Antecedentes y objetivos: la localización de la infección urinaria en el niño tiene implicaciones terapéuticas y pronósticas. La afectación gammagráfica se considera como «patrón oro» en el diagnóstico de pielonefritis aguda. Se han realizado estudios con biomarcadores urinarios con resultados controvertidos. El objetivo de este estudio ha sido determinar la utilidad de β2-microglobulina, α1-microglobulina, cistatina C, IgG y albúmina en el diagnóstico de localización, analizando la relación entre su excreción urinaria, parámetros clínicos y de laboratorio y la afectación renal gammagráfica. Pacientes y métodos: estudio observacional prospectivo realizado en 40 pacientes de un mes a 11 años de edad tras su ingreso hospitalario por sospecha de pielonefritis. Se analizaron variables clínicas y de laboratorio, realizando una ecografía renal y una gammagrafía renal en los primeros siete días del ingreso. Una vez remitida la fiebre, se analizó el cociente urinario proteína/creatinina para cada variable. Tras el proceso agudo, se realizó una gammagrafía renal en fase tardía para detectar cicatrices renales. Resultados: el filtrado glomerular y la ecografía fueron normales en todos los pacientes. Un 45% de los niños (24/80 riñones) tuvieron alteraciones gammagráficas compatibles con pielonefritis aguda, no existiendo diferencias en la proteinuria entre éstos y aquellos con gammagrafía normal. A mayor edad, mayor probabilidad de afectación gammagráfica. La sensibilidad y especificidad de los leucocitos y PCR para predecir pielonefritis fue del 77-65 y 94-52%, r = 0,70 (IC 95% 0,54-0,87) y 0,75 (IC 95% 0,60-0,90), respectivamente. No existe relación entre la temperatura máxima o duración de los síntomas y la afectación renal. Conclusiones: la gammagrafía renal sigue siendo el patrón de referencia en el diagnóstico de pielonefritis aguda en Pediatría. El uso combinado con marcadores urinarios de función renal no aumenta su sensibilidad. Es preciso realizar estudios que confirmen la utilidad de nuevos biomarcadores.
Antecedents and objectives: the location of the urinary tract infection in children has serious implications both in therapy and prognosis. Affectation in gammagraphic studies is considered the “gold standard” for the diagnosis of acute pyelonephritis. Several studies with biomarkers have been made with controverted results. The objective of this study is to set the utility of beta2-microglobuline, alfa1-microglobuline, Cistatine C, IgG and albumin in the location of the infection, through the analysis of the relation among their urinary excretion, clinical and laboratory parameters and the renal scintigraphy findings. Patients and methods: Prospective observational study made in 40 patients, aged from 1 month to 11 years, after their Hospital admission with suspicion for acute pyelonephritis. Exclusion criteria were: decrease in the glomerular filtration rate, malnutrition, massive albuminuria and history or findings of nephrourologic disease. Clinical and laboratory variables were analyzed, and renal ultrasonography and scintigraphy were performed within the first seven days after admission. Once the fever dropped, the urinary creatinine-protein ratio was analyzed. After the acute process, a renal scintigraphy was performed in order to detect renal scars. The non-parametric Mann-Whitney U test has been used as the statistical hypothesis test, and Chi-square and Fisher´s exact tests have been used to compare the qualitative variables. Results: The glomerular filtration rate, as well as the ultrasonography scan, was normal in all patients. 45% of the children (24/80 kidneys) had scintigraphic alterations that were compatible with acute pyelonephritis, and there were not differencies in proteinuria between these and those with normal scintigraphy. To greater age greater probability of scintigraphic affectation. The sensitivity and specificity of leukocytes and CRP to predict pyelonephritis were 77-65% and 94-52%, r = 0.70 (CI 95% 0.54-0.87) y 0.75 (CI 95% 0.60-0.90) respectively. An apparent relation between the maximum temperature or duration of the symptoms and the renal affectation does not exist. In all the children, the normality of urinary markers of renal function was confirmed once the acute phase had passed, even in those 3 patients with renal scars (7.5%). Conclusions: Renal scintigraphy is still being the reference pattern for the diagnosis of acute pyelonephritis in Pediatrics. The combined use of different urinary markers of renal function does not increase its sensitivity. It is necessary to do research in order to confirm the utility of new biomarkers.
INTRODUCTION
Urinary Tract Infection (UTI) is one of the commonest infections in infants and children.1 From a practical stance, it can be classified as follows: lower urinary tract infection, infection affecting the renal parenchyma (acute pyelonephritis) and asymptomatic bacteriuria.
The localization of the UTI has significant implications in terms of therapy and prognosis as only upper urinary tract infections entail risk of permanent damage to the renal parenchyma which is associated with recurrent episodes of pyelonephritis, high blood pressure, proteinuria, hypostenuria, terminal chronic renal failure and complications during gestation.1-3 Adequate diagnosis and treatment are required at the acute stage to avoid scarring and long term complications.1,2
Although symptoms of general illness and acute phase response are more frequent in Acute Pyelonephritis (APN), they are also found in the absence parenchymal inflammatory lesions.4 The use of Tc-99 Dimercaptosuccinic Acid (DMSA) scintigraphy in the acute phase of pyelonephritis for the detection of inflammatory lesions in the renal parenchyma is currently considered a “gold standard” in APN diagnosis.5-7
Other diagnostic tests had been used in UTI localisation, amongst others interleukins, tubular enzymes (NAcetylglucosaminidase [NAG]), Low Molecular Weight Proteins (LMWP), ß2 microglobulin, α1 microglobulin, and cystatin C with conflicting results making urinary biomarker comparisons difficult.1,8-20
Ninety-nine percent of the filtered LMWP are reabsorbed in the tubule under normal conditions. Therefore, any increase in urine excretion of these proteins suggests tubular disease.20,21 Another renal function marker is microalbuminuria, an established early marker of glomerular damage, although a small fraction filtered by the glomerulus is later reabsorbed in the proximal tubule.20,22,23 Several studies show an association between UTI, albuminuria and scintigraphic lesion, which implies a glomerular-interstitial interaction in the pyelonephritis inflammation.4,12,19 It has also been shown that in complex glomerular lesions, there is usually urinary loss of proteins of greater molecular weight than albumin, such as IgG.20
The main aim of this study was to assess the use of certain markers such as ß2 microglobulin, α1 microglobulin, cystatin C, IgG and albumin in the localization of a child’s first urinary infection, in relation to urinary excretory function clinical and laboratory parameters and renal scintigraphic findings.
PATIENTS AND METHODS
A prospective study was carried out between July 2006 and July 2007. An analysis of the urinary excretion of ß2 microglobulin, α1 microglobulin, cystatin C, IgG and albumin was carried out together with DMSA scintagraphy in 40 patients (one month to 11 years) who had been admitted to hospital with UTI and suspicion of acute pyelonephritis They presented with an increase in acute phase reactants (CRP > 20mg/l), fever (Temp > 38.5º C) and/or general malaise with no evidence of other bacterial infection. The average age was five months (2.5-9.8 months) and weight 7.4 kg (5.5-8.7kg). Seventeen (42.5%) were male and 23 (57.5%) female.
The acceptance criteria were: a) nursing infants under three months with suspected UTI; b) nursing infants under one year with feverish UTI; and c) feverish UTI at any age, with malaise, vomiting, dehydration and/or an unfavourable home environment. The exclusion criteria were the following: 1) alteration in kidney function with decreased GFR, according to the normal values described in the literature; 2) malnutrition; 3) massive albuminuria (albumin/Cr > 1,000mg/g); 4) patients under one month and those diagnosed with intrauterine growth retardation at birth (standard deviation of < 2); and 5) history or findings of kidney and/or nephrourologic disease: CAKUT («congenital anomalies of the kidney and urinary tract»), UTI or urolithiasis.9,16-20,24-30
The highest temperature and the duration of the symptoms were recorded. A blood culture on admission, as well as a full blood count (FBC), C-Reactive Protein (CRP) and Creatinine (Cr) were measured. Glomerular Filtration Rate (GBR) was calculated using the Schwartz formula.31 At the on-call physician’s discretion, additional tests were performed to rule out other sources of bacterial infection.
Urine samples for the UTI diagnosis in the infants without voluntary bladder control were taken via bladder catheterisation. Clean-catch midstream urine collection was used for the remainder. The samples were immediately laced in culture without bacteriostatics. UTI was diagnosed if there was pyuria (> 10 leukocytes per field in centrifuged urine), together with the isolation of bacteria in the urine culture of > 10,000CFU/ml for catheter samples and >100,000CFU/ml for midstream urine.
Written consent was obtained from parents or carers for the additional tests. Approval from the hospital Ethics Committee was also obtained.
DIAGNOSTIC IMAGING
A renal ultrasonography was performed on all of the patients with confirmed UTI on admission and a DMSA renal scintigraphy scan after the acute phase, two to seven days after UTI diagnosis using a Philips Sky device with double head, low power collimator and high resolution. The patients did not require sedation. In the scintigraphic study analysis, each kidney was divided into three segments (upper pole, middle section and lower pole). The acute pyelonephritis was defined by the presence of focal or diffuse areas of less definition showing no signs of cortical loss.4,32
A repeat scintagraphy was performed 6-12 months after the acute episode in those who had an abnormal scan initially.Renal scarring was defined by less definition associated with loss of renal contours or cortical thinning with volume decrease.4,32 A Voiding Cystourethrogram (VCUG) was performed in recurrent episodes of UTI during monitoring.
RENAL FUNCTION MARKERS
Once the patient’s temperature had fallen following intravenous antibiotic therapy in the first 48-72 hours, the overnight urine was discarded to avoid functional proteinuria interference.8,9,12 A second urine sample was taken for the measurement of protein, creatinine and pH. The samples were analysed immediately to avoid protein breakdown by proteolytic enzymes and bacterial cathepsins ensuring the stable conditions described in the analysis of the diverse proteins. 16-18,33-35 After the acute stage, the urinary markers of kidney function were during follow-up.
Urine pH was determined by reactive strip (Urifelt S®), measured by reflectance in an automated Meranini Aution Max™ urine chemistry analyser (A. Meranini Diagnosis, Italy). A Hitachi 917 (Roche, Germany) analyser determined the Cr in urine, using the kinetic Jaffé method; the Cr in blood with a modified enzymatic reaction according to Trinder; the total proteins through turbidimetry using benzethonium chloride and the albumin through turbidimetric immunoassay with polyclonal antibodies. The average IgG, α1 microglobulin and ß2 microglobulin were determined through immunonephelometry with an automatic BN ProSpec analyser (Dade Behring, Marburg, Germany). Lastly, cystatin C was measured using the same nephelometer with the N Latex Cystatin C Kit (Dade Behring).
To correct for differences in urine flow, the protein/creatinine urinary ratio was determined for the different proteins, given its good correlation with the 24 hour urine measurements and compared with the published reference ranges; protein/Cr (<442mg/g between six months and two years; <222mg/g in children over two), albumin/Cr (34[27-54]mg/g between one and twelve months; < 35mg/g in over twelve month olds), IgG/Cr (8.8[8-11.5]mg/g), α1 microglobulin/Cr (<14mg/g), ß2 microglobulin (<0.4mg/L), ß2 microglobulin/Cr (<0.36mg/g), cystatin C (0.03-0.3mg/l) and cystatin C/Cr (<0.35mg/g).16-18,20,24-26,35,36
The percentage of albuminuria and the ratio of albumin toα1 microglobulin and ß2 microglobulin were also measured. An albumin/total proteins ratio of > 40-50% was considered suggestive of predominant glomerular involvement. The albumin/ß2 microglobulin ratio is approximately 30-200 in normal urine, 1,000-15,000 in glomerular proteinuria and < 300 in tubular proteinuria. An albumin/α1 microglobulin ratio of <17 or >160 distinguishes between tubular or glomerular involvement, respectively. Mixed glomerularinterstitial kidney malfunction was considered at albumin and α1 microglobulin values of 20-40mg/g Cr and 14-30mg/g Cr, respectively.11,19,20,37
The control group for the urinary markers of renal fuction consisted of 20 healthy infants and children. The absence of a family or personal history of nephrourologic disease or UTI was confirmed. The capability of maximum urine concentration was also determined after a dry diet. Renal ultrasonography and urine dipstix, microscopy and culture were carried out producing normal results.38 Since some of infants and children had been investigated for isolated primary enuresis, their urinary excretion of calcium was determined (and found to be normal) to eliminate the possible interference in the measurement of some proteins in urine .17,26
STATISTICAL ANALYSIS
The data was analysed using the SPSS 14.0 program. The values are expressed as median and interquartile range to assure normal distribution. The non-parametric Mann-Whitney test was used to test the contrast hipótesis. Chisquare and Fisher’s exact tests were used to compare the qualitative variables, the latter was used in cases where the former could not be applied. Significant statistical differences exist when the p value is ≤ 0.05.
RESULTS
Clinical, laboratory and microbiological data
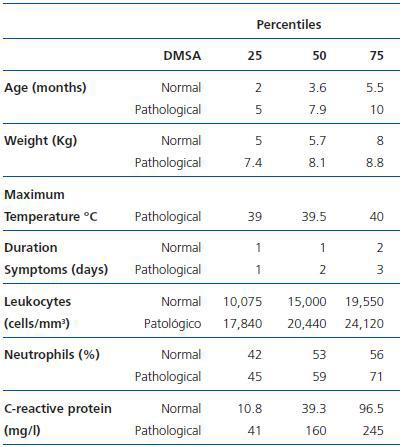
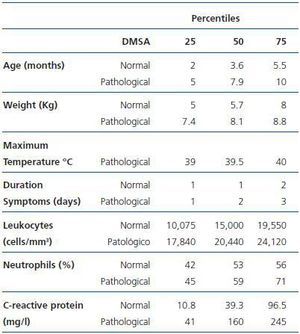
All of the patients in the study had a normal estimated GFR for their age. Table 1 compares the epidemiological (age and weight), clinical (maximum temperature and duration of the symptoms before admission) and laboratory (leukocytes, neutrophil percentage and CRP) data of the patients whether or not they showed acute scintigraphic involvement consistent with pyelonephritis. There are differences for age, leukocytes and CRP value in blood serum, with an area under the curve of 0.72 (CI 95% 0.55-0.88), 0.70 (CI 95% 0.54-0.87) and 0.75 (CI 95% 0.60-0.90), respectively. The sensibility and specificity of the leukocytes and CRP for the prediction of acute pyelonephritis was 17,495 leukocytes/mm3 (77 and 65%) and 24.5mg/l (94 and 52%), respectively.
Escherichia coli was isolated in the urine culture of 36 patients (90%), the remaining was positive for other bacteria. No bacterium was isolated in the blood cultures.
Diagnostic Imaging
The renal ultrasonography showed no pathological findings of note. Among the 40 infants and children, 18 (45%, 24/80 kidneys) presented scintigraphic defects compatible with acute pyelonephritis (focal changes in 19/24 kidneys and diffuse in 5/24 kidneys). As shown in Table 1, the probability of scintigraphic abnormality increases with age.
Regarding the segmental distribution of the scintigraphic changes, they appeared mainly in the upper pole (right, 44% of patients; left, 39% of patients) and lower pole (right, 22% of patients; left, 33% of patients) whilst 38% of the patients presented involvement in the middle section (right, 22%; left, 16%).
Urinary Parameters of Renal Function
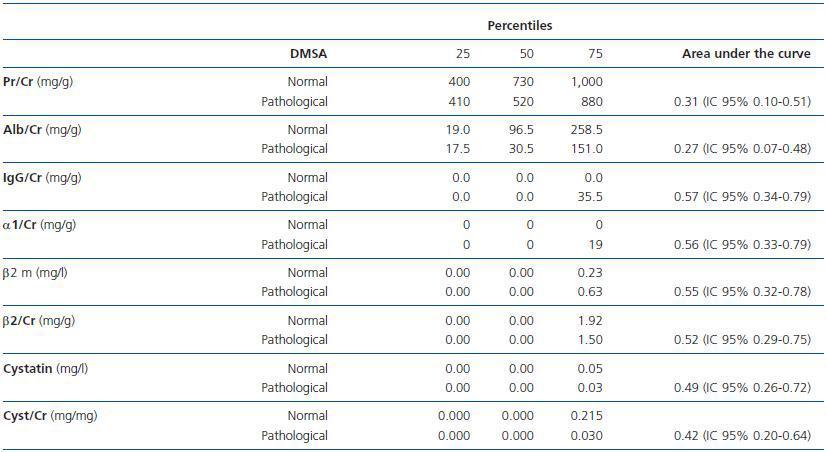
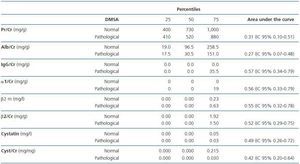
Table 2 compares the area under the estimated curve using ROC curves and the urinary markers of kidney function in patients with and without scintigraphic lesion. There were no differences between the two groups in any of the parameters studied.
There were no differences in any of the variables studied when the values were divided into normal and pathological, according to age and the presence of abnormality in renal scintigraphy. Similar results were obtained when comparing the indexes of tubular and glomerular (albumin/ß2 microglobulin, albumin/α1 microglobulin, albumin/total proteins) proteinuria with the scintigraphic changes.
Patients Evolution Results
The urinary markers of kidney function returned to normal in all the patients once the acute phase resolved. Nine patients (23%) had recurrent episodes of urinary tract infection during the study period. A VCUG was performed in these patients. Three were diagnosed with low grade (I-III) Vesicourethral Reflux (VUR), according to the classification of the international reflux study in infants and children, but there was no association between the DMSA changes and the presence of reflux in the VCUG. The only case of grade III VUR corresponded to an older girl suffering from voiding disorder that improved after specific treatment.
Subsequent renal scintigraphy was performed on 17 patients during the study period. Changes consistent with renal scars were seen in three (7.5%) patients, although the lesions were less extensive than in the initial scintigraphy.
None of these patients presented VUR in the VCUG. One patient with bilateral focal lesions in the acute phase did not undergo follow-up scintigraphy due to lack of parental consent.
DISCUSSION
In this study, none of the proteins or urinary protein/creatinine ratios used for the UTI localization diagnosis (protein/Cr, albumin/Cr, IgG/Cr, α1 microglobulin/Cr, ß2 microglobulin, ß2 microglobulin/Cr, cystatin C, cystatin C/Cr, albumin/ß2 microglobulin, albumin/α1 microglobulin and albumin/total proteins) showed correlated with the initial scintigraphic abnormality or its extension.
These findings contrast with the results obtained by other investigators endorsing the diagnostic reliability and stability of some LMWP as indicators of tubular damage measured by standard methods and at different pH values. We did not corroborate the findings of studies showing a link between UTI, albuminuria and the scintigraphic lesion accompanied by concealed tubular lesion markers.4,9,12,13,15,17,24,33,34 Nevertheless, and despite their high sensibility, some LMWP such as α1 microglobulin excretion may be increased in glomerular proteinuria, characteristically associated with urinary loss of proteins with greater molecular weight, such as albumin or IgG.9,10,20 In this connection, we did not demonstrate the superiority of cystatin C, produced and secreted shortly after a synthesis through all the nucleated cells in a constant way over other markers of renal function.35,39-41
This lack of correlation in our analysis could be only be explained by the small number of patients studied, given the reliability of the UTI diagnosis and the meticulous handling of the samples to assure protein stability and the tight entry criteria to avoid confounding factors, amongst others functional, overflow immaturity-related proteinuria. However, work carried out in a similar number of subjects did show a link between the urinary excretion of α1 microglobulin and albumin with APN although the latter was not confirmed with scintigraphy in the acute phase in all the cases.4,13,15 Other investigators did not find differences in the urinary excretion of some LMWP, such as α1 microglobulin and ß2 microglobulin, in patients with fever of non-renal origin as compared with those with suspected APN.8
Nonetheless we have shown an association between white blood cell count, serum CRP and a relationship involving renal abnormality that have not been previously reported.4,32 As in other studies, renal ultrasonography failed to detect any abnormality and like the clinical parameters such as the maximum temperature reached and the duration of the symptoms was not useful in the localization of the UTI.4,32,42
DMSA scintigraphy during the acute stage is currently considered the most sensitive method for the diagnosis of APN.5-7,42 Some authors consider its clinical usefulness doubtful in this period, while others support its use in infants < 2 years of age with febrile UTI. It has also been suggested that a normal scintigraphic scan in the acute phase could replace the cystography in the initial investigation, based on the fact that it would exclude a clinically significant VUR. If the scintigraphy is normal, the probability of scarring is very low, even if there is VUR and infections recur. Therefore, in the opinion of a number of authors, no other imaging study is needed.42-45
In this study, acute lesions were found in only 45% of the patients with clinical criteria of APN, who had no symptoms or signs of other source of bacterial infection. This contrasts with the higher percentage of other series and could be related to technical difficulties in obtaining a specimen without sedation in infants and children that made up the majority of the of the population.4,46 However, despite reported greater predisposition of infants under one to have renal lesions in UTI, some studies show a higher incidence of scintigraphic lesions in older patients, with no relationship between age and renal damage in infants and children with APN.46,47 In this sense, this study shows a positive correlation between age and scintigraphic pathology, although the majority of the patients were under one.
Normalisation of the urinary secretion of the different proteins studied after treating the infection was confirmed in every patient as described by others.4,15,19 This also occurred in patients with evidence of renal scars in the DMSA performed at a later stage, contrary to the reported relationship between microalbuminuria and reduction of renal parenchyma by pyelonephritic scarring, even in cases with normal GFR.20,22,23
On the other hand, the existence of VUR in the VCUG showed no correlation with the presence of acute scintigraphic lesions, renal scars or renal function parameters, data confirmed in studies with broader series.32,46,48
We can conclude that the DMSA renal scintigraphy in the acute phase remains the investigation of choice for the paediatric diagnosis of APN. Its sensitivity was not increased by using it in combination with renal function markers Further studies are needed to evaluate the alledged superiority of cystatin C in comparison to other parameters, and the use of new biomarkers, currently under investigation such as KIM-1, NGAL, NHE3, IL-6, IL-8, IL-18, Cyr61, actin, α-GST and π-GST.
Acknowledgements
To Dr D. Sanz from the Nuclear Medicine Department of the Virgen de la Arrixaca University Hospital (Murcia).
Table 1. Clinical and laboratory data according scintigraphic findings
Table 2. Renal function markers according to scintigraphic findings