The impact of body mass index (BMI) and body weight on hospitalization rates in haemodialysis patients is unknown.
This study hypothesizes that being either underweight or obese is associated with a higher hospitalization rate.
Observational study of 6296 European haemodialysis patients with prospective data collection and follow-up every six months for three years (COSMOS study). The risk of being hospitalized was estimated by a time-dependent Cox regression model and the annual risk (incidence rate ratios, IRR) by Poisson regression. We considered weight loss, weight gain and stable weight. Weight change analyses were also performed after patient stratification according to their baseline BMI.
A total of 3096 patients were hospitalized at least once with 9731 hospitalizations in total. The hospitalization incidence (fully adjusted IRR 1.28, 95% CI [1.18–1.39]) was higher among underweight patients (BMI <20kg/m2) than patients of normal weight (BMI 20–25kg/m2), while the incidence of overweight (0.88 [0.83–0.93]) and obese patients (≥30kg/m2, 0.85 [0.79–0.92]) was lower. Weight gain was associated with a reduced risk of hospitalization. Conversely, weight loss was associated with a higher hospitalization rate, particularly in underweight patients (IRR 2.85 [2.33–3.47]).
Underweight haemodialysis patients were at increased risk of hospitalization, while overweight and obese patients were less likely to be hospitalized. Short-term weight loss in underweight individuals was associated with a strikingly high hospitalization rate.
El impacto del índice de masa corporal (IMC) y el peso corporal sobre las tasas de hospitalización en pacientes en hemodiálisis es desconocido.
La hipótesis del estudio es que tanto el bajo peso como la obesidad se asocian con un exceso de hospitalizaciones.
Estudio observacional que incluye 6.296 pacientes europeos de hemodiálisis con recolección prospectiva de datos y seguimiento cada 6 meses durante 3 años (estudio COSMOS). El riesgo de tener una hospitalización se estimó mediante regresión de Cox dependiente del tiempo y el riesgo anual (razón de tasa de incidencia [IRR]) mediante regresión de Poisson. Se consideró la pérdida de peso, el aumento de peso y el peso estable. Los análisis de cambios de peso también se realizaron después de la estratificación del paciente, de acuerdo con su IMC inicial.
Tres mil noventa y seis pacientes fueron hospitalizados al menos una vez con un total de 9.731 hospitalizaciones. Los pacientes con bajo peso (IMC<20kg/m2) tuvieron una mayor incidencia de hospitalización (IRR completamente ajustada 1,28, IC 95% [1,18-1,39]) que los pacientes con peso normal (IMC 20-25kg/m2), mientras que aquellos con sobrepeso (0,88 [0,83-0,93]) y obesos (≥30kg/m2, 0,85 [0,79-0,92]) tuvieron una incidencia menor. El aumento de peso se asoció con menor riesgo de hospitalización. Por el contrario, la pérdida de peso se asoció con una mayor incidencia de hospitalización especialmente en pacientes con bajo peso (IRR 2,85 [2,33-3,47]).
Los pacientes con hemodiálisis con bajo peso tienen un mayor riesgo de hospitalización, mientras que el sobrepeso y la obesidad tuvieron menos probabilidades de ser hospitalizados. Las pérdidas de peso a corto plazo en individuos con bajo peso se asociaron a una tasa de hospitalización notablemente alta.
The relationship between protein energy wasting (PEW) and mortality in patients undergoing maintenance haemodialysis (HD) therapy is well documented.1,2 Paradoxical associations between various nutritional markers such as body mass index (BMI) and weight changes with mortality risk in HD patients have been described, showing better outcomes for those subjects with a higher BMI or that gain weight over time.3–7
While hospitalization is an inherently limited outcome measure in etiological and prognostic research, it is a fundamental outcome for health-economy research and for research on the quality of clinical care. The risk of hospitalization increases in a graded manner by progressive CKD stage8 and, for dialysis patients, it has been estimated to account for 40–60% of total healthcare expenditures.9,10 During the first year on dialysis, 77% of patients require hospitalization and spend 8.6% of patient treatment days in hospital.11 Given the mounting epidemics of obesity and the parallel decline in underweight and malnutrition in the dialysis population,12 identification of potentially modifiable hospitalization risk factors is important to improve the quality of clinical care in this population. Both malnutrition13,14 and obesity15–17 are important causes of hospitalization in the community, with considerable economic and social cost implications, particularly for inpatient care.18 Data concerning the association between nutritional parameters and hospitalization risk in HD patients are, however, scarce and mainly focused on the study of albumin levels.11,19–22 We here address the association between BMI and short-term body weight changes with the hospitalization risk of dialysis patients. Given the parallel risk for hospitalization of extreme nutritional categories, we hypothesize that both obesity and underweight associate with higher hospitalization rates.
MethodsStudy populationThe Current Management of Secondary Hyperparathyroidism: A Multicentre Observational Study (COSMOS) is a multicentre, prospective and open cohort study with 3 years of follow-up including patients undergoing maintenance haemodialysis from 227 facilities in 20 European countries. The original aim of the cohort was to survey mineral and bone disturbances and related clinical practice patterns. Patients and facilities were randomly selected among a complete list of hospital and satellite dialysis centres from the 20 participating countries. The number of patients recruited in each country was proportional to its dialysis population, and each centre was expected to recruit 20 patients. COSMOS initially recruited 4500 patients and followed them up with data collections every six months for up to three years. Subject dying or leaving the study for other reasons (renal transplantation, change to a non-COSMOS facility or switch to peritoneal dialysis) during that period were replaced by 2297 new patients on haemodialysis for less than 1 year, totalling 6797. Recruitment took place between February 2005 and July 2007, and follow-up ended in July 2010. Study design and ethical approval, as well as random recruitment characteristics have been described in detail elsewhere.23,24
Demographic, clinical, and laboratory dataData on age, sex, height, post-HD weight, smoking (never, former or current smoker), diabetes mellitus, primary kidney disease, cardiovascular disease, country, type of facility (public or private) and haemodialysis-related variables (vintage, modality and hours per week) were collected when the patients entered the study. Data on weight, haemodialysis modality, and hours per week were collected every 6 months during the 3-year observation period. According to protocol design, weight was measured immediately after the haemodialysis session.
Hospitalization dataDuring the 3-year observation of the cohort, all occurring hospitalization events were registered for each patient every 6 months. Time to first hospitalization was collected and annual incidence rate were also calculated and used in the analyses evaluating the association between BMI, weight changes and the hospitalization risk.
Statistical design and analysisCategorical variables are presented as percentage and continuous variables as mean±SD or median (interquartile range), where appropriate. BMI was calculated as weight in kilograms divided by the square of height in meters. BMI was analyzed after stratification by 4 categories. According to the World Health Organization (WHO) guidelines, obesity was defined as a BMI ≥30kg/m2, and overweight was defined as a BMI of 25–29kg/m2. We defined normal weight as a BMI of 20–24kg/m2, which is within the normal range according to the WHO, and underweight as a BMI <20kg/m2. Normal weight was used, a priori, as the reference category. Weight changes over a 6-month period were calculated according to the difference between consecutive weight values, expressed as a percentage. Categorical analysis of weight change was established along previous analyses of BMI we performed in COSMOS6 and 3 categories were identified weight loss (decreases >1%), weight gain (increases >1%) and stable weight (weight changes of less than ±1%). We chose this definition to allow comparison with preceding studies,6,25 where mortality associations were already observed for such small weight variations. Stable weight (within 1% variation) was considered the reference category.
In the analysis of hospitalization risks, both time-to-event analysis and incidence rates are clinically important and complementary outcomes. We performed time-dependent Cox proportional hazards regressions to assess the associations between 6-month BMI or weight changes and the risk to have one hospitalization. In these analyses patients were censored if undergoing renal transplantation (n=382), death (n=332), lost to follow-up (transfer to a non-COSMOS facility) (n=136), change to peritoneal dialysis (n=5) or end of the study, whichever occurred first. Data is presented as hazard ratios (HR) and 95% confidence intervals (CI). In addition, we used Poisson regression models to estimate the association between BMI or weight changes and the annual hospitalization rate versus the referent category, thus presenting data as incidence rate ratios (IRR) and 95% CI. In these analyses, we used either the baseline BMI or weight changes during the first 6 months of follow-up, and took into account all hospitalizations registered for each patient to estimate annual incidence rates. To both approaches, multivariate adjustment controlled for confounders including age, sex, smoking, country, type of haemodialysis centre, dialysis vintage, haemodialysis hours per week and modality. Covariates were considered as time-dependent when applicable, except sex, smoking, diabetes mellitus, cardiovascular disease, country, type of haemodialysis centre and primary kidney disease. Statistical significance was set at the level of P<0.05. All statistical analyses were performed using STATA version 12.1 (Stata Corporation College Station, TX).
ResultsThe present analysis included 6296 patients from COSMOS cohort, after excluding 485 patients (7.1%) who did not have follow-up data and 16 patients further lacking information on BMI. The majority of patients (94.5%) had undergone haemodialysis for more than 3 months. Baseline patient characteristics at study entry and incidence rates of hospitalization according to four BMI categories are displayed in Table 1 and published earlier.6 Both underweight and obese patients had similar incidence rates of hospitalization and higher than normal and overweight patients.
Patient characteristics according to basal body mass index categories.
| All patients | <20kg/m2 | 20–24kg/m2 | 25–29kg/m2 | ≥30kg/m2 | |
|---|---|---|---|---|---|
| (n=6296) | (n=690) | (n=2664) | (n=1955) | (n=987) | |
| Rates of hospitalization (admissions/100 person-years) | 105 (0, 133) | 111 (0, 157) | 105 (0, 133) | 99 (0, 133) | 110 (0, 137) |
| Age (yr) | 64±14 | 61±18 | 64±15 | 65±13 | 63±12 |
| Men (%) | 61 | 47 | 64 | 65 | 53 |
| Body mass index (kg/m2) | 25.3±4.9 | 18.4±1.3 | 22.6±1.4 | 27.1±1.4 | 33.8±3.8 |
| Diabetes mellitus (%) | 31 | 15 | 24 | 35 | 53 |
| Cardiovascular disease (%) | 72 | 67 | 71 | 74 | 74 |
| Smoking (%) | |||||
| Current | 17 | 24 | 18 | 15 | 11 |
| Former | 19 | 11 | 20 | 20 | 20 |
| Never | 64 | 65 | 62 | 65 | 69 |
| Primary kidney disease (%) | |||||
| Hypertension | 20 | 21 | 19 | 23 | 17 |
| Glomerulonephritis | 17 | 24 | 20 | 14 | 12 |
| Polycystic kidney disease | 8 | 8 | 9 | 8 | 7 |
| Diabetes mellitus | 21 | 9 | 17 | 24 | 36 |
| Obstructive/interstitial | 12 | 12 | 12 | 11 | 11 |
| Tumours | 2 | 2 | 2 | 1 | 2 |
| Unknown | 12 | 13 | 12 | 12 | 9 |
| Other | 8 | 11 | 9 | 7 | 6 |
| Vintage (months) | 20 (8–51) | 26 (8–67) | 22 (8–57) | 19 (8–47) | 16 (7–40) |
| Haemodialysis modality (%) | |||||
| Conventional low-flux | 54 | 59 | 54 | 54 | 51 |
| Conventional high-flux | 37 | 33 | 37 | 37 | 39 |
| Other | 9 | 7 | 9 | 9 | 10 |
| Haemodialysis hours per week (%) | |||||
| <12 | 21 | 28 | 22 | 20 | 15 |
| 12 | 55 | 56 | 57 | 55 | 51 |
| >12 | 24 | 16 | 21 | 25 | 34 |
Categorical data are shown as percentage; continuous data as mean±SD, except for vintage that is shown as median and interquartile range (25th and 75th percentiles).
Values in parentheses are the 25th and 75th percentiles.
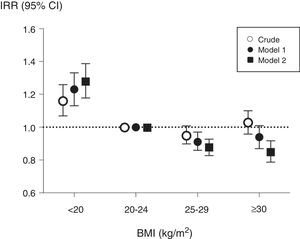
During the 3-year follow-up, 3696 patients were hospitalized at least once, and a total of 9731 hospitalizations were registered in the cohort. The analysis of hazards to a first hospitalization considered a total of 17,797 observations through time-dependent analyses (Table 2). Underweight patients had a higher risk of hospitalization than patients with normal weight, while overweight and obese patients presented a non-statistically significant lower risk (model 2, Table 2). In the analysis of annual incidence rates, the association between baseline BMI and IRRs showed similar results (Fig. 1); correction for potential confounding factors increased the IRRs estimates associated with underweight while it decreased IRRs estimates of overweight and obese patients.
Time-dependent associations (every 6 months) between body mass index (BMI) and time to hospitalization in 6296 haemodialysis from COSMOS stratified by BMI categories.
| Unadjusted | Adjusted | |
|---|---|---|
| BMI <20kg/m2 | 1.20 (1.09–1.33) | 1.22 (1.10–1.35) |
| BMI 20–24kg/m2 | 1 | 1 |
| BMI 25–29kg/m2 | 0.93 (0.86–1.01) | 0.89 (0.82–0.96) |
| BMI ≥30kg/m2 | 0.98 (0.89–1.08) | 0.93 (0.84–1.02) |
Represented are unadjusted and adjusted hazard ratios and 95% confidence intervals for the risk of one hospitalization using time-dependent Cox regression analyses. Adjusted model includes multivariate adjustment for age, sex, smoking (never, former or current), country, type of centre (public or private), dialysis vintage, haemodialysis hours per week and modality.
Associations between baseline body mass index (BMI) and annual hospitalization rates, expressed as incidence rate ratios, in 6296 haemodialysis patients from COSMOS. Normo weight individuals were taken as the reference. Model 1 includes multivariate adjustment for age, sex, smoking (never, former or current), country, type of centre (public or private), dialysis vintage, haemodialysis hours per week and modality; Model 2 includes multivariate adjustment of variables included in model 1 plus primary kidney disease, diabetes mellitus and history of cardiovascular disease.
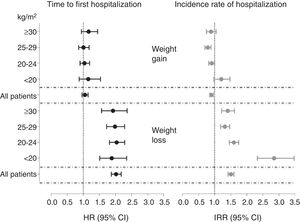
Patients experiencing weight losses within a 6-month period were at higher risk of hospitalization than patients with stable weight. No association was seen in patients gaining weight (Table 3). When patients were stratified by their initial BMI, the risks estimates associated with weight losses were statistically significant and similar in magnitude across categories, with HR ranging from 1.88 and 2.04 (Fig. 2).
Associations between weight changes, time to first hospitalization and risk of hospitalization per year in 6296 haemodialysis patients from COSMOS.
| Time to hospitalization (hazard ratios) | Annual hospitalization rates (incidence rate ratios) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Weight loss | 2.10 (1.94–2.26) | 2.05 (1.90–2.21) | 1.61 (1.52–1.71) | 1.52 (1.44–1.61) |
| Stable weight | 1 | 1 | 1 | 1 |
| Weight gain | 1.09 (0.99–1.19) | 1.07 (0.97–1.17) | 0.99 (0.93–1.05) | 0.90 (0.84–0.96) |
Represented are unadjusted and adjusted hazard ratios and 95% confidence intervals for the risk of one hospitalization using time-dependent Cox regression analyses (every 6 months), and incidence rate ratios and 95% confidence intervals for calculation of annual hospitalization rates using Poisson regression analyses. Weight changes are considered as weight loss (decreases in weight higher that 1%), weight gain (increases in weight higher than 1%) and stable weight (weight changes of ±1%), taken here as the reference category. Adjusted model includes multivariate adjustment for age, sex, smoking (never, former or current), country, type of centre (public or private), dialysis vintage, haemodialysis hours per week and modality.
Fully adjusted hazard ratios for the time to hospitalization (hazard ratios) and annual hospitalization rates (incidence rate ratios) according to 6-month weight changes and stratified by body mass index (BMI) categories. Multivariate adjustment controlled for age, sex, smoking (never, former or current), country, type of centre (public or private), dialysis vintage, haemodialysis hours per week and modality primary kidney disease, diabetes mellitus and history of cardiovascular disease.
Patients experiencing weight losses during the first 6 months of follow-up had higher annual hospitalization rates (IRR) than individuals with stable body weight. Likewise, patients gaining weight had significantly lower hospitalization rates (Table 3). When analyses were stratified by initial BMI categories, the magnitude of the association between dry weight losses and the hospitalization rates was similar among obese (IRR 1.41 [95% CI 1.22–1.62]), overweight (1.32 [1.19–1.47]) and normal weight (1.60 [1.46–1.75]) patients. However, a much higher risk was observed for underweight patients losing weight, with an IRR of 2.85 [2.33–3.47]) (Figure 2).
DiscussionCompared with other chronic illnesses, patients with CKD have more comorbidities, the highest annual number of inpatient stays, and the greatest number of hospital days.26 Heart failure and ischaemic heart disease have been suggested as the top causes of hospitalization in this patient population.8,11 Poor nutritional status, as identified by hypoalbuminemia11,19–22 may also contribute to explain such risk. To the best of our knowledge, this is the first study addressing the hospitalization risks associated with BMI and short-term body weight changes in this patient population.
Contrary to our working hypothesis which, along with mortality data,6 envisaged an U-shaped relationship between the BMI and the risk of hospitalization, in COSMOS we found that BMI is inversely associated with the risk of hospitalization. Overweight and obese individuals were less likely to be hospitalized and presented a lower annual incidence rate of hospitalizations than normal weight patients. On the other hand, underweight individuals presented a higher relative risk of hospitalization. Even though a precious instrument to investigate the effect of nutritional status in epidemiological studies, BMI is an imperfect marker of fat mass excess both at a general population level27 and in the dialysis population.28–30 High BMI in part reflects high muscle mass, a body component which is of fundamental importance for determining disability in chronic diseases.31,32 Thus our analysis likely reflects the importance of frailty and sarcopenia in engendering clinical complications eventually leading to hospitalization in long-term dialysis.33,34 Some observational in our analysis may align with that hypothesis, such as the shorter dialysis vintage across higher BMI categories. In recent years, it has become apparent however that muscle quality and strength may be as important (or maybe more) as muscle mass.35,36
Independently of baseline nutritional status, intercurrent diseases leading to hospitalization have a relevant impact upon protein-energy metabolism in muscle mass. Accordingly, we found that short-term body weight losses have a clear-cut relationship with the risk of hospitalization, increasing it by two fold. In addition, in the long term, weight losses associated with approximately 50% higher annual hospitalization rates than those maintaining a stable body weight over time, in line with the hypothesis that sarcopenia and intercurrent PEW may underlie the excess risk for hospitalization in dialysis patients. Although it is possible that short term BMI changes in dialysis patients may in part reflect subclinical volume expansion,37 BMI increases had just a minor, non-significant effect on the risk of hospitalization.
Our findings need to be interpreted considering several strengths and limitations. Strengths include the prospective and careful cohort design (including their random recruitment), the completeness of data and the record of consecutive hospitalizations to allow the analysis of annual incidence rates. Its large sample size and representativeness of the European HD population should also be remarked. As discussed, BMI does feature several weaknesses as a metric of body fat in CKD patients.38,39 We lack information on length of hospital stay, which would have allowed us to make cost-effectiveness analysis, and we have unsatisfactory collection on hospitalization causes which limits our capacity to define them. A sensitivity analysis grouping countries according to global background hospitalization rates would have been preferred but was not feasible. In addition, we lack information on the intentionality of these body weight gains or losses. Given the short time frame studied (6-month periods) and the absence of consensus guidelines on weight loss programs for dialysis patients, we are assuming them to be unintentional. Other limitations are the ancillary nature of our findings, as well as the lack of information on additional surrogates of muscle and fat stores.40 As in all observational cohort studies, residual confounding by unmeasured or unknown confounders may have played a role. The predominantly prevalent nature of the HD patients included in COSMOS makes it vulnerable to survivor bias despite the careful adjustment for dialysis vintage.
We conclude that both underweight (lower than 20kg/m2 in this analysis) and short-term body weight losses associate with increased hospitalizations rates, likely because it signals an unstable clinical situation including, but not limited to, an unfavourable nutritional status. This, and our previous report on hard endpoints,6 altogether emphasize the importance of nutritional status on HD outcomes and bring special attention to the importance of the short-term weight losses in the underweight patients, a finding that deserves additional surveillance. The high and rising health cost absorbed by the dialysis population is a reason of concern for health authorities and providers. Since hospitalization has a major share in this cost, understanding the implication of nutritional disorders for this outcome may help to set strategies aimed at limiting hospital admission in this population. Cost-effectiveness studies indicate that, along with improved wellbeing, substantial savings in health costs can be obtained by protein and energy supplementation in treatment or prevention of malnutrition in the general population.41 Analyses modelling the effect of such intervention on hospitalization in the dialysis population suggest that the potential savings in hospitalization, treatment costs and mortality may be substantial.42,43 Our observations provide further strength to the quest of performing clinical trials aimed at assessing whether nutritional interventions targeting body weight may improve clinical outcomes and reduce hospitalization in this high risk population.
FundingCOSMOS is sponsored by the Bone and Mineral Research Unit (Hospital Universitario Central de Asturias), SAFIM (Sociedad Asturiana Fomento Investigaciones Óseas), the European Renal Association-European Dialysis and Transplant Association, the National Program of I+D+I 2008–2011 and Instituto de Salud Carlos III (ISCIII), the ISCIII Retic REDinREN (RD06/0016/1013 and RD12/0021/1023), the ISCIII (ICI14/00107 and PI17/00384), Fondo Europeo de Desarrollo Regional (FEDER), Plan Estatal de I+D+I 2013–2016, Plan de Ciencia, Tecnología e Innovación 2013–2017 del Principado de Asturias (GRUPIN14-028) and Fundación Renal Íñigo Álvarez de Toledo (FRIAT). Logistics (meetings, secretarial help, printing of materials, development of Web site for data entry, etc.) have been financially supported by AMGEN Europe, Fundación Renal Íñigo Álvarez de Toledo (FRIAT) and the Spanish Society of Nephrology (Estudio Estratégico de la SEN). ICR research stay at Karolinska Institutet was supported by the Río Hortega program, Instituto Carlos III, Spain. The authors are not aware of any additional relationships, funding, or financial holding that might be perceived as affecting the objectivity of this study.
Conflict of interestsThe authors declare no conflict of interest.
We would like to acknowledge a group of persons who have collaborated in COSMOS: José Luis Motellón, Matthew Turner, Julien Chaussy, Bart Molemans, Wal Zani, Dylan Rosser, Bastian Dehmel, Bruno Fouqueray, Brian Bradbury, John Acquavella, Jennifer Hollowell, Dave Carter, Phil Holland, Ana Baños, Caroline Mattin, Cathy Critchlow, Joseph Kim, Charlotte Lewis, Antonia Panayi, Margit Hemetsberger, Stephen Croft, Philippe Jaeger, Prisca Muehlebach, Jane Blackburn, Esther Zumsteg, Silvia Rodríguez, Angel Pérez, Pau Faner, Irantzu Izco, Susana Traseira, Carmen Castro, Javier Moreno, David Calle and Francesca Pieraccini.
Miha Benedik, Willem-Jan Bos, Adrian Covic, José Luis Gorriz, Reinhard Kramar, Pierre-Yves Martin, Drasko Pavlovic, Boleslaw Rutkowski, Christian Tielemans, Dierik Verbeelen, Rudolf P. Wüthrich.