The immunosuppressive combination most commonly used in de novo kidney transplantation comprises a calcineurin inhibitor (CI), tacrolimus, a mycophenolic acid derivative and steroids. The evidence which underlies this practice is based in the Symphony trial with controlled follow-up of one year, in which no comparator group included the combination CI-mTOR inhibitor. Different high-quality clinical trials support the use of everolimus as a standard immunosuppressive drug associated with reduced exposure of a CI in kidney transplantation. This combination could improve health related outcomes in kidney transplantation recipients.
The present recommendations constitute an attempt to summarise the scientific evidence supporting this practice, discuss false beliefs, myths and facts, and offer specific guidelines for safe use, avoiding complications.
La combinación inmunosupresora más utilizada en trasplante renal de novo incluye un inhibidor de calcineurina (IC), tacrolimus, un derivado del ácido micofenólico y esteroides. La evidencia que sustenta esta práctica se basa en el ensayo clínico Symphony, de evolución controlada durante un año, en el que no existía ningún grupo comparador con IC asociado a un inhibidor de mTOR. Diversos ensayos clínicos de alta calidad sustentan la indicación del uso de everolimus como inmunosupresor básico asociado a una exposición reducida de un IC en pacientes que reciben un trasplante renal. Esta combinación podría mejorar las expectativas de resultados en salud.
Estas recomendaciones tratan de aportar la evidencia científica que apoya esta práctica, discuten las falsas creencias, mitos y realidades de la combinación y concretan pautas que permiten utilizarla con seguridad y evitar complicaciones.
The immunosuppressive combination most commonly used in de novo kidney transplantation (KT) includes a calcineurin inhibitor (CNI), tacrolimus, a mycophenolic acid derivative (mycophenolate sodium or mycophenolate mofetil [MMF]) and steroids.1 This combination has reduced the incidence of acute rejection to 12% in 1 year.2 However, this decline in acute rejection has not been reflected in a proportional improvement in long-term graft survival.3 Moreover, there is still unresolved question about the high incidence of failures due to the early death of patients with functioning grafts, mainly as a result of cardiovascular disease,4 followed closely by malignant neoplasms (with increasing impact5,6) and infections. Cytomegalovirus (CMV) infection produces a significant morbidity and mortality in organ transplantation, but its prevention and treatment requires an adequate coordination and is very costly.
Minimisation or elimination of CNI while using mTOR inhibitor (imTOR) like sirolimus or everolimus, which acts through mechanisms complementary to the CNIs, achieves a synergic immunosuppressive effect that is not nephrotoxic. This strategy allows to reduce CNIs during the early post-transplant period. A decade after the publication of a review on the use of everolimus in its various therapeutic options,7 and after a lengthy development, a group of experts have conveyed in this document a series of reflections and recommendations about the use of everolimus in the de novo KT, a way to optimise the full potential of this drug in the KT recipient.
The mTOR inhibitors have demonstrated their potent immunosuppressive effect by inhibiting the action of T cells by their anti-proliferative and anti-migratory effect through the blockage of vascular endothelial growth factor (VEGF); they also reduce the development of atherosclerosis, so confers an anti-neoplastic and cardiovascular protective profile.7–11 Thus, in addition to their immunosuppressive effect, mTOR inhibitors have a number of characteristics that make them attractive for the use in KT: a) ability to prevent renal graft dysfunction through mTOR-mediated mechanisms, such as the reduction of glomerular hypertrophy and pro-inflammatory and pro-fibrotic cytokines, and the inhibition of epithelial-to-mesenchymal transition; b) reduction of angiogenesis; c) reduction of tumour growth and in de novo neoplasms; d) cardioprotective effects; and e) reduction of viral infections due to an increase in the specificity of CD8+ T cells against pathogens such as CMV during the immediate post-transplant period.
What is the advantage of the combination CNI-de novo everolimus?The early or late conversion to an mTOR inhibitor has shown benefits on the glomerular filtration rate (GFR), however the increased rate of acute rejection and the high incidence of intolerance to mTOR inhibitors due to side effects,10,11 makes desirable to search for alternative strategies.
The use of a CNI minimisation regimen combined with everolimus from the time of transplantation is a sufficiently potent combination to prevent rejection, and due to its immunosuppressive synergy it is possible to use less doses and prevent side effects.
In controlled studies, the short term outcomes of everolimus combined with reduced-dose CNIs are similar to the classical combination of CNI with mycophenolate. However everolimus combined with reduced-dose CNIs may provide advantages in several key aspects that are crucial for medium and long term survival of graft and patient. Therefore, leaders of some transplant centres from Spain and abroad have incorporated regimens for initial KT immunosuppressive therapy based on the combination of reduced doses and exposure of CNIs and everolimus. The potential advantages are stated below.
Immunosuppressive potency and immunomodulatory propertiesEverolimus plus tacrolimus minimisation has proven effective in the prevention of acute rejection, with an efficacy similar to that observed with the tacrolimus-MMF arm in the Symphony study.2,7,9–18 In fact, the de novo mTOR inhibitor-CNI combination prevents the higher incidence of acute rejection observed in early or late conversion of CNIs by mTOR inhibitors.19 The evidence will be reviewed in detail later.
It should be considered that besides the immunosuppressive effect, mTOR inhibitors have a completely different mechanism of action that the CNIs. The mTOR inhibitor blocks the cell cycle progression of activated T cells promoting a state of anergia, even in the presence of co-stimulation, thus inducing a state of functional tolerance.20 Through this effect, the incorporation of everolimus in the immunosuppressive regimen of KT patients could favour mechanisms of immunological tolerance that may improve long-term outcomes.
Minimising calcineurin inhibitor and renal toxicityThe combination of everolimus with a minimised dose of CNI results in a GFR that is between 3 and 6mL/min higher than with the standard-exposure to CNI.7,9–18 If the combination everolimus-cyclosporine is compared with mycophenolic acid-cyclosporine, the presence of mTOR inhibitor allows a 60% reduction of the CNI, an improved GFR and reduced proteinuria.
A recent meta-analysis has reported that anti-calcineurin minimisation was more beneficial when performed prior to 6 months post-transplant, with a better GFR and a lower risk of graft loss.13
Improvement in the cardiovascular profileThe everolimus-CNI combination improves the cardiovascular profile through two mechanisms: 1) adequate minimisation of the CNI allows better blood pressure control and a reduction in the number of anti-hypertensive agents, and 2) a direct beneficial effect of everolimus on the atherosclerotic plaque, observed in animal models of transplantation and in heart transplants, in which there is a reduction in allograft vasculopathy, peripheral vascular disease and left ventricular hypertrophy.8,21–24
It is important to understand that allograft vasculopathy in heart transplantation is a lesion that can have a counterpart in the renal graft. It also has a significant prevalence and is included in the Banff classification as index chronic vascular (CV) damage. This is an accelerated fibroproliferation of the graft vascular bed that causes circumferential intimal thickening. Its pathogenesis involves immune and non-immune factors that cause endothelial inflammation, endothelial injury and fibroproliferative cellular responses.25 The fact that everolimus can reduce allograft vasculopathy in heart transplantation, especially when used in the early post-transplant period,26,27 suggests that a similar benefit in KT could have major repercussions on both the patient and graft.
Improvement of the tumour risk profileIn KT, the potential benefit of the everolimus-CNI combination is due in part to CNI minimisation which is implicated in neoplastic progression, and also to the direct anti-neoplastic effect of everolimus; this has been observed in cancers that are common in transplantation, such as basal and squamous cell carcinomas, post-transplant lymphoproliferative syndromes, Kaposi saroma28,29 and also in general, as reported in the Convert study.6 Everolimus has been used for years as an anti-neoplastic agent in diverse oncology indications.30,31
Cytomegalovirus infectionsThe mTOR inhibitors reduce the incidence, severity and recurrence of CMV infection in the post-transplant period.32,33 A significant increase in the CMV-specific CD8+ T cell count has been reported in KT patients converted from cyclosporine (CsA) to everolimus. It is also able to block the phosphatidylinositol 3-kinase pathway, which is crucial for CMV replication.
In a pooled analysis of three randomised clinical trials on KT comparing everolimus with reduced-exposure CsA vs. mycophenolic acid (MPA) with standard-dose CsA, it was observed that the rate of CMV infection/disease was significantly lower with everolimus than with MPA, even in patients who received CMV prophylaxis.34
Tedesco-Silva et al., in a randomised, prospective, single-centre study on 288 patients without pharmacological prophylaxis for CMV infection (not even in patients with high risk of D+/R− infection), showed that the use of everolimus-tacrolimus in patients with a low/moderate immunological risk and induction with reduced-dose basiliximab or anti-thymocyte globulin (ATG) (3mg/kg) was associated with a lower incidence of CMV infection and disease than the group on standard treatment with tacrolimus-MMF and basiliximab induction.35
BK virus infectionThere is evidence that everolimus with reduced-dose CsA is associated with a low rate of BK infection as compared with the standard CNI-MPA regimen16,17; this is possibly due to the suppression of viral replication through inhibition of the protein kinase pathway activated by the BK virus. In addition, mTOR inhibitors permit the development of specific CD8+ T cells against the virus.36 Switching to everolimus has produced clinical benefits.37
Indication for everolimus in combination with low doses of calcineurin inhibitor in de novo kidney transplantationEvidence in the literature supports the use of everolimus as a basic immunosuppressant combined with reduced CNI exposure in KT recipients. These recommendations try to provide scientific evidence to support this practice, in addition to the opinion of experts if the published evidence is low.
Evidence for the de novo use of everolimus with a calcineurin inhibitor. Advantages and disadvantagesFour strategies have been developed for the use of mTOR inhibitors in KT: 1) the use with reduced-dose CNI in de novo KT; 2) preventive introduction of mTOR inhibitors with withdrawal or minimisation of CNI at some point of the KT in the absence of a clinical requirement38,39; 3) conversion from CNI to everolimus in response to certain clinical circumstances – a strategy that is only based on the analysis of patient series without randomised studies; and 4) CNI-free mTOR inhibitor regimen since the time of KT, of proven inefficacy due to the high incidence of acute rejection episodes and sub-optimal renal function.11
One of the most attractive aspects of the combined use of mTOR inhibitors with CNIs is that the same immunosuppressive efficacy of each agent is obtained but at lower doses. In the case of everolimus combined with CsA, the same efficacy is obtained with a 10%–20% lower dose of everolimus and a 20%–40% lower dose of CsA.40 For both CsA and tacrolimus, an everolimus C0≥3ng/mL is related with a reduction in the onset of acute rejection episodes: the range between 3 and 8ng/mL has been found to offer the best balance between a reduction in the acute rejection risk and clinical tolerability of the drug.15
Randomised clinical trials on the use of everolimus and reduced-dose calcineurin inhibitor in de novo kidney transplantationThere are results available from several randomised clinical trials on the use of everolimus combined with reduced-dose CNIs in patients with low to moderate immunological risk, with a follow-up of 6–24 months.11 Steroid and basiliximab induction was used in all trials except in the US92, in which induction with ATG was permitted as an alternative to basiliximab.18 The CNI used in three clinical trials was tacrolimus,14,15,18 while CsA was used in the rest.16,17,41–44
Incidence of acute rejection and efficacy of the reduced calcineurin inhibitor/mTOR inhibitor regimenStudies registered in Europe, showed that the use of everolimus allowed greater CNI minimisation (in this case CsA) with respect to the combination with mycophenolate. The study A2309, registered in the USA, confirmed these results.16 This non-inferiority study prospectively evaluated two regimens in de novo KT: everolimus combined with low-dose CsA versus mycophenolate sodium combined with standard-dose CsA. The primary endpoint was composite of efficacy which included: biopsy-proven rejection that was treated, graft loss, patient death or loss to follow-up at 12 months. In one of the everolimus groups, the patients received a starting dose of 0.75mg/12h with a target blood concentration of 3–8ng/mL. In the other one, the starting dose was 1.5mg/12h, targeted to 6–12ng/mL. The third patient group received a fixed dose of Myfortic® (720mg/12h) combined with standard-dose CsA. The CsA levels at one year were 55ng/mL in the everolimus 1.5mg/day group and 137ng/mL in the Myfortic® group. The incidence of rejection was similar in both groups.
The ASSET study used the combination of everolimus and low-dose tacrolimus in de novo KT.14 The target tacrolimus levels were 4–7ng/mL for the first three months, with everolimus levels of 3–8ng/mL throughout the entire study (12 months). From month 4 onwards, patients were randomised to maintain the same exposure to tacrolimus (4–7ng/mL) or to minimise it further (1.5–3ng/mL). One year post-transplant, tacrolimus patients in the 4–7ng/mL arm had a concentration of 5.5ng/mL, while patients in the 1.5–3ng/mL arm had a level of 3.4ng/mL. The incidence of acute rejection was 7.7% in the control arm (tacrolimus 4–7ng/mL throughout the first year). In the extreme tacrolimus minimisation arm, the incidence of rejection was 18%, although curiously, all the differences occurred during the first trimester and before randomisation, when the tacrolimus levels were similar in both groups.
Based on these findings, we can conclude that the incidence of rejection in the everolimus plus tacrolimus combination (with levels between 4 and 7ng/mL) for the first year is in a range comparable with the tacrolimus group in the Symphony study. The Transform study, currently underway, compares standard-dose tacrolimus or CsA with MPA against everolimus with a very low CNI dose in 2037 de novo KT patients.45
Study outcomes according to the recipients’ immunological riskIn low- and moderate-immunological risk KT, randomised trials show the non-inferiority of everolimus with reduced-dose CNI as compared to the standard-exposure CNI. Study US92 is the only clinical trial to include a small percentage of high risk patients.18 Patients randomly received everolimus or mycophenolate with reduced- or standard-exposure tacrolimus, respectively. The rate of biopsy-confirmed acute rejection was higher in the everolimus and reduced-dose tacrolimus group, because the tacrolimus and everolimus levels were excessively low and the proportion of patients with a high immunological risk was higher in the reduced-dose tacrolimus group.
In vitro, everolimus can cause early and delayed inhibition of B cell proliferation and the subsequent differentiation into plasma cells; this is unlike MPA, which acts only on the early stages of the B cell-mediated immune response.46,47 Despite this, randomised clinical trials that have compared the de novo use of everolimus with reduced-dose CNI versus a standard regimen have excluded patients at high immunological risk based on different criteria: percentage of panel reactive antibodies, prolonged ischaemia time, chronic infections, re-transplants, elderly donors or recipients, T cell positive cross match or ABO-incompatible transplant.9–18 The conversion studies available have also excluded patients with high panel reactive antibodies or with recent acute rejection.18
To date, there are not published clinical trials in which the de novo development of donor-specific antibodies (DSA) constitutes one of the objectives, and there is virtually no comparative information on the development of a humoral response in regimens based on CNI-everolimus or more standard regimens with mycophenolate.
Outcomes according to the calcineurin inhibitor doseThe everolimus and reduced-dose CNI regimen has proven comparable to a conventional CNI and MPA regimen: it has shown non-inferiority with respect to the composite primary endpoint (treated rejection, graft failure, death or loss to follow-up),16,17 even when everolimus was used with very low levels of CNI.14
In the everolimus group a higher percentage of patients (20% vs. 12%) started with a GFR <60mL/min and increased above 60mL/min at one year post-transplant. Even with relatively high everolimus exposure (8–12ng/mL)42,44 and very reduced-dose CsA, the GFR was significantly higher than with the standard MPA plus CNI regimen, with the same incidence of biopsy-confirmed acute rejection.44
Importance of everolimus and tacrolimus levelsEverolimus is an mTOR inhibitor derivative of sirolimus with greater oral bioavailability and a shorter half life; thus monitoring four days after starting treatment is sufficient to determine any dose adjustment, this is unlike the 5–7 days required in the case of sirolimus.48 Exposure to everolimus is influenced by the type of concomitant CNI used, due to its different pharmacokinetic interactions. In fact, the administration of CsA increases exposure to everolimus two- to three-fold,49 since both drugs are metabolised by cytochrome P450 3A4 and are P-glycoprotein drug transporter substrates. In contrast, tacrolimus does not present such a major interaction with everolimus, therefore, although the mTOR inhibitor reduces the bioavailability of tacrolimus in a dose-dependent manner,50 the area under the curve (AUC) for everolimus does not change with the different doses of tacrolimus.51 Pharmacokinetic monitoring is essential to prevent adverse effects, and to detect drug interactions with their potential toxicity.49 Numerous studies have revealed that both immunosuppression and side effects are dose- and concentration-dependent.52
Everolimus presents a good correlation between C0 and the AUC, so that the C0 is a good measure of drug exposure and a good indicator of the clinical course (higher efficacy and lower toxicity).53
Adequate exposure to everolimus treatment has been set above 3ng/mL.54 To achieve this, the recommended starting dose of everolimus, if administered together with tacrolimus, is 1.5mg/12h, unlike in the case of CsA, where 0.75mg/12h is recommended.50,51 In trial US92, an inadequate starting dose of everolimus of 0.75mg every 12h (based on previous clinical trials using CsA as CNI) starting from day 5 post-transplant was too low and late, resulting in mean everolimus levels of less than 3ng/mL on day 7 post-transplant. This was the main cause of a high incidence of acute rejection observed in the everolimus/tacrolimus arm (19.1% versus 11.2% in the MPA arm).18
We believe that an everolimus dose of 1.5mg every 12h from day 1 post-transplant should be given in combination with tacrolimus to achieve levels of 3–8ng/mL in the early phase of transplantation.55 In many patients, after determination of blood levels on day 3–4° post-transplant, the dose has to be increased by 50% to reach the range.
There is not a published clinical trial comparing reduced-dose CsA with everolimus against reduced-dose tacrolimus with everolimus. However, the transform study includes patients stratified in both groups who will be analysed in the future.45 The choice of one or another regimen should be considered taking into account the risk profile of the pair/recipient for comorbidities such as diabetes, or cost restrictions in the setting in which it is to be used, given the fact that a combination with CsA means saving on the everolimus dose.
Importance of inductionWith the aim of adequately minimising the CNI and effectively preventing acute rejection, the use of antibody induction is recommended with this regimen in de novo KT. Most clinical trials have used induction with basiliximab, although induction with ATG may also be used.18,35,45
False beliefs, myths and realities of de novo use of everolimus in kidney transplantationThe mTOR inhibitors have beneficial effects on cardiovascular stability,23–26 reduced infections33–35 and a decreased incidence of neoplasms.28,29 However they are most commonly used in conversion regimens, because of the failure or adverse effects of the CNI or mycophenolate derivatives or, the onset of a neoplasm. The reality is that the de novo use of everolimus is scant. In our opinion, the fundamental reason for not using a minimised CNI and everolimus-based immunosuppression regimen is a set of myths and beliefs, perhaps due to prior experience with sirolimus using loading doses, disproportionately high levels and combined with MMF instead of CNIs. Another major reason is the result of the Symphony study, which concluded that the best immunosuppressive regimen in KT comprised tacrolimus, MMF and steroids, without having included any treatment arm combining CNI and mTOR inhibitors.2 Other additional reasons are the alleged lack of efficacy in the prevention of acute rejection, the possible increase in acute tubular necrosis, healing problems and the fear of adverse effects and the management thereof (Tables 1 and 2).
Incidence of acute rejection in patients treated with a calcineurin inhibitor combined with everolimus vs. MMF.
| Study | Cyclosporine-everolimus | Cyclosporine-MMF | Tacrolimus-everolimus | Tacrolimus-MMF |
|---|---|---|---|---|
| A230916,17 | 16.2 | 17 | ||
| A120258 | 4.9 | 8.2 | ||
| US0915 | 14 | |||
| ASSET14 | 7.7a | |||
| Symphony2,56 | 15.4 | |||
| ABOi59 | 12 | |||
| Paediatric KT60 | 6 | 13 |
Data (%).
MMF: mycophenolate mofetil.
Main complications considered to be a impediment to de novo use of an mTOR inhibitor and their incidence in various clinical trials.
| A2309 (CsA) EVE vs. MPA16,17 | US09 (tacrolimus)15 | ASSET (tacrolimus)14 | Symphony2,56 | |||||
|---|---|---|---|---|---|---|---|---|
| EVE (1.5mg/d) | MPA | EVE (1.5mg/d)+low tacrolimus | EVE (1.5mg/d)+standard tacrolimus | EVE (1.5mg/d)+very low tacrolimus | EVE (1.5mg/d)+low tacrolimus | Tacrolimus-MMF | Sirolimus-MMF | |
| Delayed graft function | 10.2 | 9.2 | 0 | 2 | NA | NA | 35.7 | 21.1 |
| Lymphocele | 6.6 | 5.1 | 4.1 | 2.3 | 7.3 | 10.9 | 4 | 11.6 |
| Surgical wound | 1.8 | 1.1 | 4.1 | 2.3 | 18.3 | 14.3 | 2.5 | 2.4 |
| Incisional hernia | 1.5 | 1.5 | 2 | 4.7 | 3.7 | 1.7 | NA | NA |
| Acute rejection | 16.2 | 17 | 14 | 14 | 18.7 | 7.7 | 15.4 | 39 |
Data (%).
EVE: everolimus; MMF: mycophenolate mofetil; MPA: enteric-coated mycophenolic acid; NA: not available.
Results from the Symphony study show that the incidence of acute rejection at 36 months was 39% in the sirolimus minimisation/MMF group without CNI compared to 15.4% in the tacrolimus/MMF group.2,56 In this study, the combination sirolimus and tacrolimus resulted in 12.2% of rejection, which is similar to that obtained with tacrolimus/MMF.57 As stated in the previous section, studies conducted with the concomitant administration of de novo everolimus plus CsA or tacrolimus show that the incidence of rejection is similar or even less than in the Symphony study (Table 1).2,14–18,56,58–60 Thus, the belief that a CNI minimisation plus everolimus regimen is associated with higher rates of rejection than the traditional MMF regimen is not supported by the available evidence.
Delayed graft functionWith respect to the hypothetical higher incidence of delayed graft function (DGF), early studies with sirolimus did not show a higher frequency of DGF; even in the Symphony study, the sirolimus group presented the lowest DGF (21.1% vs. 35.7% in the tacrolimus/MMF group).2,56 No clinical study comparing CNI-everolimus and CNI-MPA has shown differences in the onset of DGF (Table 2).
Healing problems and lymphocelesInhibition of the mTOR pathway limits cell proliferation and angiogenesis of endothelial cells and fibroblasts, and therefore it may delay the fibrotic component necessary for wound healing and the recanalisation of lymphatic vessels. However, as shown by Azzola et al.61 the inhibition of fibroblast proliferation is higher with everolimus than with tacrolimus, CsA, azathioprine and steroids, but this inhibition is still more accentuated with MMF.
The relationship of mTOR inhibitors with surgical wound complications is based on the initial findings when high doses of mTOR inhibitors were used. With the mTOR inhibitor concentrations currently used and without loading doses, accompanied by reduced-dose CNIs, there is no evidence of an increase risk of events as compared to a full-dose CNI and MMF regimen (Table 2).62
One study that compared the incidence of lymphoceles reported 19% in the MMF group compared to 10% in the azathioprine.63 In the multivariate analysis, the use of MMF was associated with a 2.6-fold higher risk of lymphoceles as compared to azathioprine.
Elimination of loading doses and very high levels of sirolimus, modification of the surgical technique and excluding patients with body mass index (BMI) >32kg/m2 has considerably reduced the incidence of wound complications and lymphoceles.64
Clinical trials with CNI-everolimus, with both CsA16,17,62,65 and tacrolimus,14,15 revealed a similar incidence of wound complications and lymphoceles in patients treated with CNI combined with everolimus or MMF.
Based on the routine clinical practice of the authors of these recommendations, and in agreement with recent reviews by experts in he field,66 no differences have been observed in the incidence of DGF or in the duration of initial hospitalisation between patients using CNI-MPA and those using CNI-everolimus at the current low doses and levels (Table 2). No special therapeutic measures are required for the care of the KT surgical wound, which should be revised daily as in any KT during the first hospital admission and thereafter at subsequent clinical visits, with the removal of sutures recommended after week 3.
The exception would be the case of obese patients, who already present a high risk of surgical wound problems and in whom it would be best to avoid the use of everolimus or sirolimus. There is no evidence that they should be used with caution in elderly or diabetic patients, who also have a greater tendency to present surgical wound complications.9–11,66
Peripheral oedemaThe onset of palpebral and lower limb oedema has been associated with the use of both sirolimus and everolimus, although some studies suggest that it is less frequent with everolimus.67
As in the previously reported side effects, the onset of oedema is related with high doses and elevated levels of mTOR inhibitors.68,69 The mechanism responsible for oedema would be an increased vascular permeability that may be related to an increase in prostacyclin and a decrease in VEGF. Current studies combining everolimus with CsA report an incidence of oedema between 2.6%16,17 and 32%58 (Table 3). The first study refers to moderate–severe oedema and the second one to mild–moderate oedema, which in no case led to discontinuation of the drug. With the combination everolimus and tacrolimus, the incidence of oedema is 10%, similar to that reported in the Symphony study.14,15 In our routine clinical practice, de novo use is not associated with oedema of higher frequency or intensity than with other regimens. After ruling out other causes of oedema, mTOR inhibitor dose adjustment is recommended and, if CsA is combined with everolimus, adjust CsA to the lowest dose, since CsA increases tissue exposure to everolimus.
Incidence of the main adverse events classically related to mTOR inhibitors.
| A2309 (CsA) Eve vs. MPA16,17 | US09 (tacrolimus)15 | ASSET (tacrolimus)14 | Symphony2,56 | |||||
|---|---|---|---|---|---|---|---|---|
| EVE (1.5mg/d) | MPA | EVE (1.5mg/d)+low tacrolimus | EVE (1.5mg/d)+standard tacrolimus | EVE (1.5mg/d)+very low tacrolimus | EVE (1.5mg/d)+low tacrolimus | Tacrolimus-MMF | Sirolimus-MMF | |
| Oedema (%) | 2.6 | 1.4 | 9.3 | 10.2 | 9.2 | 10.9 | 12 | 32 |
| Hyperlipidaemia (%) | 21 | 16 | 10.2 | 9.3 | 26 | 21 | 9.9 | 15.8 |
| Proteinuria [>0.5g/24h (%)] | 9.1 | 7.3 | 0 | 2.3 | 7 | 11 | 5.3 | 5 |
| Diabetes (%) | 14 | 16 | 24 | 38 | 15.1 | 12.8 | 10.6 | 7.8 |
EVE: everolimus; MMF: mycophenolate mofetil; MPA: enteric-coated mycophenolic acid.
Hypercholesterolemia and hypertriglyceridemia are adverse effects commonly reported in KT and become more evident when mTOR inhibitors are used, since these drugs decrease apolipoprotein B-100 catabolism and reduce lipoprotein lipase activity. The incidence is higher when combined with CsA (up to 45% vs. 16% in the control group)16,17,58,65 and somewhat less when combined with tacrolimus (9.3%–21%)14,15 (Table 3). In our groups, the incidence of hypercholesterolemia was 40%–50% at month 6, using statin in around 70% (unpublished data).
In regimens with everolimus, the incidence of post-transplant diabetes mellitus (PTDM) ranges between 11.5% and 14% if it is combined with CsA,16,17,53 or 12.8% and 24% if combined with tacrolimus,14,15 this similar to that observed in the Symphony study2,56 (Table 3). There is no evidence that minimised CNI-everolimus regimen is associated with more frequent or more severe PTDM than with use of CNI-MPA regimens.
Mouth ulcersMouth ulcers appear to result of a direct toxic mechanism of mTOR inhibitors on the oral and nasal mucous membranes.70 They usually appear at the beginning of the therapy, approximately after one week of exposure, and are usually dose-dependent. In the authors’ personal experience, the incidence is much lower if the drug is used de novo rather than in conversion. Ulcers usually begin to heal after the dose adjustment of the mTOR inhibitor or topical corticosteroid treatment.
Haematological effectsThe use of these drugs has been associated with an increased risk of leucocytopenia and thrombocytopenia as compared with CNIs,71 an effect that may not be dose dependent. In the case of conversions from CNIs to mTOR inhibitors, the increased in the dose of MMF may be in relation with haematological toxicity. There are studies in which conversion to mTOR inhibitors is associated with greater leucocytopenia and thrombocytopenia; by contrast the A2309 trial, show a higher leucocyte and platelet count in de novo everolimus and reduced-dose CNIs than in a standard regimen with MPA.16 The use of mTOR inhibitors may also affect the risk of anaemia, but low haemoglobin is not usually an exclusion criterion for its use. In the opinion of the authors of this review, anaemia is not usually a significant clinical problem with the reduced doses used in combination with CNIs, so it is always essential to rule out other causes of bone marrow suppression.
Development of proteinuriaThe mechanism by which mTOR inhibitors may cause proteinuria in the KT recipient is not well understood.72 They may produce proteinuria by inhibiting VEGF, which alters endothelial and podocyte function. Although the most well known mTOR inhibitor-associated proteinuria is presented after conversion of a CNI to an mTOR inhibitor,70 it has also been observed with de novo sirolimus.73 In studies on de novo everolimus, the incidence of proteinuria is low or similar to the control group (Table 3). In fact, in trials A2306 and A2307 on de novo everolimus with CsA, less than 5% of patients presented proteinuria in a spot urine sample.9,74 In the A2309 trial, no differences were observed between the mycophenolate arms and low everolimus doses with levels of 3 to 8ng/mL.75 The incidence of everolimus associated proteinuria reported in the Symphony study was 12% vs. 8% in the tacrolimus-MMF group.
PneumonitisThe potentially most serious adverse effect associated with mTOR inhibitors is interstitial pneumonitis. It is an immune-mediated process. The initial incidence associated with sirolimus was 5%–15% in solid organ transplant,76 which is somewhat higher than that described by Spanish groups in KT (4%–7%).77,78 Smoking and pre-existing lung disease have been found to predispose the patient to pneumonitis. Likewise, greater exposure to the drug has been related with a higher degree of lung toxicity. Although clinical presentation varies from minimal symptoms to severe respiratory failure, the reported cases generally mention mild–moderate signs and symptoms that subside after withdrawal of the drug.
A case series with the largest number of everolimus-induced interstitial pneumonitis was published recently.79 This was a case-control study in which 13 KT recipients (12.7% of the cohort) developed the disease after conversion from CsA-MPA-prednisone to everolimus-prednisone after 6 months post transplantation. The median time of everolimus exposure until pneumonitis was 5.5 months. Although the authors did not find predisposing factors, there was a higher incidence of previous lung disease in those who developed pneumonitis (31% vs. 17%). Moreover, while the blood everolimus levels of those who developed the complication were not greater than those who did not, the exposure was high in all cases (trough levels of around 10ng/mL in both groups). However, the description of this event seems much more unusual in the de novo use of everolimus combined with reduced-dose CNIs. In fact, in a retrospective review of trial A2309, the authors were only able to identify one patient who developed pneumonitis out of a total of 556 patients exposed to everolimus.80
Appearance of de novo anti-HLA antibodiesThe scant evidence on the potential development of DSAs in patients treated with mTOR inhibitors is limited to studies on conversion to an mTOR inhibitor after 3–4.5 months of CNI-MPA therapy. The experience of one transplant centre in Berlin was that conversion from a CsA-MPA-based regimen to everolimus-MPA appeared to be associated with an increase in the de novo appearance of DSAs and humoral acute rejection.81 In the aforementioned study, the selection bias and under-immunosuppression of patients converted to everolimus was notable, particularly concomitant steroid withdrawal.82 Other similar studies have failed to demonstrate the same outcomes.83–85
In ABO-incompatible KT treated with everolimus and CsA, 4 out of 25 patients developed DSA, 3 in relation to previous KTs.59 In paediatric KT, 11.4% of patients treated with everolimus developed DSA compared to 17.9% in the low tacrolimus-MMF control group.60 There is no evidence from controlled clinical trials that describe DSAs in de novo KT with CNI minimisation and everolimus-based regimens.
Safety in the management. Recommendations for preventing complicationsContraindications in relation to the de novo use of everolimus in kidney transplantationThe best strategy to avoid serious drug-related adverse effects is to refrain from using the drug in patients with a special risk for develop adverse effects (Table 4). The de novo use of everolimus should be avoided in KT patients with hypersensitivity to the drug or its excipients, severe hyperlipidaemia that cannot be controlled using standard treatment, history of interstitial lung disease or severe chronic obstructive pulmonary disease, obese patients with a BMI >35, cases of primary focal segmental glomerulosclerosis or atypical haemolytic–uraemic syndrome, and in those who require complex vascular surgery during the renal transplant. Finally, although there is positive unpublished experience about its use, it should be applied with caution in patients requiring high doses and elevated levels of CNI, such as patients with a very high immunological risk.
Relative contraindications to the use of everolimus in de novo KT and reasons for advising against it.
| Relative contraindication | Reason |
|---|---|
| Interstitial lung disease or severe chronic obstructive pulmonary disease | To avoid exposure to the drug in recipients susceptible to suffering mTOR-inhibitor-associated pneumonitis |
| Obesity with a body mass index greater than 35kg/m2 | To avoid exposure to the drug in subjects with a greater tendency to suffer surgical wound complications and lymphoceles due to their obesity |
| Primary focal segmental glomerulosclerosis as underlying nephropathy | Potential of the drug to cause proteinuria by podocyte damage and development of focal segmental sclerosis |
| Atypical haemolytic–uraemic syndrome | Since the aetiopathogenic association of mycophenolate with thrombotic microangiopathy syndromes has not been described, its combination with CNIs is considered more advisable in de novo kidney transplantation |
| Complex vascular surgeries (e.g. renal artery anastomosis to a Gore-Tex iliac stent) | To avoid high risk of suture dehiscence |
| Need for use of high doses and elevated levels of CNI, such as for example, those with a very high immunological risk | To avoid nephrotoxicity with maximisation of the CNI |
CNI: calcineurin inhibitor.
Early experiences with de novo sirolimus tell us that the starting dose of mTOR inhibitors should be low from day 1 post-transplant. The desired levels of everolimus when used in combination with a CNI, are between 3 and 8ng/mL.55 When used in combination with a CNI, potential pharmacological interactions between the two drugs should be monitored; the everolimus dose of 1.5mg/12h indicated in combination with tacrolimus frequently requires an increase in the dose of everolimus to reach an adequate level; thus, a mean dose of 2.5mg/12h after the first month and 2mg/12h after 12 months is needed.51 This increase is usually unnecessary, or in any case is much less, when the CNI used is CsA.
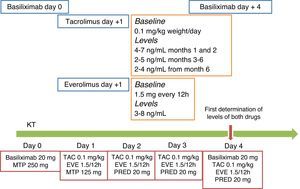
As shown in Fig. 1, levels of everolimus should be maintained around 4ng/mL, within a range of 3–8ng/mL, during the first three weeks to facilitate surgical wound healing and not to contribute to lymphocele formation.
Proposed protocol to start immunosuppression therapy using reduced-dose CNI and de novo everolimus. As in most published studies that use this combination, patients should be induced with anti-IL2r monoclonal antibodies (basiliximab). Although the experience is limited, induction can also be applied with anti-thymocyte globulin following a standard or minimised protocol. The initiation of everolimus days or weeks after the transplant surgery has not shown any benefits, so starting on day 1 post-transplant is recommended. The everolimus starting dose is 1.5mg every 12h if the CNI is tacrolimus, and 0.75 every 12h if the CNI is cyclosporine. Blood levels should be measured at the 3rd or 4th post transplant day, and the dose should be increased by 50% if levels are less than 3ng/mL. The therapeutic range is 3–8ng/mL; the dose should be maintained close to 3ng/mL for the first 3 weeks to subsequently maintain levels of 5–7ng/mL during the first trimester. CNI should be started on the day of the transplant or within the first 24h, maintaining a tacrolimus level of 4–7ng/mL during the first two months, 2–5ng/mL thereafter, and even dropping to 2–4ng/mL after the 6th month. A simple rule that can be applied is that the total sum of tacrolimus and everolimus concentration may be around 10mg/ml, prioritising tacrolimus the first month and everolimus thereafter. The regimen outlined in the figure is the protocol currently applied at the Hospital del Mar. ATG: anti-thymocyte globulin; EVE: everolimus; CNI: calcineurin inhibitor; MTP: methylprednisolone; PRED: prednisone; TAC: tacrolimus.
Tacrolimus levels with concomitant everolimus use should be focused on reducing CNI exposure and avoid CNI-associated adverse effects such as infections, neoplasms, and nephrotoxicity. However, as mentioned with respect to everolimus but in the opposite direction, the tacrolimus levels in the first three months post-transplant should be maintained at around 7ng/mL, with a subsequent dose reduction to optimise protection against acute rejection. A simple rule that can generally be applied is that the tacrolimus and everolimus levels may always total around 10ng/mL, prioritising tacrolimus in the first month and everolimus thereafter.
No special measures are required in the care of the KT surgical wound, which should be examined daily as in any KT during the first hospital admission and thereafter at subsequent visits, with removal of sutures after week 3.
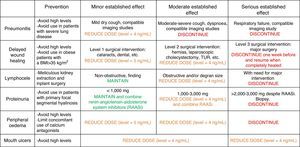
Management of complications: the timing to reduce the dose and when to discontinue the drugIn general, if a serious adverse effect that can compromise patient safety occurs, the correct practice is to discontinue the drug that appear to be related with adverse effect. However, in many occasions, one of us is faced with a mild or moderate adverse effect –tolerance issues that worsen the patient's quality of life and prolong the medical attention, which make us to discontinue the drug (Fig. 2).
Management of the adverse effects and clinical situations associated with the use of everolimus. In each of these situations, causes unrelated to everolimus must always be ruled out before withdrawing the drug without a solid reason. BMI: body mass index; RAASi: renin–angiotensin–aldosterone system inhibitors; TUR: transurethral resection.
Knowing when to reduce the dose or when to discontinue the drug depends on the physician's experience and on the tolerance that both doctor and patient may have in a given situation. The balance adverse effect/potential benefit should be applied, as with any other immunosuppressive drug. Curiously, the rate of dose reductions and withdrawals at the authors’ transplant units are no different in everolimus- and MPA-based regimens, as concomitant drugs to CNIs.
The “easy” response to any adverse situation that might be potentially associated with everolimus is to discontinue the medication, even if the observed effect is not drug-related or when no benefit is expected after its discontinuation. It is therefore necessary to discern between the need for a simple adjustment of doses and levels and complete withdrawal of the drug, which often, it is not indicated (Fig. 2).
ConclusionsThe myths associated with the use of mTOR inhibitors do not match the current reality in which doses are used to obtain levels of between 3 and 8ng/mL together with low doses and levels of CNI., In patients with a low to moderate immunological risk, this combination:
- -
Allow a reduced exposure to CNIs during the first few weeks post-transplant, without increasing the risk of acute rejection and with a potential medium- to long-term improvement of renal function.
- -
Has not been used systematically in patients with a high immunological risk, and information on its relationship with the development of DSAs has been controversial.
- -
Is associated with a reduced risk of viral infections in the immediate post-transplant period: in patients with a moderate risk of CMV infection, even in the absence of prophylaxis for CMV, or by BK.
- -
Has not been associated with an increase in surgical wound complications; however its use is not recommended in patients with increased risk, such as patients with a BMI > 35kg/m2.
- -
Dyslipidaemia that cannot be controlled with standard treatments is a relative contraindication for the use of an mTOR inhibitor.
- -
If it does not contraindicate the transplant, moderate–severe lung disease is another relative contraindication to the use of everolimus, due to the possible increase in the incidence of pneumonitis.
- -
Has not been associated with an increased risk of proteinuria as compared with standard regimens, although it is not recommended in patients with focal segmental glomerulosclerosis.
- -
The absence of significant pharmacological interactions with everolimus, together with its higher immunosuppressive effect, makes it advisable the use of tacrolimus, rather than CsA, in combination with everolimus.
- -
Although cardiovascular and neoplastic complications are of multifactorial origin, the antiproliferative and anti-tumour effect of mTOR inhibitors may be an advantage for long-term patient survival.
- -
Other problems related with the use of high-dose CNIs, such as neurotoxicity, could be improved with the combination of CNI and everolimus, which enables a reduction in the doses and levels of CNI.
This document of recommendations is a product of the joint work of the authors and a collaboration agreement between Novartis Farma and the Spanish Society of Nephrology, which endorses it. Novartis did not have access to its content and has not influenced the elaboration of the manuscript.
Please cite this article as: Pascual J, Diekmann F, Fernández-Rivera C, Gómez-Marqués G, Gutiérrez-Dalmau A, Pérez-Sáez MJ, et al. Recomendaciones para el uso de everolimus en trasplante renal de novo: falsas creencias, mitos y realidades. Nefrologia. 2017;37:253–266.