To the Editor,
The development of abdominal hernias is a factor that can lead to peritoneal dialysis (PD) failure. Infusing dialysis fluid causes intraabdominal pressure to increase, which promotes abdominal hernias in PD patients. The aim of this study is to understand the risk factors that contribute to abdominal hernia in PD patients, so as to inform the clinician of preventative measures. We will also review how hernias can be treated and how they evolve.
The study included all incident and prevalent PD patients at the Arnau de Vilanova Hospital, Lleida (Spain), over the past 6 years (2003-2009).
The following variables were analysed: age, sex, primary kidney disease, diabetes mellitus (DM), body mass index (BMI), PD modality, PD time, interval between dialysis start-up and catheter insertion, maximum daytime infusion volume (MDV) and body surface (BS)-adjusted MDV (MDV/BS), maximum night-time infusion volume (MNV) and BS-adjusted MNV (MNV/BS), abdominal surgery and hernias prior to PD.
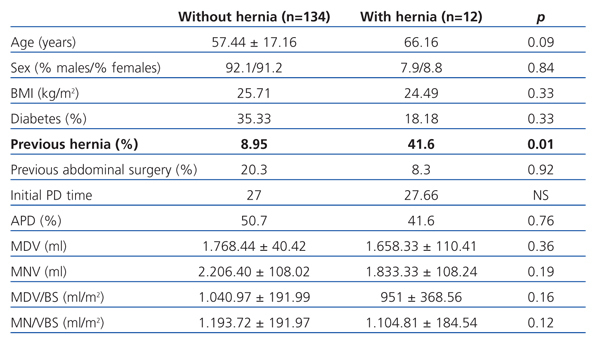
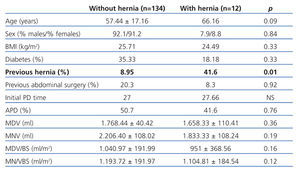
We included a total of 146 patients with an average age of 58.15±1.4 years; 101 men (69.2%) and 45 women (30.8%); 33.56% were diabetic. Accumulated PD monitoring time was 3738.2 months and PD time was 26.26±22.75 months. PD modality: 73 patients (50%) were treated with continuous ambulatory PD (CAPD) and 73 (50%) with automated PD (APD). Our patients’ primary kidney diseases were: diabetic nephropathy (26.71%), nephroangiosclerosis (24%), systemic diseases (19.86%), interstitial nephropathy (14%), unknown (12.31%), polycystic kidney disease (2%) and other diseases (2%). Twelve patients (8.3%) developed abdominal hernias (seven were inguinal, four umbilical, and one crural). No incisional or pericatheter hernias were observed in the follow-up period. Hernia rate was 0.04/patient/year. Hernias developed after an average of 5.3 months of PD. All hernias were intervened by placing a mesh; none were recurrent. Table 1 shows data comparing patients with and without hernias.
The hernia rate in our PD population is comparable to those published in other studies.1,2 The only factor that predisposed patients to hernia development was history of abdominal hernias before inclusion in the study. However, patients with hernias were elderly, almost ten years older than those patients that did not develop hernias during PD. As it has been observed in other studies,1,3 the infusion volume did not influence abdominal hernia development during PD. Intraperitoneal hydrostatic pressure proportionally increased with augmenting the infused volume. Furthermore, it could not predict whether the patient will develop abdominal hernias,3 although pressure should not exceed 20cm H2O. Despite these results, infusion volume increase should be limited, especially in older patients, those with a history of abdominal hernia or ultrafiltration problems. In our unit, the volume prescription varied, according to the patient’s needs, from 1-1.5l/m2 of the body surface-area.
A higher incidence of hernias has been described for patients undergoing PD with polycystic disease,4,5 due to an intraabdominal pressure increase caused by polycystic kidneys or primary collagen disorders. In our study, only 2 patients had polycystic kidney disease, which did not allow us to analyse the impact that the polycystic disease has on the risk of developing hernias during PD.
Some studies1,2 state that there is lower prevalence of hernias in APD-treated patients than in CAPD-treated patients. We have not found any statistically significant difference between the two techniques. However, APD patients used the same daytime volume as the CAPD patients (1756+ml compared with 1763+ml, respectively; P=0.92/0.45). These results do not correlate with those of other studies where greater daytime volumes were used for APD patients, which could explain why the results were different. Furthermore, it supports the hypothesis that increasing intraabdominal pressure in a sitting and decubitus position could cause an increase in hernia development during CAPD.
We treated all hernias with surgery, placing a mesh, and evolution was successful, with no recurrences. A mesh significantly reduces recurrence6 and should be used when repairing hernias in patients at risk of recurrence or new hernias, such those undergoing PD.
To summarise, PD patients with a history of hernia have a greater risk of developing abdominal hernias upon starting treatment. We recommend systematically examining for abdominal hernias in patients before starting PD treatment, and if patients present with clinical signs compatible with abdominal hernia, this complication should be suspected. If surgical repair is necessary, a mesh should be placed. Although abdominal hernias are a relatively frequent complication during PD, if treated correctly the patient’s condition improves, proving that that this complication should not be a motive for not using this technique.
Table 1. Comparative data between populations with and without hernia