Chronic kidney disease is associated with an increased risk of cardiovascular events. In recent years, protein-bound toxins have become more important due to their association with increased morbidity and mortality, characterised by inadequate clearance during dialysis. The purpose of this study is to assess the influence of high convective volumes on postdilution online haemodiafiltration (OL-HDF) on the removal of medium-sized molecules, small molecules and protein-bound molecules.
Material and methodsIn forty postdilutional OL-HDF sessions, the reduction rates of toxins of different molecular weights were measured in 13 patients, including protein-bound molecules such as p-cresyl sulphate, indoxyl sulphate and homocysteine.
ResultsTotal convective volume was 28.3 (5.1)l (range 16.3–38.0l). Mean reduction rate of protein-bound molecules was 44.4% (15.7%), 48.7% (14.1%) and 58.6% (8.8%) for p-cresyl sulphate, indoxyl sulphate and homocysteine, respectively. Moreover, a statistically significant direct association was found between the reduction rates of all three molecules, the replacement volume and the Kt/V.
ConclusionHigh convective volumes during postdilution OL-HDF are associated with increased removal of protein-bound uraemic toxins.
La enfermedad renal crónica tiene mayor riesgo de eventos cardiovasculares. En los últimos años, han ido adquiriendo mayor importancia las toxinas unidas a proteínas, que han sido asociadas a mayor morbimortalidad y que se caracterizan por la dificultad para su depuración en diálisis. El objetivo de este estudio es valorar la influencia de altos volúmenes convectivos en HDF-OL posdilucional sobre la eliminación de medianas moléculas, pequeñas moléculas y moléculas unidas a proteínas.
Material y métodosSe realizaron 40 sesiones de HDF-OL posdilucional en 13 pacientes y se midió el porcentaje de reducción de toxinas de distinto peso molecular y entre ellas, moléculas unidas a proteínas como el p-cresyl sulfato, indoxyl sulfato y homocisteína.
ResultadosEl volumen convectivo total fue de 28,3(5,1) litros con un rango entre 16,3 y 38,0 litros. La reducción media de moléculas unidas a proteínas fue de 44,4(15,7) % para el p-cresyl sulfato, de 48,7(14,1) % para el indoxyl sulfato y de 58,6(8,8) % para la homocisteína. Además, se encontró una relación directa y estadísticamente significativa entre el porcentaje de reducción de las tres moléculas con el volumen de sustitución y con el Kt/V.
ConclusiónAltos volúmenes convectivos totales en HDF-OL en posdilución se asocian a una mayor eliminación de toxinas urémicas unidas a proteínas.
The chronic kidney disease population has higher risk of fatal and non-fatal cardiovascular events than the general population, even after controlling for traditional cardiovascular risk factors.1 Uraemic toxins are risk factors in patients at any stages of chronic kidney disease.2
Uraemic toxins have been divided according to molecular size and protein binding.3 Small molecules are preferentially eliminated by diffusive transport, while mid-sized molecules are eliminated by convective transport. Recent studies demonstrate that online haemodiafiltration (OL-HDF) with volumes over 20l are capable of decreasing mortality.4–7
However, there is a third group of uraemic toxins consisting of small molecules bound to proteins, including p-cresyl sulphate, indoxyl sulphate, or homocysteine, which are associated to high cardiovascular morbidity and mortality in chronic kidney disease patients.8–11 Removal of these toxins with conventional haemodialysis or OL-HDF techniques remains very limited. Some authors demonstrate that both total p-cresol levels and free p-cresol levels are associated with cardiovascular events,9 though other studies show that only high levels of free p-cresyl sulphate are associated with higher mortality in patients with chronic kidney disease in any stage.12,13
Furthermore, modern dialysis monitors with the ability to perform OL-HDF, through automated biocontrol systems, enable us the substitution of volumes greater than 25l in a 4-hour session that is obtained in patients with good vascular access.
The objective of this study is to assess the influence of high convective volumes in OL-HDF on removing mid-sized molecules, small molecules, and protein-bound molecules.
Material and methodsPopulation and dialysis techniqueThis is an observational study of patients in renal replacement therapy with post-dilution OL-HDF (post-OL-HDF). Data was collected in 40 post-OL-HDF sessions in 13 prevalent patients with advanced chronic kidney disease in regular haemodialysis. Data was analysed from three weekly sessions for all the patients studied, except for one in which four sessions were collected. The patients signed an informed consent form. All the procedures were performed in accordance with the Declaration of Helsinki and its later revisions.
Before the study, all the patients were on post-OL-HDF treatment. The monitors used were Fresenius 5008 CorDiax®, AK-200 Gambro with ultra-control® and Artis Gambro®, in which the substitution rate is automatically controlled. The dialyser used in all cases was the FX-1000 CorDiax (FMC®), manufactured with a Helixone® membrane, with an ultrafiltration coefficient of 75ml/h x mmHg, 2.2m2 effective surface area, 35μm wall thickness, and 210μm internal capillary diameter. The haemodialysis sessions lasted 240min.
Information about the characteristics of each session were collected: real blood flow, arterial blood pressure, venous blood pressure, substitution litres, total convective volume, defined as the sum of the substitution volume and ultrafiltration, and Kt/V, with K obtained by ionic dialysance and V analysed using bioimpedance spectroscopy (BMC® by FMC, Bad Homburg).
Laboratory testsThe pre-dialysis and post-dialysis mid-sized molecule concentration (alpha-2 macroglobulin, beta-2 microglobulin, prolactin, myoglobin, and interleukin-6), the small molecule concentration (urea, creatinine, and phosphorus), and protein-bound molecule concentration (total p-cresyl sulphate, indoxyl sulphate, and homocysteine) were measured in each session. The post-dialysis samples were obtained from the artery once the session was completed and before the needles were removed.
The samples of mid-sized molecules, homocysteine, and small molecules were sent at the time of extraction to the biochemistry laboratory and were analysed using conventional methods in an automated analyser. To determine IL-6, p-cresyl sulphate, and indoxyl sulphate, the serum samples were frozen at −35°C and sent to an external laboratory. IL-6 was analysed using immunonephelometry. To determine the p-cresyl sulphate and indoxyl sulphate, the samples were deproteinised and analysed using high-performance liquid chromatography (HPLC).
To assess the molecule clearance efficacy, the reduction ratio was calculated for each using the formula:
The post-dialysis plasma concentrations of protein-bound molecules and mid-sized molecules were adjusted to the degree of haemoconcentration based on the changes in the extracellular volume as assessed by the pre- and post-dialysis weight according to the equation14:
Statistical analysisThe quantitative variables were expressed as mean and standard deviation for variables with a normal distribution and as the median and interquartile range for the rest. The qualitative variables were expressed as percentages.
The means were compared using a Student's t-test or ANOVA when dealing with more than two samples. Pearson's correlation coefficient was used to assess the bivariate correlations between quantitative variables. It was considered statistically significant if p<0.05. SPSS V.17.0 was used (Chicago, Illinois).
FindingsThe characteristics of haemodialysis session are shown in Table 1.
The total convective volume was 28.3 (5.1) liters ranging from 16.3 to 38.0 litres. Eighty-five per cent (85%) of the patients received dialysis via an rteriovenous fistulae (AVF) while the remaining 15% through a permanent tunnelled catheter. The mean arterial blood flow was 426 (70) ml/min ranging from 250 to 500ml/min.
Table 2 shows the molecule reduction ratio during the dialysis session and its correlation with the convective transport.
Bivariate correlations between the total convective transport and the molecule reduction ratio, represented as the mean (standard deviation).
| Molecules (MW Da) | Pre-dialysis serum levels | % Reduction | Pearson coefficient of correlation | p |
|---|---|---|---|---|
| Low-molecular-weight molecules | ||||
| Phosphorus (31) | 4.2(1.5)mg/dl | 57.1(17.3) | −0.004 | 0.981 |
| Urea (60) | 121(40)mg/dl | 79.0(23.12) | 0.320 | <0.050 |
| Creatinine (113) | 9.6(3.7) mg/dl | 75.9(7.8) | 0.678 | <0.001 |
| Mid- and high-molecular-weight molecules | ||||
| Beta-2 microglobulin (11,800) | 23(7)mg/l | 81.3(6.4) | 0.607 | <0.001 |
| Myoglobin (17,000) | 236(114)ng/ml | 60.0(11.5) | 0.431 | <0.010 |
| Prolactin (22,000) | 28(22)μg/l | 60.1(14.3) | 0.395 | <0.050 |
| Interleukin-6 (26,000) | 5.6(3.0)pg/ml | 28(17.2) | 0.113 | 0.613 |
| Alpha-2 macroglobulin (72,000) | 153(30)mg/dl | −2.2(12.2) | 0.270 | 0.960 |
| Protein-bound molecules | ||||
| Homocysteine (135) | 26.8(9.5)μmol/l | 58.6(8.8) | 0.492 | <0.005 |
| P-cresyl-sulphate (187) | 13.7(3.7)mg/dl | 44.4(15.7) | 0.630 | <0.001 |
| Indoxyl-sulphate (212) | 42.2(14.9)mg/l | 48.7(14.1) | 0.461 | <0.050 |
For small molecule clearance, the mean Kt/V was 1.76 (0.64). The creatinine and urea reduction ratios showed a significant correlation with the total convective volume (p<0.001 and p<0.050, respectively), whereas the phosphorus clearance did not show a correlation with the convective volume.
The mid-sized and large molecule reduction ratios in OL-HDF were highly variable, varying between 81.3% for beta-2 microglobulin and −2.2% for alpha-2 macroglobulin. We found a significant correlation between the beta-2 microglobulin, prolactin, and myoglobin clearance rate with the total convective volume, while there was no correlation between the IL-6 and alpha-2 macroglobulin levels and the convective volume.
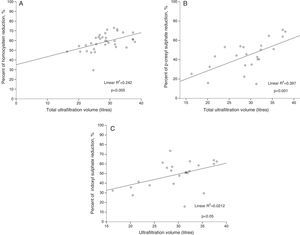
The protein-bound molecule reduction ratio was significantly correlated with the total convective volume in all analysed molecules (Table 2). Likewise, the clearance of the three molecules was correlated with the Kt/V (r=0.425, p=0.014 for homocysteine; r=0.554, p=0.009 for p-cresyl sulphate, and r=0.579, p=0.006 for indoxyl sulphate). Furthermore, the percentage of protein binding the analysed molecules (70% for homocysteine,15 90% for indoxyl sulfate,16 and 95% for p-cresol16) showed an inverse relationship with the reduction ratio of these molecules (r=−0.996, p=0.058). The linear relationship between the analysed protein-bound molecules and the total convective volume is presented in Fig. 1.
DiscussionOur study demonstrates that high-volume convective transport in OL-HDF is associated with higher protein-bound toxin clearance and confirms a high small- and mid-sized molecule clearance, as demonstrated in other studies.
The importance of removing these protein-bound toxins has increased in recent years, as we have been learned about the association between high serum levels of these molecules and increased cardiovascular risk.11,13,17 Two of the most studied protein-bound molecules, in both metabolism and action, are p-cresol and indoxyl sulphate.
P-cresol is generated in the intestinal flora and is subsequently metabolised into p-cresyl sulphate and p-cresyl glucuronide. Both molecules are strongly bound to proteins.18 Free p-cresyl sulphate (not protein-bound) appears to be associated with overall and cardiovascular mortality in patients with chronic kidney disease and in dialysis.10 Free p-cresyl glucuronide, the other p-cresol metabolite, is also associated with cardiovascular mortality in chronic kidney disease patients.19
Indoxyl sulphate belongs to the indol family and is also a protein-bound toxin that has been associated with endothelial damage and overall and cardiovascular mortality, as well as vascular calcification.10
The problem with protein-bound toxins is the difficulty to remove them with conventional dialysis techniques. Several studies have been conducted with different clearance techniques showing non-uniform results. Meert et al. compared pre- and post-dilution OL-HDF with pre-dilution haemofiltration. They found a higher protein-bound toxin clearance in the two OL-HDF modalities than in pre-dilution haemofiltration, but no differences between pre- and post-dilution OL-HDF.20 In contrast, Bammens et al. shoed greater protein-bound toxin clearance in post-dilution OL-HDF, with convective transport equivalent to 20l (substitution flux of 87ml/min over 230min per session), than with high-flux haemodialysis.21 Nevertheless, other authors found, in only eight patients, that mean substitution volumes of 21.5l have little significance on removing these solutes,22 our results demonstrate that a 40% higher substitution volumes manage to significantly increase clearance of this type of toxin.
The three protein-bound solutes studied have a similar molecular weight, but different protein-binding rates, 70%, 90% and 95% for homocysteine, indoxyl sulphate and p-cresyl sulphate respectively.15,16 We found that the reduction ratio in these molecules is usually inversely proportional to the protein-binding, such that p-cresyl sulphate, which is the solute with the tightest protein bond, is the hardest to remove by convection, whereas homocysteine is the one that shows the greatest reduction ratio. Nevertheless, it is important to point out that the clearance of the three toxins studied show a direct correlation with the convective volume, with a higher correlation coefficient for p-cresyl sulphate. It has been suggested that protein-bound toxins are removed nearly exclusively by removing the free fraction, such that the protein-bound fraction is displaced into its free form during the dialysis session, enabling these toxins to cross the membrane.21 It is clear that in our study, small molecule clearance is high, as the sum resulting from the convective and diffusive transport. The result may be that more of the free fraction of the protein-bound toxins are removed, which may increase the displacement of the protein-bound fraction to the free fraction, thereby enabling their continued clearance. However, this hypothesis would need to be confirmed.
Encouraging studies have recently appeared which demonstrate that incorporating resins with the ability to remove protein-bound molecules by adsorption may have an additive effect on the efficacy of conventional techniques. Thus, haemodiafiltration using resin adsorption to regenerate the ultrafiltrate (HFR, Bellco®), which combines adsorption with convective transport and reinfusion of the patient's own ultrafiltrate, has been shown to be effective in removing protein-bound toxins.23–25 Nevertheless, more studies are necessary to assess the amount of resin that needs to be incorporated into the technique to prevent premature saturation.
Studies have also been conducted with absorbents, which have been demonstrated to decrease the levels of these toxins26 although not significantly, and especially not in in vitro studies. Moreover, using probiotics and symbiotics has been demonstrated to be able to reduce the levels of protein-bound toxins.26,27 More recently it has been demonstrated that it is also possible to decrease the levels of indoxyl sulphate and possibly p-cresyl sulphate by increasing the fibre content in the diets of haemodialysis patients.28
Three randomised, controlled studies conducted in recent years have demonstrated increased survival in patients treated with post-OL-HDF with substitution volumes greater than 17.4, 20.7, and 22l, respectively,5–7 possibly in relation to a higher uraemic toxin clearance, especially mid-sized toxins, although with the findings from our study, it is possible that this decrease in morbidity and mortality is also partly due to greater protein-bound toxin clearance.
Modern dialysis monitors with the possibility of performing OL-HDF incorporate automatic biomonitoring systems, which enable substitution volumes over 25l to be obtained in four-hour sessions. This requires good vascular access, and preferably using 14G needles to increase blood flow. In our study, the mean blood flow was 426ml/min, which enabled more than 100l of blood to be dialysed in a 4-hour session, reaching a convective volume over 28l on average with no technical problems (approximate filtration fraction was 28%). However, one of the largest bottlenecks for increasing convective transport is that not all the potentially beneficial substances that may be removed with the ultrafiltrate have been quantified, such as amino acids, minerals, vitamins, antioxidants, and other nutrients, whose clinical relevance is still unknown.
The possible albumin loss is one of the most studied aspects. These losses occur especially during the first 30min of each session.29,30 Nevertheless, recent studies demonstrate that the losses are higher in haemodiafiltration than in haemodialysis and depend on the type of dialyser chosen.22,31 Incorporating nanotechnology into the manufacture of more modern dialysers has enabled the membrane pore characteristics to be modified, such that these losses are much smaller30 with low clinical significance. In a previous study, we found that the albumin losses with the dialyser used in this study are limited, although with significant differences between the 20- and 30-l substitution volumes per session.
In our results, we found that the reduction ratio of the alpha-2 macroglobulin levels, with a molecular weight of 72,000Da, is slightly negative despite having adjusted the post-dialysis levels to the haemoconcentration, which indicates that it is hardly removed or not removed at all.
This study has some limitations such as the small number of sessions analysed, that we only determined the reduction rate in protein-bound toxins without measuring the losses by the dialyser and that we used a single type of dialyser, although we chose a dialyser with suitable characteristics for the technique used.32
In summary, our study demonstrates that with high-volume convective transport can achieve higher protein-bound toxin removal. Nevertheless, more studies with the ability to confirm our hypothesis are needed to analyse the possible effect that this behaviour may have in the longer term on the progression of patients thus treated.
Conflicts of interestThe authors declare that there are no conflicts of interest.
Please cite this article as: Abad S, Vega A, Quiroga B, Arroyo D, Panizo N, Reque JE, et al. Toxinas unidas a proteínas: valor añadido en su eliminación con altos volúmenes convectivos. Nefrología. 2016;36:637–642.