los 24 meses. La situación de DPE es dinámica, como demuestra que un 26-36 % de los pacientes sin DPE lo desarrollan de novo cada año, y un 12-30 % se recuperan anualmente de esta situación. La presencia de DPE se asoció a mayor índice de resistencia a la eritropoyetina (irEPO) y a mayor presión de pulso al final de la diálisis. En el modelo de regresión multivariable, las variables clínicas predictoras de DPE fueron sobrehidratación, irEPO, agua intracelular y ratio agua extracelular/agua intracelular. Veintiséis (21 %) pacientes fallecieron. La curva de Kaplan-Meier no mostró diferencias en el riesgo de mortalidad entre pacientes con y sin DPE, pero la pérdida de masa muscular se asoció con mayor mortalidad. Conclusión: El presente estudio observacional subraya la alta prevalencia de DPE y tiene un carácter dinámico en pacientes en hemodiálisis. Solo el criterio pérdida de masa muscular (aumento del catabolismo proteico) se asoció a un incremento de mortalidad, mientras que el resto de los criterios de DPE según la clasificación ISRMN no se asoció a un incremento de la mortalidad. Igualmente hemos observado un estado de sobrehidratación en los pacientes con DPE. Dicho estado de sobrehidratación (aumento de agua extracelular por ocupación de la pérdida de músculo, sin aumento del agua corporal total) no es valorable ni por el peso seco ni por el índice de masa corporal. Son necesarios estudios de intervención para evaluar si la prevención de la sarcopenia mejora la supervivencia.
Introduction: Malnutrition has been described in patients with chronic kidney disease as well as its association with cardiovascular risk and mortality in haemodialysis patients. Recently, the new term ‘protein energy wasting’ has been proposed with new diagnostic criteria (biochemical and anthropometric markers) for early identification of patients at risk for protein energy wasting and mortality. The aim of this study was to examine the prevalence, evolution over time and prognostic significance of PEW in a Spanish dialysis centre for the first time in Spain. Patients and methods: an observational study that included 122 prevalent haemodialysis patients at our centre. Between January 2010 and October 2012, three visits were carried out in which clinical, biochemical, anthropometric and body composition parameters were collected using BIS (bioelectrical impedance spectroscopy) along with their respective dialytic characteristics, in accordance with the criteria of the new definition. We analysed the prevalence of PEW in each visit, progression of the malnutrition parameters and factors potentially associated with PEW. After a mean follow-up period of 461 days, we analysed survival. Statistical analysis was performed using the R software. Results: The prevalence of PEW remained constant over time: 37% at baseline visit, 40.5% at 12 months and 41.1% at 24 months. With the introduction of the dynamic variable muscle mass loss, included in the definition of PEW, prevalence increased to 50% at 24 months. The PEW situation is dynamic, as demonstrated by the fact that 26%-36% of patients without PEW develop it de novo each year and 12%-30% annually recover from this situation. The presence of PEW was associated with higher rates of resistance to erythropoietin (irEPO) and higher pulse pressure at the end of dialysis. In the multivariable regression model, PEW predictive clinical variables were over-hydration, irEPO, intracellular water and the extracellular water/intracellular water ratio. Twenty-six (21%) patients died. The Kaplan-Meier curve did not show any differences in mortality risk between patients with and those without PEW, but the loss of muscle mass was associated with increased mortality. Conclusion: The present observational study highlights the high prevalence of PEW, which has a dynamic nature in haemodialysis patients. Only the criterion of muscle mass loss (increased protein catabolism) was associated with increased mortality, while the other PEW criteria according to the ISRNM classification were not associated with increased mortality. We also observed a state of over-hydration in patients with PEW. This state of over-hydration (increased extracellular water due to occupation of muscle loss without an increase in total body water) cannot be evaluated by dry weight or the body mass index. Intervention studies are necessary in order to assess whether or not the prevention of sarcopaenia improves survival.
INTRODUCTION
In recent years, chronic kidney disease (CKD) has become a public health problem worldwide due to its frequency and high morbidity and mortality.1,2 Cardiovascular disease is the primary cause of death in CKD patients on haemodialysis.3 Despite correcting the traditional cardiovascular factors such as hypertension, dyslipidaemia and left ventricular hypertrophy and improving dialysis techniques, a high rate of unexplained mortality remains, as has been demonstrated on randomised controlled studies such as the HEMO study.4 New non-traditional risk factors for morbidity and mortality such as malnutrition have been described.5 The classic concept of uraemic or cachexic uraemic malnutrition6,7 has evolved in recent years thanks to better understanding of the physiopathological mechanisms involved such as inflammation, protein hypercatabolism and anorexia.
The study of the impact of malnutrition on CKD has been difficult due to a wide variety of diagnostic criteria and definitions. In the last meeting of the International Society of Renal Metabolism and Nutrition (ISRMN), protein energy wasting (PEW) syndrome as been defined8 in an effort to unify the different terminologies associated with the concept of malnutrition in CKD. Recently, the Nutrition Working Group of the Spanish Society of Nephrology has proposed the term desgaste proteico-energético (DPE by its Spanish abbreviation) as the definition that best defines in Spanish the Anglo-Saxon concept of PEW.9 PEW is defined as a pathological state where there is a continuous decrease or wasting of both protein deposits and energy reserves.8 In other words, PEW syndrome includes a simultaneous loss of fat and muscle in the uraemic patient. It was clear from the beginning that the concept of PEW is dynamic and different from the classic more static concepts since it includes the concept of protein-energy loss over a long period of time.
Using the classic definitions, it has been estimated that the prevalence of malnutrition in the haemodialysis population is 18%-75%.10 However, the prevalence of PEW in haemodialysis patients in Spain has not yet been described using the ISRMN criteria. The parameters proposed for defining PEW were established in the American population, so there is a question as to whether these findings can be extrapolated to other geographic distributions and lifestyles such as in Europe, Asia or in our case, Mediterranean countries. This study describes the prevalence of malnutrition using the PEW concept for the first time in a Spanish haemodialysis population, its progression over time and the possible impact that PEW can have on mortality.
PATIENTS AND METHOD
Patients
Ours was an observational, prospective study with no intervention. 122 prevalent patients on haemodialysis from the dialysis satellite centre of the Fundación Renal Íñigo Álvarez de Toledo-Hospital Fundación Jiménez Diaz in Madrid were included. All stable patients who had not been hospitalised in the previous two months and were on chronic haemodialysis on January 1, 2010 were included. The mean age at the start of the study was 63.6±14.3 years and the median time on dialysis was 26 (11-79) months. All patients were informed of the analysis and agreed to take part in the study. Patients who were hospitalised at the start of the study were excluded. Recruiting began on January 1, 2010 and survival was calculated until October 1, 2012, with a median follow-up of 461.4 days (240-931 days). The presence of PEW was evaluated annually (baseline, 12 and 24 months). The aetiology of CKD was glomerulonephritis in 21.8%, interstitial nephritis in 9.7%, polycystic kidney disease in 11.3%, nephroangiosclerosis in 27.4%, diabetic nephropathy in 15.3% and other causes in 14.5%. The degree of comorbidity as measured using the Davies criteria11 was high (two or more criteria) in 37% of subjects and medium (1 Davies criterion) in 51%. Twenty-seven patients (21.8%) were diabetic and 51 (41%) had a history of cardiovascular disease. Vascular access was an arteriovenouos fistula in 74%, prosthetic in 18% and tunnelled catheter in 8% of patients. None of the patients had a temporary catheter for more than 24 hours. Dialysis therapy included a time of at least 4 hours three times a week. The calcium concentration of the dialysis bath was 1.25-1.5mmol/l with a Kt/Ve of 1.42 (1.24-1.6) at a bath temperature of 36.5ºC. The dialysers used were high-flow polysulfone in 27%, low-flow polysulfone in 41% and high-flow polynephron in 32%. The water met criteria for ultrapure water throughout the study.
Body composition and nutritional status
The anthropometric and body composition measurements were performed immediately after the middle dialysis session of the week by a single observer on the same day that blood samples were collected. The PEW criteria were calculated with these measurements (Table 1).8
The body mass index (BMI) was expressed in kg/m2. Weight was calculated as dry weight, defined as post-dialysis weight in which the patient was normotensive and with no signs of overhydration (OH). The triceps fold (TF) was measured in millimetres with a plicometer in triplicate (lipocalibre Holtain, Crymych, United Kingdom) in the arm contralateral to the vascular access. The brachial circumference (BC) was measured in centimetres in the middle third with a flexible tape measure (Holtain Ltd., Crymych, United Kingdom). The TF and BC were used to calculate the mid-upper arm muscle circumference (MUAMC) using the formula MUAMC = BC – (0.314 x TF).12
Body composition analysis was done using bioimpedance spectroscopy (BIS). The Fresenius Medical Care BCM body composition monitor was used for this task. It was done post-dialysis after a 15-minute rest period in a short time in dialysis with no metallic objects, always with the electrodes placed in the contralateral side to vascular access location. The body composition measurement by BIS was done every six months. The three compartment: LTM (lean tissue, primarily muscle), ATM (adipose tissue) and OH were identified from the weight, height, intracellular water (ICW) and extracellular water (ECW) measurements taken by BIS.
The presence of PEW was defined as the patient meeting at least three criteria in the four different categories for malnutrition markers at baseline (Table 1).8 At 12 months (visit 1) and at 24 months (visit 2), the variables from the four categories were once again analysed, in addition to adding a new variable done by BIS, loss of muscle mass, which defined the concept of wasting. This variable was not introduced at the baseline visit since it requires measuring changes compared to the previous evaluation.
Biochemical analysis and other samples
The blood samples were collected at the start of the second dialysis of the week, the same day as the anthropometric data. Twenty minutes after drawing blood, the samples were centrifuged at 4 °C and those samples which were not immediately analysed were stored at -40 °C until analysis. Albumin (bromocresol technique, reference range 3.8-4.4g/dl), creatinine, C-reactive protein (CRP) and cholesterol were measured by an automatic analyser. Prealbumin was measured using the Nephelometry technique (Qm 300 nephelometer, Kalestad Diagnostic, reference range 10-40 mg/dl), transferrin by immunoturbidimetry using an ADVIA 2400 automatic analyser, 25-hydroxyvitamin-D was measured by immunoassay (DiaDorin LIASON®) and intact parathyroid hormone (PTH) was measured by electrochemiluminescence (Elecsys-2010, Roche Diagnostics) in the Biochemistry Department Laboratory at Hospital Fundacion Jimenez Diaz in Madrid. The erythropoietin (EPO) resistance coefficient was defined as the weekly EPO-alpha dosage (U/kg of pre-dialysis weight) divided by the haemoglobin level (g/dl) (erythropoietin resistance index; ERI).
All patients underwent a urea kinetics study. Blood urea nitrogen (BUN) was measured before (BUN1) and after (BUN2) the mid-week dialysis session. The normalised protein catabolism rate (PCRn) was used as an indirect indicator of protein intake and was obtained using the following formula: PCRn = (9.35 × G + 0.294 × V [litres])/ideal weight (kg). Where G is the generation of urea in the period between dialysis: G (mg/dl) = (BUN pre - BUN post) × V/time between dialysis (min). The Kt/Ve used was the Daugirdas bicompartmental definition.13
Weight gain between dialysis, total ultrafiltration rate, pulse pressure at the start and end of dialysis (PPi, PPf) defined as the difference between systolic blood pressure and diastolic blood pressure, the indirect measurement of vascular rigidity and vascular calcification were also collected. In addition, losses to follow-up, including deaths, were recorded and their cause analysed.
Statistical analysis
The statistical analysis was done using R software. Variables with a normal distribution were described using the mean ± standard deviation and those without a normal distribution were described using the median and range (minimum-maximum) or interquartile range (25-75 percentile, IQR). The categorical variables were described using their frequency distribution. Comparison between quantitative variables was done using the Mann-Whitney test for two-group comparisons and the Kurskal-Wallis test for comparing more than two groups. Comparison of the same variable at different visits was carried out using the Wilcoxon test for related samples. The Spearman correlation coefficient was used to evaluate the associations between malnutrition markers and the selected parameters. In order to study the possible factors that contribute to the presence of PEW in haemodialysis patients, a multivariate logistical regression model was fitted, the results of which were summarised using the odds ratio (OR) and the 95% confidence interval (95% CI). For the analysis of mortality, the survival curves were estimated using the Kaplan-Meier method and comparison was done using the log-rank test. In addition, a Cox multivariate regression model was adjusted, the results of which were summarized using the hazard ratio and the 95% CI. All comparisons were done with bilateral tests with a 0.05 level of significance.
RESULTS
Prevalence of malnutrition according to protein-energy wasting criteria
At the baseline visit, 46 patients (37%) had PEW according to the ISRMN definition by meeting three criteria in the four different categories. Table 1 shows the distribution of the different malnutrition markers according to the PEW criteria. At the start of the analysis, 49.6% of the population had an albumin level <3.8g/dl, 36.7% had prealbumin levels <40mg/dl and only 5.7% had cholesterol levels <100mg/dl. Among the body composition and anthropometric parameters, almost half (44.7%) had a BMI <23kg/m2 and 41/7% had a MUAMC below the 50th percentile for the population. 73.4% had an insufficient protein intake, defined as a PCRn <0.8g/kg/day. We observed a significant positive correlation between LTM and plasma creatinine (rho = 0.34; P=.013) and between LTM and albumin (rho = 0.3; P=.011).
Progression of protein-energy wasting over time
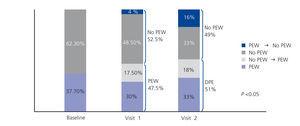
The prevalence of PEW remained constant over time: 40.5% at 12 months (visit 1) and 41.1% at 24 months (visit 2). However, when the muscle mass loss variable was introduced, the prevalence of PEW increased significantly to 47.3% at visit 1 and 50% at visit 2 (Figure 1). Of the 49 patients who did not have PEW at the baseline visit, 13 (26%) progressed to PEW at visit 1. Of the 28 patients who did not have PEW at visit 1, 10 (36%) progressed to PEW at visit 2. Recovery from PEW without any therapeutic intervention occurred in 3 (12%) of 25 patients with previous PEW at visit 1 and in 9 (30%) of 27 patients with previous PEW at visit 2. As can be seen in Table 1, the percentage of patients with malnutrition parameters according to the ISRMN criteria did not show any significant change between the the baseline visit, visit 1 and visit 2. However, when progression of the malnutrition biomarker values is analysed at the different visits, significant progression in the malnutrition status was observed (Table 2).
A significant decrease in prealbumin and plasma creatinine was seen at visit 1, as well as a significant increase in serum calcium, transferrin and type-B natriuretic peptide (pro-BNP) (Table 2). There were also significant increases in BMI, OH, total body water (TBW), ECW and ICW. ATM decreased significantly and LTM was unchanged.
A significant decrease in albumin, cholesterol and PTH levels were seen at visit 2, as was a significant increase in alkaline phosphatase, gamma-glutamyl transpeptidase (GGT) and pro-BNP levels. With regards to the anthropometric parameters, the BMI was unchanged, ICW and muscle mass decreased significantly and there was an insignificant tendency towards increased OH (Table 2).
Factors associated with protein-energy wasting
The clinical characteristics and phenotypes of patients with PEW were analysed (Table 3). Patients with PEW had significantly higher ERI, higher PPf, and greater OH despite having a lower TBW, lower ICW and ECW; lower triglyceride and transferrin levels were also seen. Despite there being no significant difference in inter-dialysis weight gain, they had an insignificant tendency towards a lower ultrafiltration rate with a significantly greater PPf. There were no significant differences with regards to age, gender or presence of cardiovascular disease or diabetes mellitus among the patients with and without PEW.
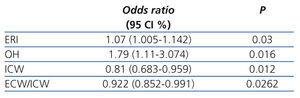
On the univariate regression model, the factors associated with PEW were ERI (OR: 1.07 [95%CI: 1.031-1.12]; P=.0005), triglycerides (OR: 0.994 [95%CI: 0.98-0.99]; P=.0075), pro-BNP (OR: 1.022 [95%CI: 1.005-1.045]; P=.0102), ECW (OR: 0.72 [95%CI: 0.569-0.877]; P=.00008), ICW (OR: 0.88 [95%CI: 0.78-0.97]; P=.0182), ultrafiltration (OR: 0.53 [95%CI: 0.308-0.885] P=.0145), diastolic blood pressure at the start of dialysis (OR: 1.021 [95% CI: 1.004-1.04]; P=.0146), PPf (OR: 1.038 [95% CI: 1.015-1.064]; P=.0008). On the multivariate regression model, the only clinical variables that were significant predictors for PEW were the level of OH, ERI, ICW and the ECW/ICW ratio (Table 4).
Protein-energy wasting and mortality
Of the 122 patients who started the study, 74 patients were alive at the end of the study, 56 of these 74 completed 34 months of follow-up, 26 died, 8 (31%) due to cardiovascular cause, 1 patients secondary to an underlying tumour, 5 (19%) due to infectious cause, 6 (23%) patients secondary to deterioration in their general status, 4 (15%) due to other causes and 2 (7%) due to unknown cause, 17 received transplants and 5 changed centres.
Fourteen (53.8%) of the patient who died had PEW. The Kaplan-Meier curve showed no differences in the risk of mortality between patients with and without PEW (Figure 2A). Given that PEW is the sum of several malnutrition criteria, we analysed each variable individually according to the ISRMN definition. The only malnutrition criteria which was associated with mortality was loss of muscle mass (Figure 2B).
DISCUSSION
This observational study underscores the high prevalence of PEW in haemodialysis patients and the dynamic aspect of a malnourished state over time. It is noted that the ERI is a malnutrition-associated factor. In addition, our results suggest that individual elements of the diagnostic criteria for PEW may have greater prognostic value than PEW itself in Spanish haemodialysis patients. Therefore, of the ISRMN malnutrition criteria, only loss of muscle mass is associated with mortality.
This study analysed for the first time the prevalence of PEW in haemodialysis in Spain. 40% of our dialysis population met the criteria for PEW. To date, there are not sufficient studies that evaluate the prevalence of PEW in other populations according to these criteria. This prevalence is slightly lower than that seen in other European or American studies that define malnutrition according to albumin, MIS (malnutrition and inflammation scale) or overall subjective evaluation: 74% of European patients in haemodialysis in the CONTRAST study,14 38% out of 331 patients in the United States15 or 39% of 221 patients on haemodialysis in the north of Europe.16 Similar data are seen for peritoneal dialysis, with 65% of 199 patients in peritoneal dialysis in Brazil.9 20 years ago in Spain, 65% of 29 patients in haemodialysis had protein-caloric malnutrition, defined using other anthropometric criteria, visceral proteins such as albumin, PCRn and protein intake.17 The reductions in fat and protein reserves allowed for the identification of malnourished patients who were not identified by relative body weight, which only showed a deficit in 38% of patients. In the same year, the Cooperative Study on Nutrition in Haemodialysis analysed malnutrition in 761 haemodialysis patients based on anthropometric parameters and biochemical markers (albumin, transferring, lymphocyte count) and concluded that the prevalence of moderate-severe malnutrition was 52% in men and 46% in women.18,19 These results are very similar to those obtained in this study using different criteria. However, none of the previous studies analysed the impact of their respective definitions of malnutrition on mortality.
PEW defines malnutrition dynamically and takes into account the loss or deterioration criterion over time. This implies that a static analysis may underestimate its prevalence. This study analysed PEW statically at the baseline visit and then dynamic criteria were added at subsequent visits. By adding the dynamic criterion of loss of muscle mass, the prevalence of PEW increases significantly and provides more information on the prevalence of malnutrition than MUAMC and BMI data as these data do not vary based on the ISRMN criteria. In addition, there is progression of the biochemical and anthropometric parameters without meeting the ISRMN criteria, which is a signal of progression of malnutrition.
Among the biochemical parameters, a decrease in prealbumin was observed in the first year, leading to a decrease in albumin levels, which was observed a year later. These data confirm that prealbumin (thyroxine/retinol-binding protein transporter protein), which has a shorter half life than albumin, is an earlier indicator of protein malnutrition that recovers quickly at the start of supplementation therapy.20,21 Both albumin and prealbumin are considered good biomarkers for malnutrition and have been associated with mortality in dialysis populations.22,23 Another biochemical parameter for malnutrition and muscle mass is plasma creatinine.24 In this study, plasma creatinine gradually and significantly decreased over the first years and was accompanied by a significant decrease in muscle mass as measured by bioimpedance (LTM) at the end of the study. We observed a positive correlation between LTM and biochemical markers such as plasma creatinine and albumin. In this regard, a formula has recently been published for calculating LTM based on plasma creatinine in haemodialysis patients without residual diuresis,25 as well as measurement of plasma creatinine kinetics as a marker of muscle loss as a good predictor of mortality in haemodialysis.26 The decrease in plasma creatinine in our observation preceded the loss of muscle mass by several months when evaluated by LTM; meanwhile, according to the ISRMN criteria, patients with PEW had lower, though insignificantly, levels compared to patients without PEW. The individual tendency towards a decrease appears to us to be of high diagnostic value.
The association between volume overload and malnutrition has been described previously,27 though more in patients on peritoneal dialysis than on haemodialysis. Post-dialysis OH in our study was not only a risk factor independently associated with the presence of PEW, but also increased progressively on subsequent visits. It is true that this increase was never greater than one liter when compared to dry weight and, therefore, was not considered a pathologic finding. The OH measurement indicates excess ECW. There are different formulas for expressing OH, such as the phase angle,28 ECW/TBW ratio29 or the ECW/ICW ratio.30 A new formula has recently been postulated for calculating OH given that the haemodialysis patient suffers changes in both volume and location between the different compartments. This is known as ECW over dry weight (ECW/body weight).31 This concept is important because malnourished uraemic patients have, by definition, a lower BMI, which implies less ATM and less LTM. One has to remember that ICW and ECW exist in both the ATM and LTM compartments, though in different proportions. On the univariate analysis, having low ECW and ICW is a risk factor for malnutrition. However, the OH indicator on multivariate analysis (excess ECW over patient weight) was associated with a higher risk of malnutrition and the ECW/ICW ratio was even a protective factor, as has been shown in previous studies.32 The ECW/ICW ratio is not a good indicator of OH since it does not take variability between the ATM and LTM compartments into account. For this reason, after our analysis we can say that malnutrition is associated with OH, an increase in ECW (due to interstitial water) due to occupation of the lean mass compartment, which is the first compartment to be altered, far sooner than fat since the body demands immediate energy which only muscle can provide.
Interpreting the OH status of malnourished patients is difficult in daily clinical practice. Hypotension and muscle cramps reflect a reduction in plasma volume but not necessarily ECW. When the ultrafiltration rate is greater than the refilling rate, blood pressure falls, even if there is excessive ECW.33 Our patients with PEW criteria had a higher total OH status, but the desired liquid was able to be filtered during the study period without a reduction in blood pressure. The difficulty in ultrafiltering excess ECW in malnourished patients may be due to less passage of the water from the interstitial to the intravascular space due to decreased oncotic pressure (decreased albumin).24 Blood pressure may not drop since the refilling capacity is maintained despite PEW and the onset of a vasoconstriction response. It appears clear that the PEW situation does not mean less refilling in all the cases. In addition, the OH status carries with it an increase in pulse pressure in patients with significantly higher PEW compared to patients without PEW,35 and as has been described in previous studies, the increase in pulse pressure is associated with an increase in the risk of cardiovascular mortality.36 We did not find a higher PPi, though we did find a higher PP afterwards. Nevertheless, the drop in pressure with the dialysis session was similar between patients with and without PEW and no significant differences were seen in inter-dialysis weight gain while an insignificant tendency towards less total ultrafiltration was seen. The malnutrition state in our study did not imply poorer tolerance to ultrafiltration in dialysis, although it represents total OH.
Another independently associated factor to malnutrition in our study was the ERI. Interest has grown in recent years due to the association between dialysis, inflammation, malnutrition and low response to EPO. The presence of high levels of inflammation markers and biochemical parameters for malnutrition (low albumin and prealbumin) is associated with a low response to EPO.37 The decrease in systemic inflammation may increase the response to EPO, as has been demonstrated in randomised studies in which changes in the dialysis technique resulted in a significant reduction in CRP or interleukin-6 levels and an improvement in response to EPO.38 A deficit in micronutrients such as folic acid may also increase resistance to EPO.39 The presence of PEW is strongly associated with systemic inflammation and a cardiovascular risk in dialysis patients.40
Finally, we studied whether malnutrition according to the ISRMN criteria may be a good mortality marker in our population. No association was seen between PEW and mortality, confirming previous studies such as CONTRAST, which lead us to believe that this classification arose for diagnostic purposes for patients at risk of malnutrition and not for prognostic purposes. The CONTRAST study observed that individual nutritional parameters such as albumin and plasma creatinine in 560 haemodialysis patients were independently associated with mortality to the same degree and magnitude as several combined nutrition factors.14 In our population, the only malnutrition marker which was associated with mortality according to the ISRMN criteria was loss of muscle mass, while a BMI <23kg/m2 (figure 2 C) or a low MUAMC was not associated with mortality. This suggests that BMI or MUAMC may not be good indicators of loss of muscle mass on haemodialysis.41 According to recent studies, patients who have a greater risk of mortality are those who have more muscle mass loss. Kalantar et al. examined changes in weight over time in 121 762 patients and they observed that the risk of mortality increased with the BMI, plasma creatinine and weight decreased, but the risk of mortality was reduced in patients in which weight decreased but plasma creatinine increased.42 For these authors, the commonly used BMI or even dry weight were poor markers of nutritional status since they did not differentiate between protein loss or water gain. In addition, Agarwal et al.43 observed that BMI is not a good indicator for distinguishing between fat and muscle mass and that patients with a high BMI had a higher survival due to an increase in muscle mass.44 The loss of muscle mass seen in our study as measured by bioimpedance was more relevant than BMI and MUAMC for identifying patients with higher protein catabolism and risk of mortality. The criterion BMI <23kg/m2 according to the PEW concept may not reflect the true malnutrition status for our population given that it was described based on a United States population. Re-evaluation is needed for a Mediterranean-European population.
In conclusion, PEW was highly prevalent and has a dynamic character in haemodialysis patients. A dynamic concept, the loss of muscle mass over time, reflects the wasting suffered by haemodialysis patients and is associated with greater mortality. Our study suggests an association between loss of muscle mass and mortality. Intervention studies are needed in order to evaluate if the prevention of sarcopaenia improves survival. As pointed out by other authors, we may need to rethink the definition of the diagnostic criteria used in PEW in European and Mediterranean patients in the future, as our results appear to suggest.
Acknowledgements
RETIC REDINREN of the ISCIII (RD12/0021/0001).
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Prevalence of protein-energy wasting based on International Society of Renal Metabolism and Nutrition criteria in 122 prevalent haemodialysis patients.
Table 2. Progression of malnutrition and body composition parameters in 122 patients in haemodialysis over three years
Table 3. Clinical characteristics of the 122 haemodialysis patients according to the presence or absence of protein-energy wasting
Table 4. Odds ratios and 95% confidence intervals for the presence of protein-energy wasting in 122 prevalent haemodialysis patients.
Figure 1. Prevalence of protein-energy wasting in 122 patients analyzed at three visits
Figure 2. A) Kaplan-Meier survival curve based on the presence of protein-energy wasting or B) based on loss of muscle mass or C) based on a body mass index <23kg m2 in 122 haemodialysis patients