Objetivos: Conocer el estado actual del seguimiento de la función renal realizada a los pacientes tratados con antiinflamatorios no esteroideos (AINE). Material y métodos: Se seleccionaron los pacientes adultos atendidos en un centro de Atención Primaria de la Comunidad de Madrid que recibieron algún AINE por primera vez. Se analizó si durante los 2 meses previos y los 6 posteriores a la prescripción del AINE se conocía la función renal. Resultados: Durante el período de estudio se registraron 42.822 prescripciones. Un total de 8611 figuran como nuevas prescripciones, 482 de las cuales (5,6 %) fueron prescripción de AINE y se realizaron en pacientes mayores de 14 años. Recibieron algún AINE 450 pacientes (64 % mujeres). Ibuprofeno (66,0 %) fue el más frecuentemente prescrito. El grupo de edad con más prescripciones de AINE fue el de 14-45 años. Solo 168 (37,1 %) cuentan con alguna analítica solicitada durante el estudio (68 % mujeres). Antes de recibir AINE, solo en el 14 % (63 pacientes) se conocía el valor de creatinina sérica. Dos pacientes recibieron AINE pese a tener cifras elevadas de creatinina. Tras la prescripción se solicitó creatinina sérica en 129 pacientes (28,7 %). Conclusiones: Se prescribe un número importante de AINE. El más utilizado es el ibuprofeno. Las prescripciones son más frecuentes en mujeres y en personas de entre 14-45 años de edad. El dolor musculoesquelético es la causa principal de esta indicación. Solo en el 14 % de los pacientes a los que se les trató con AINE se conocía el valor de creatinina, que no siempre se tuvo en cuenta a la hora de la prescripción. El control de la función renal tras prescribir AINE fue porcentualmente bajo.
Objectives: To determine the current state of renal function monitoring carried out on patients treated with NSAIDs. Material and Method: We selected patients from a Primary Care Centre who had received NSAIDs for the first time. We checked if renal function was measured and/or controlled 2 months pre/6 months post-NSAID administration in order to assess if patient renal function was known at the time of prescription and afterwards. Results: During the study period, there were 42 822 prescriptions made. Of these, 8611 were new drug prescriptions, of which 482 (5.6%) were NSAIDs in patients older than 14 years of age. A total of 450 patients (64% female) were treated with NSAIDs. Ibuprofen (66.0%) was the most commonly prescribed. NSAIDs were more frequently used in patients between 14-45 years of age. Only 168 (37.1%) patients underwent any analytical tests over the course of the study (68% female). Before prescription, renal function was measured in only 14% of cases (63 patients). Two patients received NSAIDs despite having high serum creatinine levels. During the follow-up, serum creatinine was measured in 129 patients (28.7%). Conclusions: In primary care, NSAIDs represent a substantial percentage of the drugs prescribed (5.6%). Ibuprofen is the most commonly prescribed. NSAIDs are more frequently used in women between 14-45 years. Musculo-skeletal pain is the main indication for prescription. Only 14% of patients receiving these drugs had previously measured levels of serum creatinine. These values are rarely taken into account when prescribing NSAIDs. Control of renal function after NSAID prescription was unusual.
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) constitute a heterogeneous group of medications that are widely used with varying levels of analgesic, anti-pyretic, anti-platelet, and anti-inflammatory activities. These drugs are the first step in providing analgesic treatment based on the recommendations of the World Health Organisation (WHO).
The consumption of NSAIDs in Spain has increased in recent years from 23.67 daily doses per 1000 inhabitants per day (DHD) in the 1990s to 45.8DHD in 2003.1 In our country, acetyl-salicylic acid is one of the top 10 most widely sold drugs (source: IMS EMF audit, December 2010). Given the characteristics of our population, the use of these drugs will probably continue to increase.
NSAIDs often produce alterations in renal function and various organ systems, with gastrointestinal, haematological, and cardiological impacts, particularly in elderly patients, where the presence of other pathologies such as diabetes, chronic kidney disease, arteriosclerosis, and simultaneous consumption of other drugs, especially those that affect renal function and vascularisation (angiotensin II receptor blockers [ARB], ACE inhibitors, diuretics, etc.), all magnify the deleterious effects of NSAIDs. As such, these drugs are not considered to be innocuous, and must be prescribed with caution in high-risk patients.2-7
Currently, we have no information regarding whether the state of renal function or possible consequences of NSAIDs are taken into account prior to prescription, especially in patients who might be considered to be particularly more vulnerable, whether due to elevated comorbidity or concomitant prescriptions with potential toxicity implications.
The objective of our study was to evaluate the current state of monitoring of renal function in patients treated with NSAIDs, both at the moment of prescription and during treatment.
MATERIAL AND METHOD
We designed an observational, retrospective study that was approved by the clinical research ethics committee of the Hospital Universitario Ramon y Cajal, and that was carried out in accordance with the standard regulations for this type of study.
We chose to evaluate patients at a health institution that would be representative of the entire population in the health area of the Community of Madrid. We received collaboration from the directors of the primary care department, and using the data registered in the Digital Medical Office system, we selected all patients who had received a prescription during the study period, analysing cases in which NSAIDs were prescribed. The study period was October-November 2006, so as to avoid vacation periods in which the population might show substantial variations during the programmed follow-up.
We examined whether or not renal function was measured and/or controlled prior to and/or after the prescription of NSAIDs. For this analysis, we included all patients older than 14 years of age who had been prescribed an NSAID during the study period, as long as there was no other registry of a different NSAID being prescribed to the same patient for at least 8 weeks prior to the date of the prescription in question. This “new prescription” also had to be maintained for at least 7 days.
Once the study population was selected, we identified which patients had been tested for renal function during the study period. We considered renal function to have been evaluated if the clinical history included a registry of creatinine values during a six-month period prior to the prescription. We examined whether or not renal function was monitored over time in the form of creatinine measurements during the 3 months following the prescription. For this analysis, we received the collaboration of the Clinical Biochemical Department of the Hospital Universitario Ramon y Cajal, whose laboratory performs all such analyses for the health area that treats the patients from our study region. By using digital medical information, we had access to the diagnosis associated with each prescription and laboratory analysis request, as well as information regarding comorbidity, concomitant treatment, and the cause for all hospitalisations.
As secondary objectives, we analysed the type of patients in which renal function was monitored, as well as factors that may have influenced whether or not this parameter was measure in our patients: type of NSAID prescribed, reason for prescription based on age, sex, associated comorbidity (diabetes, hypertension, chronic pulmonary obstructive disease, cirrhosis, heart failure, gastropathy), and concomitant treatment (beta-blockers, ACE inhibitors, ARB, diuretics, calcium channel blockers, digitalis, gastric protectors etc.). We described all complications that appeared between the start of treatment and 6 months afterwards. For this analysis, we defined renal failure as a 50% increase in creatinine values over the baseline measurement, as long as these data were available, or an absolute value of creatinine >1.3mg/dl in females or >1.5mg/dl in males. Hospitalisation was categorised as Yes or No. We considered hospitalisations to be all periods spent in the hospital of at least 24 hours within the department to which patients were sent from the emergency department. We also examined the cause for the original decompensation and subsequent hospitalisation.
Finally, we analysed all summary of product characteristics for the NSAIDs approved for use in Spain and that were available on the website of the Spanish Agency of Medicines and Medical Devices (www.aemps.es), and evaluated whether these data contained contraindications, special precautions, or dosage modifications for use in patients with renal failure.
RESULTS
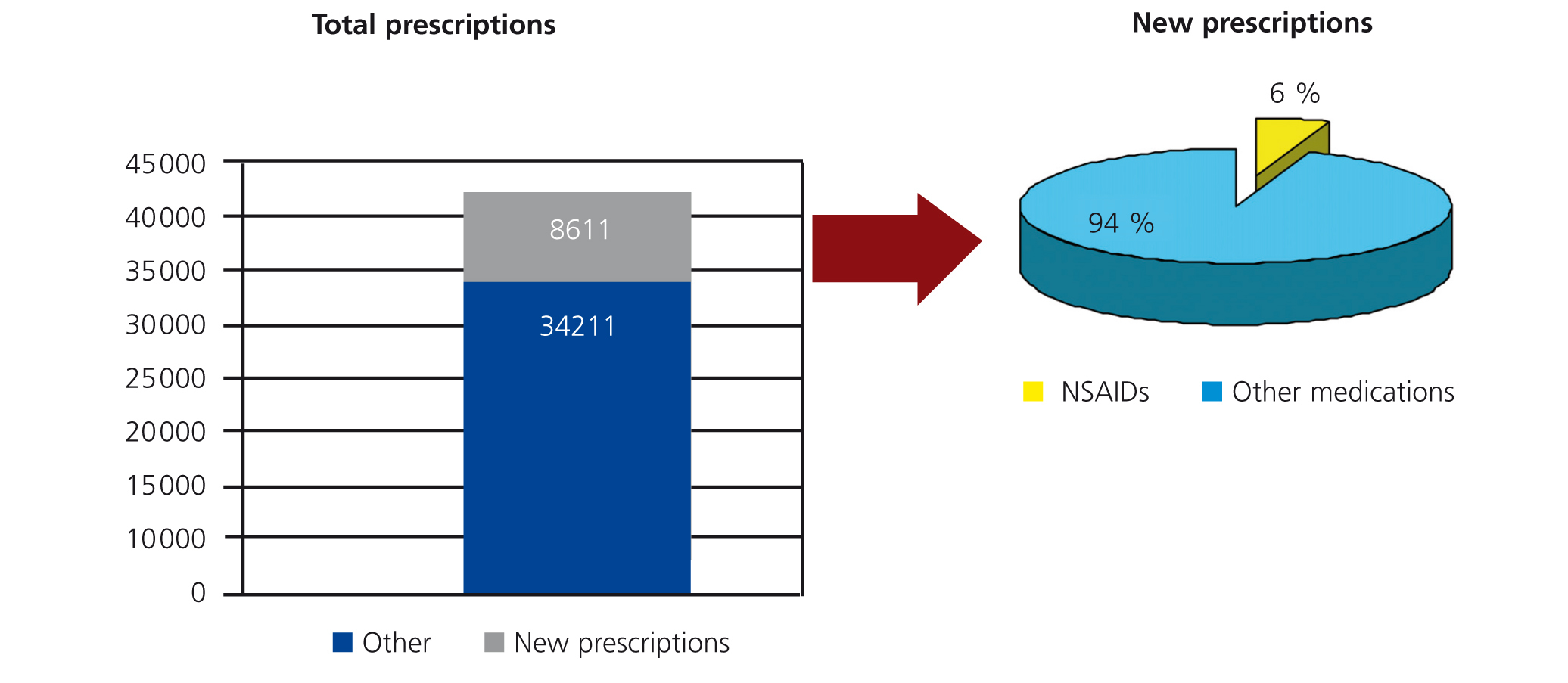
During the months of October and November 2006, a total of 42 822 new prescriptions were registered in the study hospital. A total of 8611 were classified as “new prescriptions” based on the study criteria, and 482 of these (5.6%) were new NSAID prescriptions (Figure 1). These prescriptions were applied to a total of 450 patients, 64% of which were female and 36% male. We analysed the distribution of prescriptions based on patient age: 51% of new prescriptions were for patients aged 14-45 years, 21% were in patients older than 65 years, and 14% each were in patients aged 46-55 and 56-65 years.
The most commonly prescribed NSAID was ibuprofen (66.0%). At the time of our analysis (2008), there were 58 pharmaceutical registries approved for use in our country with ibuprofen as the active ingredient. This was followed in decreasing order by diclofenac (10%), naproxen (8%), dexketoprofen (5%), and aceclofenac (3%). Currently (2011), a total of 79 pharmaceutical compounds have been registered in Spain with ibuprofen as the active ingredient (Medimecum 2011).
In association with these prescriptions, we registered approximately 200 different pathologies that we have classified into 4 general groups in order to simplify our analysis. The most relevant group, corresponding to 54.6% of all prescriptions, was what we have called musculo-skeletal pain. The second group corresponded to 25.5% of all prescriptions, and constituted inflammatory processes of the upper respiratory tract. This was followed by generalised pain (dysmenorrhea, etc.) at 10% and other processes not included in the other sections at 9.9%.
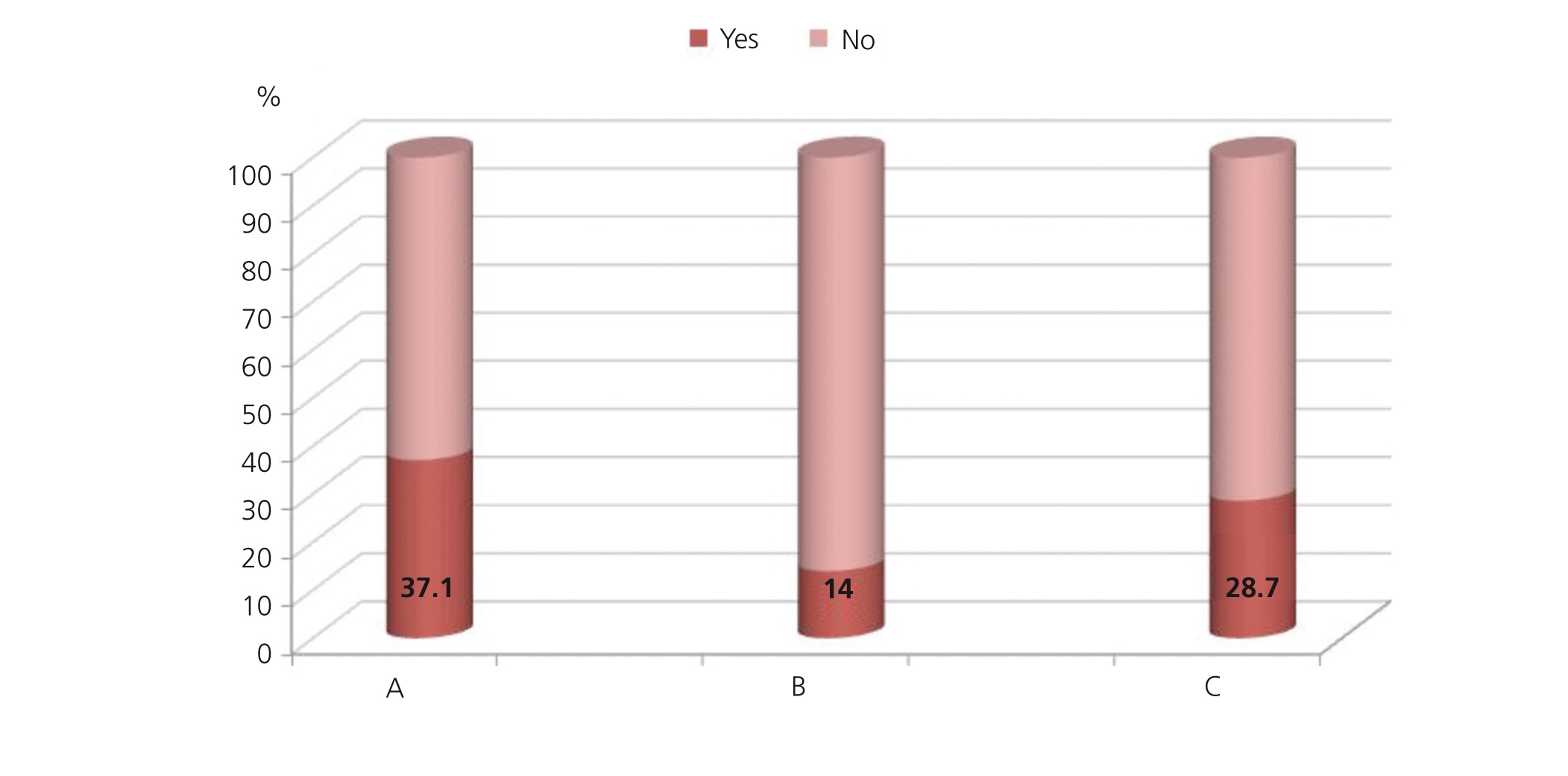
In 168 patients, we observed registries of serum creatinine measurements during the study period, and only 42 patients (9%) had more than one measurement available. The majority of patients in which creatinine values were measured were female (68%). Renal function was more commonly measured in patients older than 65 years of age (39%) and in the youngest group (31%). Only 14% of patients had creatinine values measured during the 6 months prior to NSAID prescription (63 patients), and 129 patients (28.6%) had creatinine values measured at least once following NSAID prescription (Figure 2). Two patients were prescribed NSAIDs despite creatinine values of 1.8mg/dl and 2.46mg/dl, respectively.
In terms of monitoring renal function parameters, 123 patients were tested for creatinine values at least 7 days after the prescription of NSAIDs: on one occasion in 105 patients, on two occasions in 15 patients, and on 3 occasions in 3 patients. We did not observe a relationship between underlying pathology (renal failure or diabetes mellitus) and the frequency of renal function assessments.
We did not observe creatinine values indicative of renal failure as defined in our study design in any of the patients with creatinine values measured before or after NSAID prescription. Nor did we observe any female patients with creatinine >1.3mg/dl or males with creatinine >1.5mg/dl following the prescription of NSAIDs, except for the two aforementioned patients, and in both cases, the creatinine values indicative of renal failure were measured prior to NSAID prescription.
Twenty-six patients were hospitalised during the 6 months following NSAID prescription. None of the final diagnoses that caused hospitalisation involved pathologies related to NSAID use.
We reviewed 19 summary of product characteristics corresponding to NSAIDs approved for use in Spain (those available on the website of the Spanish Agency of Medicines and Medical Devices), evaluating whether information was provided in terms of warnings or precautions, contraindications, and recommendations for modification of dosage in patients with altered renal function. In section 4.2 of the medication technical data sheets regarding dosage and mode of administration, 68% of the drugs evaluated recommended reducing dosage in patients with renal failure, although no specifications were provided regarding the exact amount of decrease; in section 4.3 (contraindications), 47% of drugs reported contraindications in patients with severe renal failure, 15.5% in patients with moderate/severe renal failure, 10.5% in patients with creatinine clearance <30, and 5.5% in patients with severe renal failure in which dialysis is not an option; in section 4.4 (warnings and special precautions), 79% of drugs mentioned warnings for use in patients with renal failure, although severity was not specified.
DISCUSSION
In our study, which took place at a hospital representative of the population in the health area of the Community of Madrid, the prescription of NSAIDs constituted a substantial percentage of all new prescriptions made during the study period (5.6%). Ibuprofen was the most commonly prescribed active ingredient, with 58 different pharmaceutical compounds on the market at the time of the study (2008). These results coincide with those published in 2007 in a report from the Spanish Agency of Medicines and Medical Devices regarding the use of NSAIDs in Spain, which showed that the global use of NSAIDs in extra-hospital patients in Spain increased significantly from 26.3DHD in 1992 to 45.8DHD in 2006. This report also demonstrated that this increase was primarily due to the prescription of ibuprofen, which “came to represent 46% of all NSAID consumption in Spain in 2006 (the most heavily consumed NSAID in this year)”.8
In this study, NSAIDs were more frequently used by females and patients aged 14-45 years. Musculo-skeletal pain was the most common indication for NSAID prescriptions.
Although it might seem surprising that the population comprising the lower age range (14-45 years) constituted more than 50% of all prescriptions, a review of the common pathologies that warranted these prescriptions shows that these are typical of this age range: dysmenorrhea and injury caused by physical exercise.
In our study, it was uncommon for the renal function of these patients to be monitored either prior to or after NSAID prescription. In fact, only 14% of patients who received these drugs had undergone an analysis of serum creatinine values prior to prescription. Two patients were even prescribed NSAIDs despite elevated serum creatinine levels (1.8mg/dl and 2.4mg/dl). We believe that the summary of product characteristics for these medications should be modified to reflect current clinical recommendations.
Our study did involve certain limitations, such as its retrospective design and the difficulties inherent in collecting information regarding patient comorbidity and concomitant treatments, primarily since the information was provided by a database organised by syndrome in the majority of cases. Due to the retrospective nature of the study, we were unable to conclude whether patients with altered creatinine levels who were not prescribed NSAIDs did not receive prescriptions precisely for this reason. We must also add that, although we initially intended to evaluate renal function based on estimated glomerular filtration rates, we were forced to modify this criterion and use creatinine values instead due to insufficient data in the digital database for this type of analysis. Although proteinuria could be affected by the use of NSAIDs, we did not take this into account for our study.
A prospective study would have allowed us to analyse whether a more strict control of renal function could have identified a higher rate of renal failure in patients who received NSAIDs. Although pathologies related to the use of NSAIDs were not present in the final diagnoses registered for our study patients, we cannot conclusively rule out this phenomenon, since we did not perform an exhaustive review of all clinical histories. Ours was a pilot study, and its conclusions must be assessed with this in mind. However, it would certainly be desirable to observe a greater degree of control of renal function parameters prior to and following the prescription of NSAIDs.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Figure 1. Relationship between new prescriptions and all prescriptions. Percentage of non-steroidal anti-inflammatory drug (NSAID) prescriptions out of the total of all prescriptions
Figure 2. Serum creatinine (Cr) measurements in 450 patients who received non-steroidal anti-inflammatory drugs (NSAID) for the first time