Dialysis fluid (DF), an essential element in hemodialysis (HD), is manufactured in situ by mixing three components: treated water, bicarbonate concentrate and acid concentrate. To avoid the precipitation of calcium and magnesium carbonate that is produced in DF by the addition of bicarbonate, it is necessary to add an acid. There are 2 acid concentrates that contain acetate (ADF) or citrate (CDF) as a stabilizer.
ObjectiveTo compare the acute effect of HD with CDF vs. ADF on the metabolism of calcium, phosphorus and magnesium, acid base balance, coagulation, inflammation and hemodynamic stability.
MethodsProspective, multicenter, randomized and crossed study, of 32 weeks duration, in patients in three-week HD, AK-200-Ultra-S or Artis monitor, 16 weeks with ADF SoftPac®, prepared with 3mmol/L of acetate, and 16 weeks with CDF SelectBag Citrate®, with 1mmol/L of citrate. Patients older than 18 years were included in HD for a minimum of 3 months by arteriovenous fistula. Epidemiological, dialysis, pre and postdialysis biochemistry, episodes of arterial hypotension, and coagulation scores were collected monthly during the 8 months of the study. Pre and post-dialysis analysis were extracted: venous blood gas, calcium (Ca), ionic calcium (Cai), phosphorus (P), magnesium (Mg) and parathyroid hormone (PTH) among others.
ClinicalTrials.gov NCT03319680.
ResultsWe included 56 patients, 47 (84%) men and 9 (16%) women, mean age: 65.3 (16.4) years, technique HD/HDF: 20 (35.7%)/36 (64.3%).
We found differences (p<0.05) when using the DF with citrate (C) versus acetate (A) in the postdialysis values of bicarbonate [C: 26.9 (1.9) vs. A: 28.5 (3)mmol/L], Cai [C: 1.1 (0.05) vs. A: 1.2 (0.08)mmol/L], Mg [C: 1.8 (0.1) vs A: 1, 9 (0.2)mg/dL] and PTH [C: 255 (172) vs. 148 (149)pg/mL]. We did not find any differences in any of the parameters measured before dialysis. Of the 4416 sessions performed, 2208 in each group, 311 sessions (14.1%) with ADF and 238 (10.8%) with CDF (p<0.01), were complicated by arterial hypotension. The decrease in maximum blood volume measured by Hemoscan® biosensor was also lower [−3.4 (7.7) vs −5.1 (8.2)] although without statistical significance.
ConclusionDialysis with citrate acutely produces less postdialysis alkalemia and significantly modifies Ca, Mg and PTH. CDF has a positive impact on hemodynamic tolerance.
El líquido de diálisis (LD), elemento esencial en la hemodiálisis (HD), se fabrica in situ mezclando 3 componentes: agua tratada, concentrado de bicarbonato y concentrado ácido. Para evitar la precipitación de carbonato cálcico y magnésico que se produce en el LD por la adición de bicarbonato, es necesario añadir un ácido. Existen 2 concentrados ácidos según contengan acetato (LDA) o citrato (LDC) como estabilizante.
ObjetivoComparar el efecto agudo de la HD con LDC vs. LDA sobre el metabolismo del calcio, fosforo y magnesio, el equilibrio ácido base, la coagulación, inflamación y la estabilidad hemodinámica.
MétodosEstudio prospectivo, multicéntrico, aleatorizado y cruzado, de 32 semanas de duración, en pacientes en HD trisemanal, monitor AK-200-Ultra-S o Artis, 16 semanas con LDA SoftPac®, elaborado con 3mmol/l de acetato, y 16 semanas con LDC SelectBag Citrate®, con 1mmol/l de citrato. Se incluyeron pacientes mayores de 18 años en HD durante un mínimo de 3 meses mediante fístula arteriovenosa. Se recogieron datos epidemiológicos, de diálisis, bioquímica pre- y posdiálisis, episodios de hipotensión arterial, y scores de coagulación mensualmente durante los 8 meses de estudio. Se extrajeron pre- y posdiálisis: gasometría venosa, calcio (Ca), calcio iónico (Cai), fósforo (P), magnesio (Mg) y hormona paratiroidea (PTH), entre otros.
ClinicalTrials.gov NCT03319680.
ResultadosSe incluyeron 56 pacientes, 47 (84%) hombres y 9 (16%) mujeres de edad media: 65,3 (16,4) años, técnica HD/HDF: 20 (35,7%)/36 (64,3%).
Encontramos diferencias (p<0,05) cuando utilizamos el LD con citrato (C) frente a acetato (A) en los valores posdiálisis de bicarbonato [C: 26,9 (1,9) vs. A: 28,5 (3) mmol/l], Cai [C: 1,1 (0,05) vs A: 1,2 (0,08) mmol/l], Mg [C. 1,8 (0,1) vs A: 1,9 (0,2) mg/dl] y PTH [C: 255 (172) vs. 148 (149) pg/ml]. No encontramos diferencias en ninguno de los parámetros medidos prediálisis. Se registraron menos episodios de hipotensión arterial durante las sesiones con el LDC; de las 4.416 sesiones de HD, 2.208 en cada grupo, cursaron con hipotensión 311 sesiones (14,1%) con LDA y 238 (10,8%) con LDC (p<0,01). También fue menor la caída de volumen sanguíneo máximo medido por biosensor Hemoscan® [−3,4(7,7) vs. −5,1 (8,2)], aunque sin significación estadística.
ConclusiónLa diálisis con citrato produce de forma aguda menor alcalemia posdiálisis y modifica de forma significativa el Ca, el Mg y la PTH. El LDC tiene un impacto positivo sobre la tolerancia hemodinámica.
The dialysis fluid (DF) is an essential element in the dialysis procedure since there is transference of different components between DF and patient. Therefore, the physical, microbiological and chemical composition of LD is essential for treatment success or failure. The recommendations on DF quality and purity are included in the Dialysis Liquid Quality Management Guidelines of the Spanish Society of Nephrology.1
From the technical point of view, to avoid the precipitation of calcium and magnesium carbonate that occurs in the DF when adding bicarbonate, it is necessary to add an acid, so the generation of DF system needs 2 concentrates, one with bicarbonate and the other with acid. The acid is usually acetic acid, at concentrations ranging between 3 and 10mmol/L. This small amount of acetate is transferred to the patient during HD, increasing its concentration in blood, since DF has concentrations 30–40 times higher than normal values in blood (0.1mmol/L). This exposure to acetate increases in techniques of online hemodiafiltration (OL-HDF)2 due to the greater amount of fluid infused. Among the side effects described with acetate are hemodynamic instability produced by vasodilation mediated by the release of nitric oxide3 and hypoxia induced activation of proinflammatory cytokines.4 Even with DF with small concentrations of acetate (3mmol/L), hemodynamic complications are present if compared with acetate-free DF.5
For years other acids have been sought as DF stabilizers. The first attempts to replace the acetate were made with hydrochloric acid. However, the relationship between sodium concentration and conductivity was modified, producing changes in the serum ions, so it was necessary to change the total and partial conductivities of bicarbonate, and its use was not clearly standardized.
The use of citrate in the DF (CDF) was developed in an attempt to improve biocompatibility by substituting acetic acid for another more physiological compound, citric acid. Citrate chelates calcium (Ca), in fact, it is used as anticoagulant by decreasing ionic calcium (Cai). It is estimated that it produces a 10% decrease in Cai, this is why most authors recommend to supplement DF with calcium when citrate is being used as an acid. As demonstrated by Steckiph et al.,6 for each mmol of citrate, the calcium concentration should increase 0.15mmol/L to maintain the calcium balance during treatment and prevent hypocalcemia. The use of CDF is increasing and presently in Spain is a therapeutic option. There are 2 CDFs, one without acetate, which is the CDF that we have use in the present study: SelectBag Citrate®, with 1mmol/L of citrate. Another CDF, Citrasate® contains small amounts of acetate, 0.3mmol/L and 0.8mmol/L of citrate. Although the CDF has been commercialized for more than 15 years, initially in the USA,7 there is limited information on the possible metabolic, coagulation or hemodynamic effects based on randomized studies. Several long-term beneficial effects have been described in relation to the use of citrate including reduced thrombogenicity,8 increased clearances,9,10 improvement of inflammation,11 nutrition,12 tolerance,13 and acid-base control with less predialysis acidosis.14 Our multicenter, randomized and controlled study aims to provide evidence to some of these issues.
Citrate is used as an anticoagulant in continuous dialysis therapies. Monchi et al.15 conclude that anticoagulation with citrate was superior to heparin to preserve the dialyzer function and reduce transfusion requirements in Intensive Care patients on continuous substitution therapies in which citrate concentration is much higher than in CDF. During dialysis with CDF, citrate binds to the circulating calcium so less calcium is available for the coagulation cascade, and the heparin requirement are reduced8; consequently, the useful surface of the dialyzer will be preserved improving clearance and dialytic efficacy. It is necessary to confirm the potential benefits of this anticoagulant effect and the impact of CDF on hemodynamic tolerance, acid base balance and metabolism of calcium and phosphate.
The objective of the present study is to compare the acute effect of HD with CDF vs. ADF on acid base balance, calcium, phosphorus and magnesium metabolism, dialytic efficacy, coagulation, inflammation and hemodynamic stability.
Material and methodsStudy designProspective, multicenter, randomized and cross-over study, performed in 8 Spanish Hemodialysis Units. All patients signed informed consent. The study protocol was approved by the Clinical Research Committee of the Community of Madrid and each participating center, and it was developed according to the Declaration of Helsinki (ClinicalTrials.gov, NCT03319680).
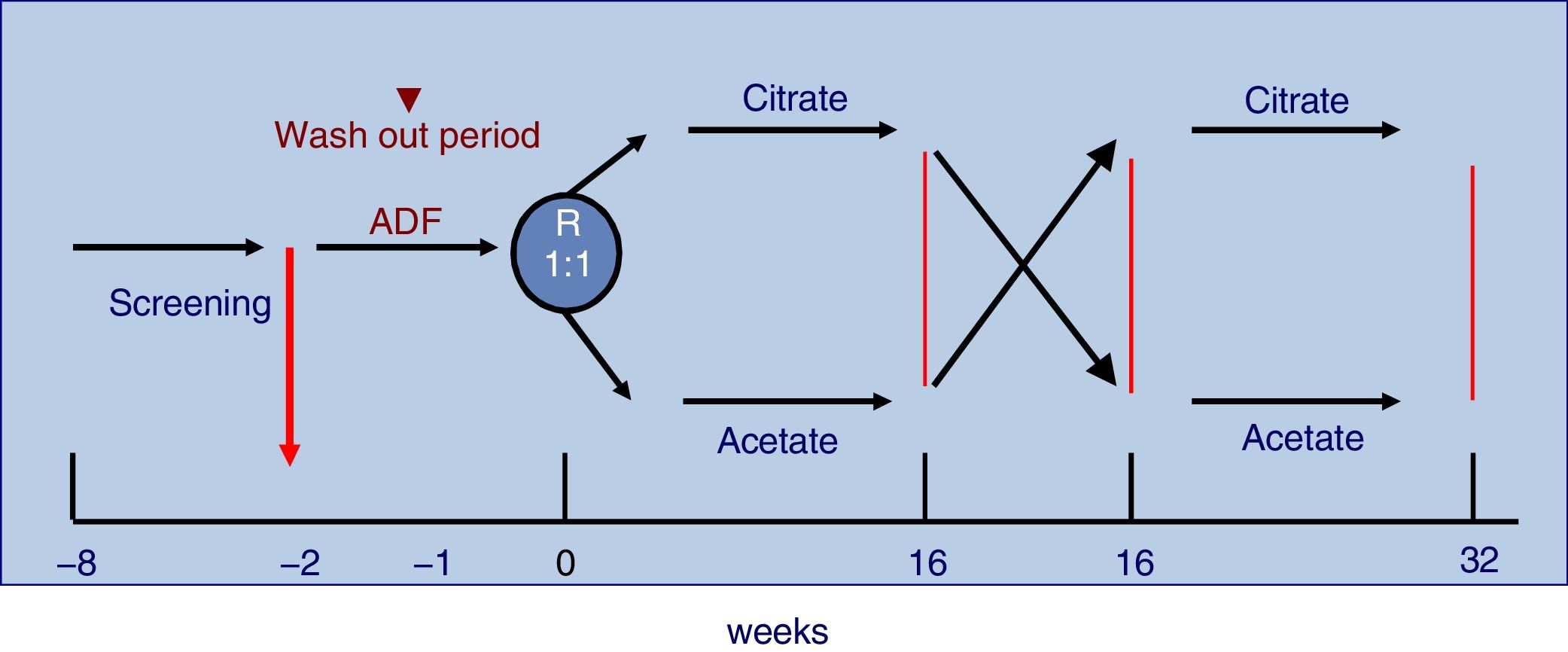
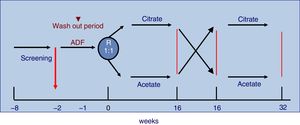
The study was 32-week long including patients on three times per week HD with monitor AK-200-Ultra-S or Artis. Patients were randomly assigned to receive 16 consecutive weeks CDF, SelectBag Citrate®, with 1mmol/L of citrate, followed or preceded by another 16 weeks with ADF, SoftPac®, containing 3mmol/L of acetate. Before the randomization process, all patients were dialyzed for two weeks with ADF. Each patient followed the usual work schedule and served as its own control. No changes were made in the dialysis regimen during the study except for DF. The study design is shown in Fig. 1.
Patients were older than 18 years and had been on regular HD for more than 3 months. All had arteriovenous fistula (native or prosthetic) and signed the informed consent. Exclusion criteria were allergy or citrate intolerance, intercurrent inflammatory diseases, HD through catheter and cognitive impairment. In the design of the study, given that one of the objectives was to assess the dialysis efficacy and the study duration was 32 weeks, it was decided to exclude patients with a catheter to avoid the possibility of dysfunction or loss of the vascular access.
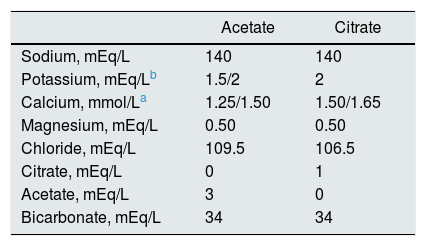
The characteristics of the dialysis fluid containing citrate and acetate are shown in Table 1. Two different calcium concentrations were used in the dialysis fluid; such a concentration depended on the regular calcium concentration that each patient used before entering the study. If the previous calcium in the ADF were 1.25 or 1.50mmol/L, the concentrations in the CDF were increased to 1.50 and 1.65mmol/L respectively.
Parameters collectedDemographic and dialysis parameters: age, underlying disease, weight, time in renal replacement therapy (TRR) and residual kidney function (RKF) as previously defined,16 dialysis technique: high-flow HD (HF-HD) or on line hemodiafiltration (OL-HDF), blood flow (Qb), sodium and bicarbonate conductivities, temperature of the DF, infusion volumes in OL-HDF (post-dilution), the Kt automatically measured by the Diascan® biosensor, interdialysis weight gain (WG), maximal reduction of blood volume (MRBV) during the dialysis session as measured by Hemoscan® biosensor, ultrafiltration volume (UF) per session and blood pressure (BP) before and after dialysis.
The number of hypotension episodes, defined as any acute decrease in blood pressure that requires the intervention of nursing personnel, was also collected.
Analytical determinations: All blood samples were obtained from the arterial line of the vascular access in the midweek dialysis session. Blood was drawn pre-dialysis, immediately before starting the procedure and post-dialysis after reduction of the Qb to 50mL/min for 60s at the end of the session.
Blood parameters: Venous blood acid–base parameters were determined by gasometer (pH, bicarbonate and excess of base in the extracellular fluid (BEecf)). Biochemical parameters: serum concentrations of sodium (Na), chloride (Cl), total calcium (Ca) and ionized (Cai), phosphate (P), parathyroid hormone (PTH), urea, beta2 microglobulin (β2m), albumin and prealbumin. Blood count and coagulation parameters
The methods used were standard and similar in all centers. No modification in methodology was allowed during the study.
Evaluation of the nutritional status included not only blood parameters but also analysis of body composition by multifrequency bioimpedance (BCM®). These measurements were made immediately before the midweek dialysis session (Wednesday or Thursday). Patients remained in the supine position for 10min, without metallic objects. The electrodes were placed in the part of the body contralateral to the vascular access. The hydration (OH) and body composition data were collected: fat tissue index (FTI) and lean tissue index (LTI), are defined respectively as fat and lean tissue adjusted to the patient's body surface area (kg/m2).
The state of inflammation was assessed by serum of C-reactive protein (CRP) and the index of resistance to erythropoietin (equivalent to EPOalpha) (ERI) using the formula: ERI=EPO dose (U/kg/wk)/Hb (g/dl).
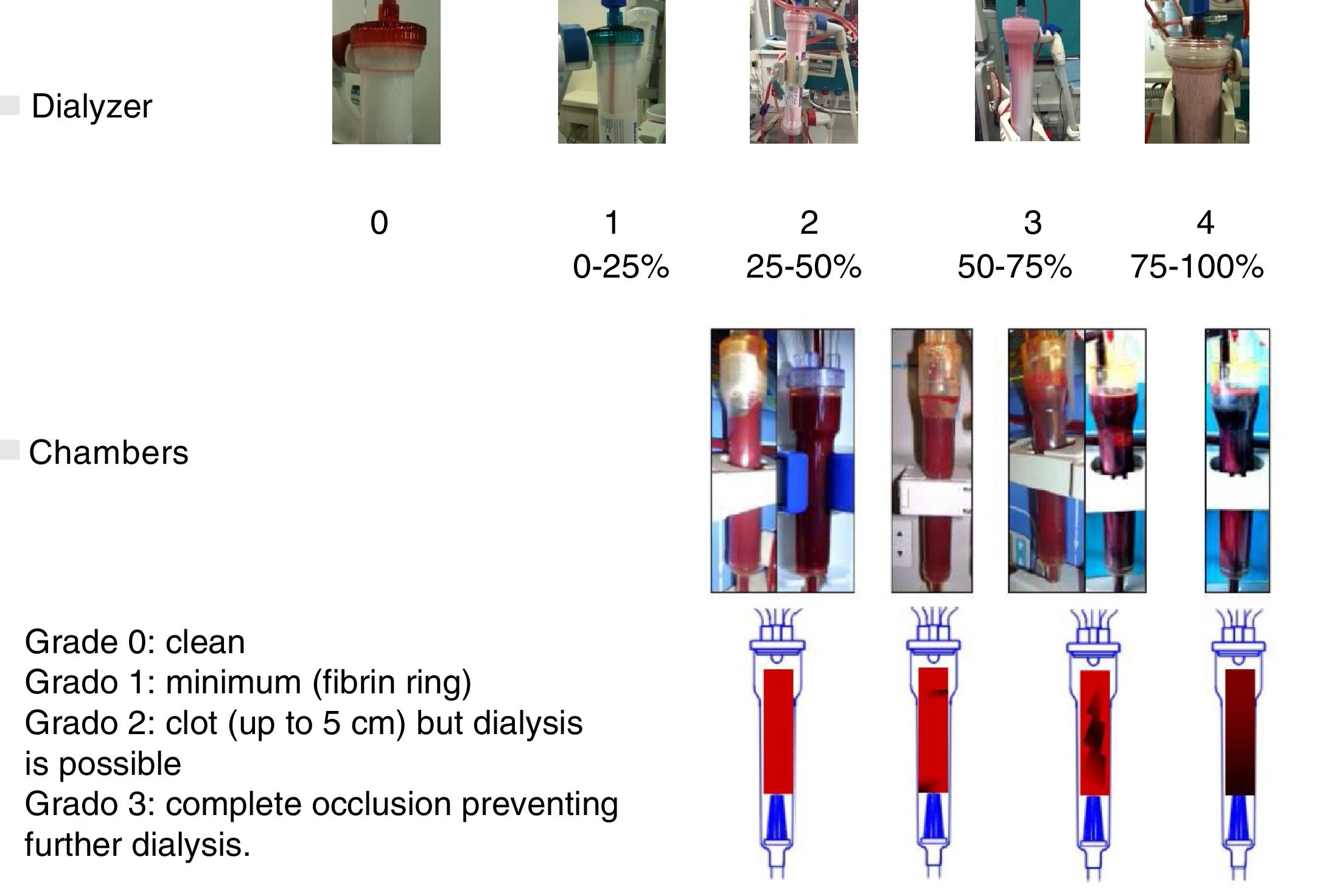
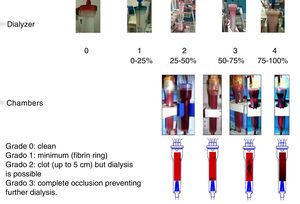
The effect of CDF on coagulation was evaluated using two coagulation scores. A visual scale of the dialyzer, managed by nursing staff, graded from 0 to 4 depending on the percentage of coagulated fibers at the end of the dialysis session. No evidence of clotted fibers, score 0; less than <25%, score 1; from 25 to 50%, score 2; from 50 to 75%, score 3; and from 75 to 100%, with impossibility to continue the dialysis, score 4. The coagulation of the chambers was scored separately: 0 if they were clean, 1 if the coagulation was minimal (fibrin ring), 2 if a clot was present (up to 5cm) but did not prevent the continuation of the dialysis procedure, and 3 if there was a complete occlusion that prevented dialysis. The chamber and dialyzer coagulation scores are shown in Fig. 2. The time of hemostasis was also collected, quantifying the minutes that were needed for the coagulation of the vascular access punctures site.
The study was registered at ClinicalTrials.gov NCT03319680.
Statistical analysisThe statistical analysis performed with the SPSS 15.0 program (SPSS INC., Chicago IL, USA). Descriptive data are presented as the mean and standard deviation (SD).
The Student t test for paired samples was used to compare of two continuous variables. ANOVA was used to compare o more than two quantitative variables. A p value <0.05 was considered statistically significant.
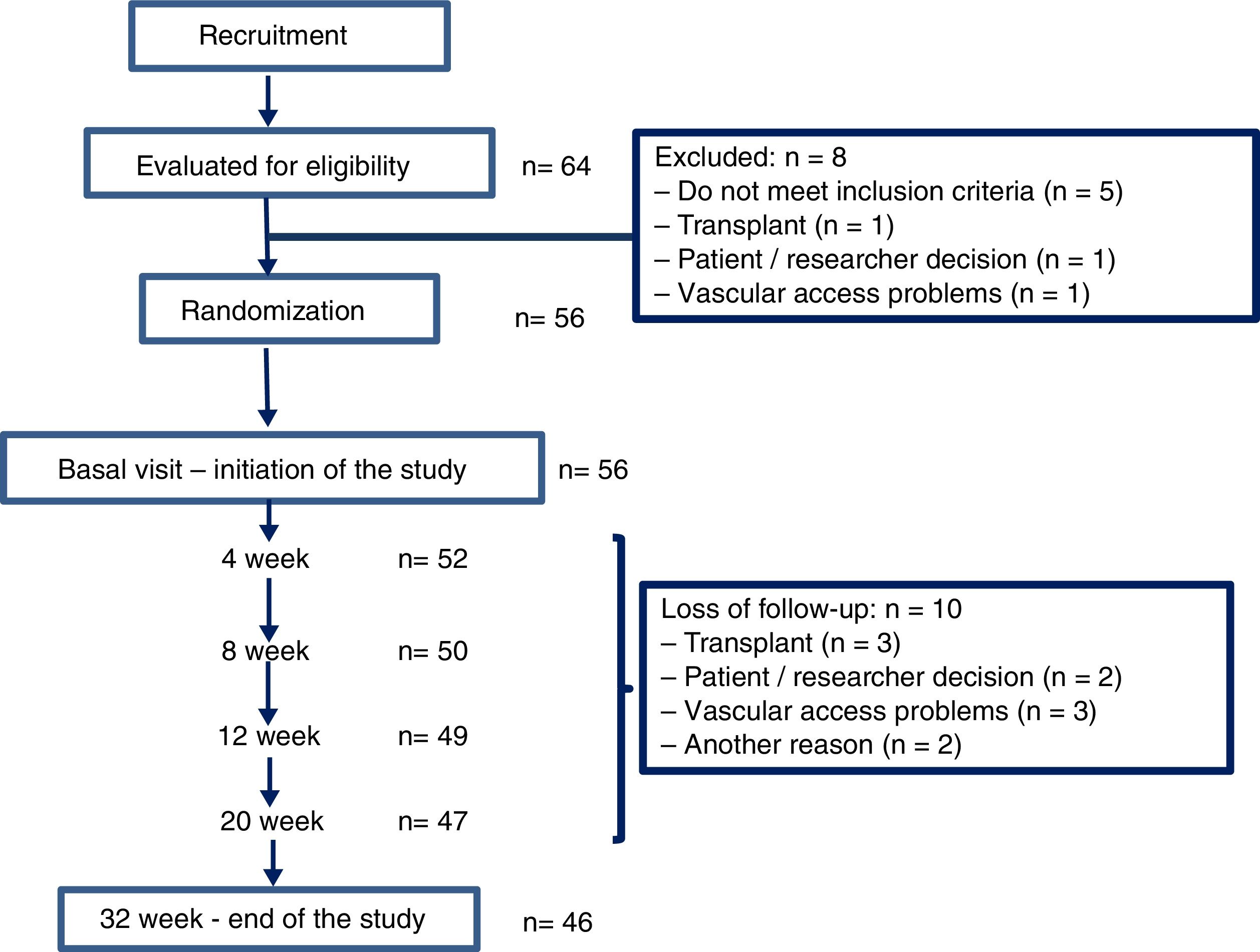
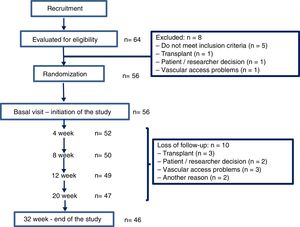
ResultsThere were 64 hemodialysis patients initially evaluated in 2015. Fiftysix patients met the inclusion criteria and were included in the study. The flow chart illustrating the progress of the patients during the trial is depicted in Fig. 3. Their average age was 65.3 (16.4) [23–93] years, 47 men and 9 women, with an adjusted Charlson index of 6.2 (2.5). The etiology of CKD was 16 glomerulonephritis, 10 diabetes, 9 vascular disease, 4 hereditary disease, 4 interstitial nephritis, 9 other causes and 4 unknown etiology. Fifty one patients (91.1%) had native AVF and 5 (8.9%) a prosthetic AVF.
Thirty six patients were on OL-HDF and 20 on HF-HD.
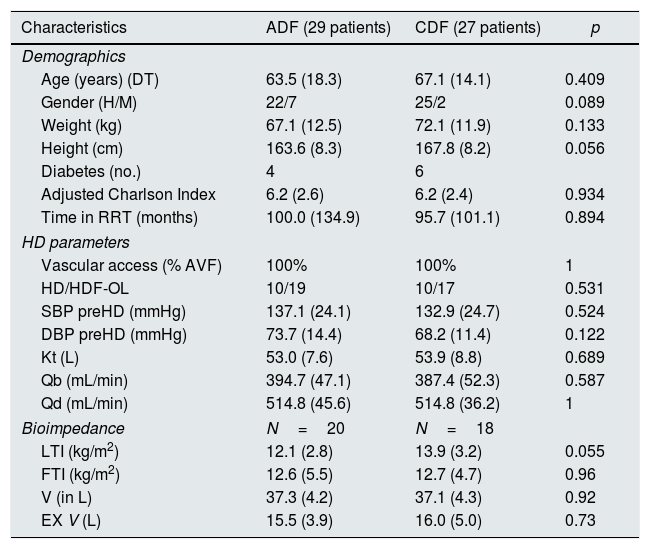
Randomization assigned 29 patients to the ADF and to 27 to CDF. At week 16 the DF were switched from ADF to CDF and vice versa for another 16 additional weeks. Table 2 describes the baseline characteristics of the patients according to the group to which they were assigned.
Baseline characteristics of the patients according to the group to which they were randomized, ADF/CDF (N=56).
| Characteristics | ADF (29 patients) | CDF (27 patients) | p |
|---|---|---|---|
| Demographics | |||
| Age (years) (DT) | 63.5 (18.3) | 67.1 (14.1) | 0.409 |
| Gender (H/M) | 22/7 | 25/2 | 0.089 |
| Weight (kg) | 67.1 (12.5) | 72.1 (11.9) | 0.133 |
| Height (cm) | 163.6 (8.3) | 167.8 (8.2) | 0.056 |
| Diabetes (no.) | 4 | 6 | |
| Adjusted Charlson Index | 6.2 (2.6) | 6.2 (2.4) | 0.934 |
| Time in RRT (months) | 100.0 (134.9) | 95.7 (101.1) | 0.894 |
| HD parameters | |||
| Vascular access (% AVF) | 100% | 100% | 1 |
| HD/HDF-OL | 10/19 | 10/17 | 0.531 |
| SBP preHD (mmHg) | 137.1 (24.1) | 132.9 (24.7) | 0.524 |
| DBP preHD (mmHg) | 73.7 (14.4) | 68.2 (11.4) | 0.122 |
| Kt (L) | 53.0 (7.6) | 53.9 (8.8) | 0.689 |
| Qb (mL/min) | 394.7 (47.1) | 387.4 (52.3) | 0.587 |
| Qd (mL/min) | 514.8 (45.6) | 514.8 (36.2) | 1 |
| Bioimpedance | N=20 | N=18 | |
| LTI (kg/m2) | 12.1 (2.8) | 13.9 (3.2) | 0.055 |
| FTI (kg/m2) | 12.6 (5.5) | 12.7 (4.7) | 0.96 |
| V (in L) | 37.3 (4.2) | 37.1 (4.3) | 0.92 |
| EX V (L) | 15.5 (3.9) | 16.0 (5.0) | 0.73 |
ADF: dialysis liquid with acetate; CDF: dialysis liquid with citrate; RRT: renal replacement therapy; AVF: arteriovenous fistula; HD: hemodialysis; OL-HDF: online hemodiafiltration; SBP: systolic blood pressure; DBP: diastolic blood pressure; Qb: blood flow; Qd: flow of dialysis fluid; LTI: lean mass index; FTI: fat mass index; V: volume of body water; EXV: extracellular volume.
The results being presented correspond to the acute effect of DF, which we consider to occur during the dialysis session, such as hypotension or coagulation and changes in blood parameters from pre to post dialysis. For this we have taken the results of the baseline visit corresponding to the pre/postdialysis values after the washout period with acetate, once the randomization was done that corresponded to 56 patients. Later some of the results these pre/post values throughout the study will be commented.
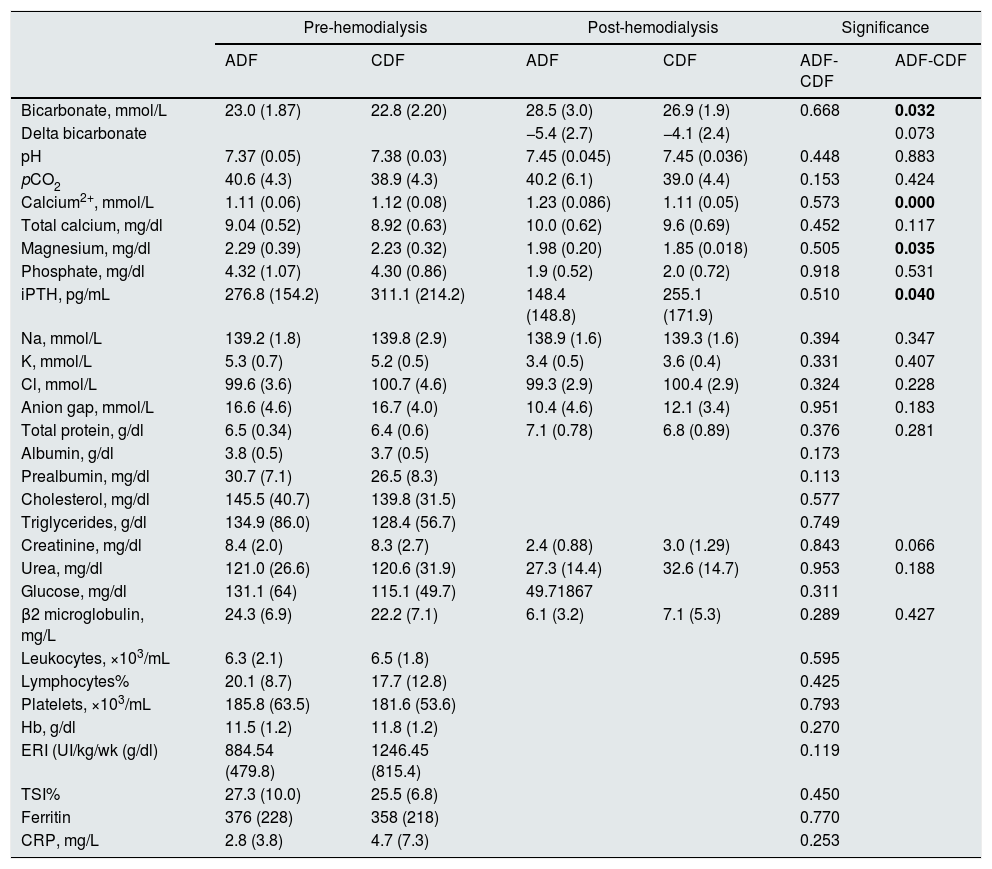
Table 3 shows the pre-hemodialysis acid-base values, biochemistry, PTH, and hematological parameters in ADF and CDF groups. Likewise, the post-hemodialysis acid base values, some biochemical and PTH parameters are also shown.
Laboratory data, pre and post HD, in 56 patients on ADF or CDF.
| Pre-hemodialysis | Post-hemodialysis | Significance | ||||
|---|---|---|---|---|---|---|
| ADF | CDF | ADF | CDF | ADF-CDF | ADF-CDF | |
| Bicarbonate, mmol/L | 23.0 (1.87) | 22.8 (2.20) | 28.5 (3.0) | 26.9 (1.9) | 0.668 | 0.032 |
| Delta bicarbonate | −5.4 (2.7) | −4.1 (2.4) | 0.073 | |||
| pH | 7.37 (0.05) | 7.38 (0.03) | 7.45 (0.045) | 7.45 (0.036) | 0.448 | 0.883 |
| pCO2 | 40.6 (4.3) | 38.9 (4.3) | 40.2 (6.1) | 39.0 (4.4) | 0.153 | 0.424 |
| Calcium2+, mmol/L | 1.11 (0.06) | 1.12 (0.08) | 1.23 (0.086) | 1.11 (0.05) | 0.573 | 0.000 |
| Total calcium, mg/dl | 9.04 (0.52) | 8.92 (0.63) | 10.0 (0.62) | 9.6 (0.69) | 0.452 | 0.117 |
| Magnesium, mg/dl | 2.29 (0.39) | 2.23 (0.32) | 1.98 (0.20) | 1.85 (0.018) | 0.505 | 0.035 |
| Phosphate, mg/dl | 4.32 (1.07) | 4.30 (0.86) | 1.9 (0.52) | 2.0 (0.72) | 0.918 | 0.531 |
| iPTH, pg/mL | 276.8 (154.2) | 311.1 (214.2) | 148.4 (148.8) | 255.1 (171.9) | 0.510 | 0.040 |
| Na, mmol/L | 139.2 (1.8) | 139.8 (2.9) | 138.9 (1.6) | 139.3 (1.6) | 0.394 | 0.347 |
| K, mmol/L | 5.3 (0.7) | 5.2 (0.5) | 3.4 (0.5) | 3.6 (0.4) | 0.331 | 0.407 |
| Cl, mmol/L | 99.6 (3.6) | 100.7 (4.6) | 99.3 (2.9) | 100.4 (2.9) | 0.324 | 0.228 |
| Anion gap, mmol/L | 16.6 (4.6) | 16.7 (4.0) | 10.4 (4.6) | 12.1 (3.4) | 0.951 | 0.183 |
| Total protein, g/dl | 6.5 (0.34) | 6.4 (0.6) | 7.1 (0.78) | 6.8 (0.89) | 0.376 | 0.281 |
| Albumin, g/dl | 3.8 (0.5) | 3.7 (0.5) | 0.173 | |||
| Prealbumin, mg/dl | 30.7 (7.1) | 26.5 (8.3) | 0.113 | |||
| Cholesterol, mg/dl | 145.5 (40.7) | 139.8 (31.5) | 0.577 | |||
| Triglycerides, g/dl | 134.9 (86.0) | 128.4 (56.7) | 0.749 | |||
| Creatinine, mg/dl | 8.4 (2.0) | 8.3 (2.7) | 2.4 (0.88) | 3.0 (1.29) | 0.843 | 0.066 |
| Urea, mg/dl | 121.0 (26.6) | 120.6 (31.9) | 27.3 (14.4) | 32.6 (14.7) | 0.953 | 0.188 |
| Glucose, mg/dl | 131.1 (64) | 115.1 (49.7) | 49.71867 | 0.311 | ||
| β2 microglobulin, mg/L | 24.3 (6.9) | 22.2 (7.1) | 6.1 (3.2) | 7.1 (5.3) | 0.289 | 0.427 |
| Leukocytes, ×103/mL | 6.3 (2.1) | 6.5 (1.8) | 0.595 | |||
| Lymphocytes% | 20.1 (8.7) | 17.7 (12.8) | 0.425 | |||
| Platelets, ×103/mL | 185.8 (63.5) | 181.6 (53.6) | 0.793 | |||
| Hb, g/dl | 11.5 (1.2) | 11.8 (1.2) | 0.270 | |||
| ERI (UI/kg/wk (g/dl) | 884.54 (479.8) | 1246.45 (815.4) | 0.119 | |||
| TSI% | 27.3 (10.0) | 25.5 (6.8) | 0.450 | |||
| Ferritin | 376 (228) | 358 (218) | 0.770 | |||
| CRP, mg/L | 2.8 (3.8) | 4.7 (7.3) | 0.253 | |||
ADF/dialysis fluid with acetate, CDF/dialysis liquid with citrate; pCO2: partial pressure of carbon dioxide; iPTH: intact parathyroid hormone; Na: sodium; K: potassium; Cl: chloride; Hb: hemoglobin; ERI: Erythropoietin resistance index; TSI: transferrin saturation index; CRP: C-reactive protein.
In bold the results that reached the statistical significance.
In relation to acid base parameters, post-dialysis bicarbonate levels were significantly lower in the CDF group, with no differences in the delta of bicarbonate.
Regarding the effect on mineral metabolism, the increase in Cai and post-hemodialysis Mg was lower in CDF than ADF and the post dialysis iPTH value was greater in CDF than ADF (Table 3).
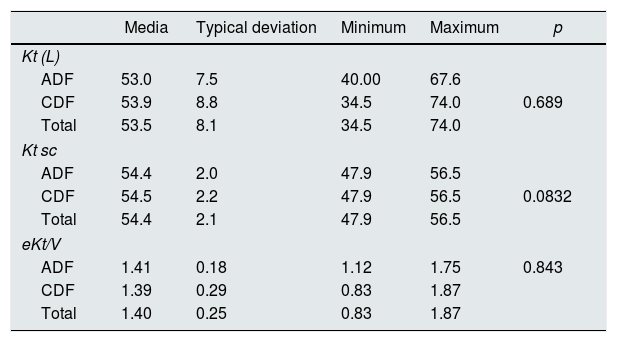
No differences were observed with LDA or LDC in the dialysis efficacy assessed by pre and post hemodialysis urea (Table 4). Neither when analyzed by treatment group (HD vs. HDF); the respective KT with ADF and CDF were: in HD 52.7 (9)L vs 48.7 (10.6)L, and in HDF 53.1 (7.2)L vs 57 (5.9)L.
Effect of the dialysis fluid (CDF with respect to the ADF), on the dialysis efficacy: Kt, Kt sc and eKt/V.
| Media | Typical deviation | Minimum | Maximum | p | |
|---|---|---|---|---|---|
| Kt (L) | |||||
| ADF | 53.0 | 7.5 | 40.00 | 67.6 | |
| CDF | 53.9 | 8.8 | 34.5 | 74.0 | 0.689 |
| Total | 53.5 | 8.1 | 34.5 | 74.0 | |
| Kt sc | |||||
| ADF | 54.4 | 2.0 | 47.9 | 56.5 | |
| CDF | 54.5 | 2.2 | 47.9 | 56.5 | 0.0832 |
| Total | 54.4 | 2.1 | 47.9 | 56.5 | |
| eKt/V | |||||
| ADF | 1.41 | 0.18 | 1.12 | 1.75 | 0.843 |
| CDF | 1.39 | 0.29 | 0.83 | 1.87 | |
| Total | 1.40 | 0.25 | 0.83 | 1.87 | |
K: fractional clearance of urea; t: time; Kt sc: KT adjusted to body surface; eKt/V: fractional clearance of urea divided by volume.
The doses of heparin remained stable during the study. None of the 56 basal HD sessions had significant coagulation problems of the dialyzer or the venous chamber. The thromboplastin time was analyzed as a coagulation parameter only pre HD and no differences were found between the two groups (p=0.388).
There were no significant differences in the coagulation score of the cameras between the HD performed with ADF and CDF; it was 0 (absent) or 1 (minimum) in 80% of the sessions; likewise no differences were observed between the dialysis techniques. The hemostasia time was 1.4 (2.7)min longer in CDF than in ADF, p<0.05.
No significant difference was observed in the C-reactive protein values (2.8 (3.8) vs 4.7 (7.3), (p = 0.253) in ADF and CDF respectively.
Regarding the tolerance of HD sessions, there were fewer episodes of hypotension during the sessions at the baseline visit with the CDF (1 vs 3) (p=0.04), and the MRBV as measured by hemoscan® biosensor was also lower with the CDF [−3.4 (7.7) vs −5.1 (8.2)] although without statistical significance. The results are the same throughout the total of the study sessions. The 46 patients who completed the study performed 4416 HD sessions, 2208 with ADF and 2208 with CDF citrate. Hypotension occurred in 311 sessions (14.1%) with ADF, and in 238 (10.8%) with CDF (p<0.01).
In the 29 patients in whom pre hemodialysis bio-impedance was performed, no significant differences were found in the total extracellular water or in relative overhydration (overall overhydration adjusted to the extracellular water, measured in % (OH/AEW)), being the values of 2.06 (1.25) vs 1.7 (1.19)L and 12.4% and 11.15% with the ADF and CDF respectively.
DiscussionAs in some previous work,17 our study found that CDF improves the control of acid base balance, avoiding or decreasing postdialysis alkalosis. Other authors such as Kuragano et al.18 found greater alkalization during dialysis with citrate versus acetate (from 7.38 to 7.50 and 21 to 29.2mmol/L vs 7.39 to 7.45 and 22.4 to 24.3 for the pH and bicarbonate values with DF with citrate and acetate respectively). Schmitz et al.19 also found less overcorrection of acidosis (bicarbonate>32mmol/L) with CDF than with ADF. The acute alkalemia induced by the gain of bicarbonate during dialysis is a problem of considerable clinical importance, since it has been related to important adverse effects such as hemodynamic instability,20–22 cardiac arrhythmias, paresthesia-cramps,23 decreased cerebral blood flow, respiratory depression,24,25 headache and a procalcifying effect.26 Finally, also a higher concentration of bicarbonate in the dialysis fluid has been associated with increased mortality,27 which led the FDA 28to alert physicians about such a risk and to consider the impact of acetate and other sources of alkali when the DF is prescribed. However, despite a lower postdialysis alkaline with CDF, the delta bicarbonate was not statistically different: −5.4 (2.7) vs −4.1 (2.4) in ADF vs CDF respectively. And the post-HD bicarbonate levels, although with statistically significant differences, in none of the groups was very high (28.5 vs 26.9 in ADF vs CDF). This is probably related to a fine adjustment of the DF bicarbonate concentration that was done in a protocolized manner prior to randomization.
Chemical characteristics of citrate may explain a reduced alkalemic effect whose metabolism is fundamentally hepatic and also muscular, being metabolized to generate bicarbonate and energy. Unlike what happens with acetate, the metabolism of citrate is not completed during the dialysis, since the hepatic and muscular metabolism occurs partly after the end of the technique. In addition, there are fast and slow metabolizing patients depending on liver function and muscle mass, factors that must be taken into account. In patients who are given citrate as a regional anticoagulation, plasma citrate levels are 1mmol/L, and HD patients have levels similar to those with normal renal function,29 but patients with liver insufficiency in the clearance of citrate is reduced by 50%.30
In relation to calcium and phosphate metabolism, with CDF the values of Cai and Mg postHD were significantly lower than preHD, and PTH values were increased after dialysis with CDF. This did not happen with ADF. As previously mentioned, citrate binds calcium and as such it causes alterations in the concentrations of this ion. It is known that low calcium in dialysis favors hypocalcemia in plasma, which stimulates PTH, bone turnover and the appearance of cramps, arrhythmias and the increased risk of hypotensive episodes, and therefore, when patients treated with CDF we increased the concentration of Ca in the DF. Despite this correction, the use of CDF resulted in a decrease in post dialysis Cai. However, other authors have not found the same results, Gabutti et al.,9 after calcium supplementation to the dialysate bath did not appreciate significant differences between the Cai values pre-dialysis and post-dialysis in patients treated CDF, while those treated with ADF with Ca supplementation there is a significant increase in post-dialysis calcium, which may have inhibitory effect on PTH secretion. Although hypomagnesemia has been shown in the regional anticoagulation with citrate,31 this is the first time that a reduction in Mg is described in relation with the use of CDF. Although not previously described, this result is not a surprise, since citrate may chelate ions such as Mg. A CDF formulation with a Mg concentration higher than the usual (0.5mEq/L) should solve the problem.
No differences were observed in the dialytic efficacy measured by Kt, Ktsc or eKt/V in ADF vs CDF. However, other authors such as Ahmad et al. found an improvement in dialysis adequacy measured by Kt/V in the citrate group (1.23±0.19 vs 1.34±0.20, p=0.01) in a study that included 25 patients,7 as well as Kossman et al.10 in a study of 146 patients (1.51±0.01 vs 1.57±0.01, p<0.0001). Although the mechanism underlying this beneficial effect are not known, some authors propose the anticoagulant effect of citrate that would prevent the coagulation of dialyzer fibers as a cause of the improvement in dialysis efficacy.
Regarding coagulation, we did not find differences in the coagulation scores, however the time of hemostasia 1.4 (2.7)min higher with the CDF than ADF. A possible explanation for the absence of differences in the coagulation scores could be that the dose of heparin used is high and small differences in coagulation may not have an impact on the scores analyzed, which showed absence or minimal alterations in 80% of the sessions. Although the hemostasis time is a gross measure of coagulation, this result is in line with that described by most authors and is due to the anticoagulant effect of citrate.
Finally, the result that appears to be most important to the study investigators is the effect of CDF on hemodynamic stability, with fewer episodes of arterial hypotension. And without significant differences in total extracellular water or relative overhydration. The MRBV measured by Hemoscan® biosensor was also lower in CDF than ADF, although without statistical significance. Daimon et al.13 describe a reduction in the frequency of hypotensive episodes in a sample of 44 patients during hemodialysis with CDF, especially in the most symptomatic and severe episodes of hypotension. Gabutti et al.9 describe greater hemodynamic stability with CDF, with a significant reduction in peripheral resistances and systolic blood pressure. However, other studies32 have found significant variations in tolerance. This greater hemodynamic stability seems to be related to the absence of acetate, which produces vasodilation mediated by the release of nitric oxide3 and the activation of proinflammatory cytokines by hypoxia.4 Smith et al.33 found a moderate and constant increase in acetate values of around 1mmol in the blood obtained in patients after dialysis with Acetate dialysis fluid concentrations of 4 and 8mEq/L.
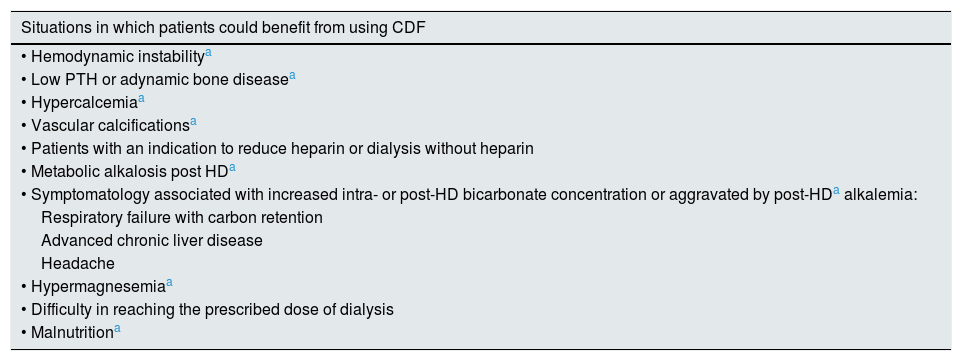
In summary, from our results by from previously published reports there some situations in which patients could benefit from using CDF. These have been detailed in Table 5. Patients who could be harmed by the use of CDF could be summarized as follows: in patients with hypocalcemia, hypomagnesemia and uncontrolled secondary hyperparathyroidism.
Situations in which HD patients could benefit from using CDF.
| Situations in which patients could benefit from using CDF |
|---|
| • Hemodynamic instabilitya |
| • Low PTH or adynamic bone diseasea |
| • Hypercalcemiaa |
| • Vascular calcificationsa |
| • Patients with an indication to reduce heparin or dialysis without heparin |
| • Metabolic alkalosis post HDa |
| • Symptomatology associated with increased intra- or post-HD bicarbonate concentration or aggravated by post-HDa alkalemia: |
| Respiratory failure with carbon retention |
| Advanced chronic liver disease |
| Headache |
| • Hypermagnesemiaa |
| • Difficulty in reaching the prescribed dose of dialysis |
| • Malnutritiona |
CDF: citrate dialysis fluid; PTH: parathyroid hormone.
The limitations of the study should be highlighted: the sample size and the selection bias that is given by the definition of one of the inclusion criteria: patients who are dialyzed through an arteriovenous fistula (AVF). Thus, the etiology of CKD of the patients included in the study is not representative of the population on dialysis, since the first cause was glomerulonephritis, followed by diabetes, while in the Spanish population and in most of the countries the leading cause of CKD in dialysis patients is diabetes. This is due to the bias of selecting patients with AVF, which has excluded a percentage of diabetic patients who are dialyzed with a catheter.
ConclusionsIn conclusion, the results of the present work show that dialysis with citrate achieves a better control of post-dialysis acid base balance by decreasing/avoiding postdialysis alkalemia. Post-dialysis levels of Cai and magnesium decrease with CDF and the PTH increases. These results together with lower alkalemia support a less calcifying profile of LD with citrate. Compared with the ADF, the CDF offers greater hemodynamic stability producing fewer episodes of hypotension.
Conflict of interestsP. de S., R.P.G., M.M. they have received honoraria for participation as a speaker at meetings of Fresenius and Baxter-Gambro and sponsorships of scientific congresses by Nipro, Fresenius and Baxter-Gambro.
To the nursing staff of the Dialysis Units of the Hospitals participating in the study for their valuable collaboration.
The promoter of the study was the Foundation of the Spanish Society of Nephrology (SENEFRO) and the funding from an independent research grant from Baxter.
P. de Sequera Ortiz (Servicio de Nefrología, Hospital Universitario Infanta Leonor, Madrid).
R. Pérez García (Servicio de Nefrología, Hospital Universitario Infanta Leonor, Madrid).
M. Molina Nuñez (Hospital General Universitario Santa Lucía, Cartagena).
R.I. Muñoz González (Hospital Galdakao,Vizcaya).
G. Álvarez Fernández (Servicio de Nefrología, Hospital Universitario Infanta Leonor, Madrid).
E. Mérida Herrero (Hospital Universitario Doce de Octubre, Madrid).
M.J Camba Caride (Complexo Hospitalario Universitario de Ourense).
L.A. Blázquez Collado (Hospital Universitario de Guadalajara).
M.P. Alcaide Lara (Hospital Universitario Virgen del Rocío, Sevilla).
R. Echarri Carrillo (Hospital Universitario Infanta Sofía, Madrid).
I. Gallardo (Hospital Galdakao,Vizcaya).
E. Hernández Martínez (Hospital Universitario Doce de Octubre, Madrid).
A. Otero González (Complexo Hospitalario Universitario de Ourense).
M. Sánchez Heras (Hospital Universitario de Guadalajara).
G. de Arriba de la Fuente (Hospital Universitario de Guadalajara).
L. Gil Sacaluga (Hospital Universitario Virgen del Rocío, Sevilla).
A. Cirugeda García (Hospital Universitario Infanta Sofía, Madrid).
V. Barrio Lucía (Hospital Universitario Infanta Sofía, Madrid).
The names of the components of the ABC-treat study group are listed in Appendix A.
Please cite this article as: Ortiz PS, García RP, Nuñez MM, Muñoz González RI, Fernández GÁ, Herrero EM, et al. Estudio prospectivo aleatorizado multicéntrico para demostrar los beneficios de la hemodiálisis sin acetato (con citrato): Estudio ABC-treat. Efecto agudo del citrato. Nefrologia. 2019;39:424–433.