In this study, we show the results of the subset of Spanish patients of the VERIFIE study, the first post-marketing study assessing the long-term safety and effectiveness of sucroferric oxyhydroxide (SFOH) in patients with hyperphosphatemia undergoing dialysis during clinical practice.
Patients and methodsPatients undergoing hemodialysis and peritoneal dialysis with indication of SFOH treatment were included. Follow-up duration was 12–36 months after SFOH initiation. Primary safety variables were the incidence of adverse drug reactions (ADRs), medical events of special interest (MESIs), and variations in iron-related parameters. SFOH effectiveness was evaluated by the change in serum phosphorus levels.
ResultsA total of 286 patients were recruited and data from 282 were analyzed. Among those 282 patients, 161 (57.1%) withdrew the study prematurely and 52.5% received concomitant treatment with other phosphate binders. ADRs were observed in 35.1% of patients, the most common of which were gastrointestinal disorders (77.1%) and mild/moderate in severity (83.7%). MESIs were reported in 14.2% of patients, and 93.7% were mild/moderate. An increase in ferritin (386.66ng/mL vs 447.55ng/mL; p=0.0013) and transferrin saturation (28.07% vs 30.34%; p=0.043) was observed from baseline to the last visit (p=0.0013). Serum phosphorus levels progressively decreased from 5.69mg/dL at baseline to 4.84mg/dL at the last visit (p<0.0001), increasing by 32.2% the proportion of patients who achieved serum phosphorus levels ≤5.5mg/dL, with a mean daily SFOH dose of 1.98 pills/day.
ConclusionsSFOH showed a favorable effectiveness profile, a similar safety profile to that observed in the international study with most adverse events of mild/moderate severity, and a low daily pill burden in Spanish patients in dialysis.
En este estudio presentamos los resultados del subgrupo de pacientes españoles del estudio VERIFIE, primer estudio post-autorización prospectivo que evalúa la seguridad y efectividad a largo plazo del oxihidróxido sucroférrico (OHS) en pacientes en diálisis con hiperfosfatemia durante la práctica clínica habitual.
Pacientes y métodosSe incluyeron pacientes en hemodiálisis y diálisis peritoneal con indicación de tratamiento con OHS. La duración del seguimiento fue de 12 a 36 meses desde el inicio del tratamiento con OHS. Las variables primarias de seguridad fueron la incidencia de reacciones adversas a medicamentos (RAMs), eventos médicos de interés especial (EMIEs) y variaciones en los parámetros del hierro. La efectividad del OHS se evaluó mediante el cambio en los niveles de fósforo sérico.
ResultadosSe reclutaron 286 pacientes y se analizaron los datos de 282. De estos 282 pacientes, 161 (57,1%) abandonaron el estudio de manera prematura y un 52,5% recibieron tratamiento concomitante con otros captores de fósforo. Un 35,1% reportaron RAMs y la mayoría fueron de tipo gastrointestinal (77,1%) y de intensidad leve/moderada (83,7%). Un 14,2% de los pacientes presentaron EMIEs, de los que el 93,7% fueron leves/moderados. Se observó un incremento de la ferritina (386,66ng/mL vs 447,55ng/mL; p=0,0013) y saturación de la transferrina (28,07% vs 30,34%; p=0,043) desde el inicio hasta la última visita. Los niveles de fósforo sérico disminuyeron progresivamente desde 5,69mg/dL al inicio hasta 4,84mg/dL en la última visita (p<0,0001), aumentando la proporción de pacientes con niveles de fósforo ≤5,5mg/dL un 32,2%, y con una dosis diaria media de 1,98 comprimidos/día.
ConclusionesOHS presentó un perfil de efectividad favorable, un perfil de seguridad similar al observado en el estudio internacional con una mayoría de efectos adversos de severidad leve/moderada, y un número reducido de comprimidos diarios en pacientes españoles en diálisis.
- •
This sub-analysis of the VERIFIE study evaluates the long-term safety and effectiveness of sucroferric oxyhydroxide (OHS) in Spanish patients.
- •
The main adverse events associated with OHS were gastrointestinal disorders of mild/moderate intensity.
- •
No clinically relevant alterations were observed in variables related to iron metabolism.
- •
OHS was effective in lowering serum phosphorus levels progressively.
- •
The mean daily dose during the observational period (988.1mg) corresponded to a reduced number of daily tablets (1.98 tablets/day).
Hyperphosphatemia is a common complication of chronic kidney disease (CKD) that worsens as the disease progresses due to the gradual loss of the capacity to excrete urinary phosphorus.1 In patients with CKD, high phosphorus levels are associated with cardiovascular complications, vascular calcification, bone abnormalities, worsening of secondary hyperparathyroidism, and increased risk of mortality.2,3 Since 2006, all these alterations, together with greater bone fragility, have been known by their acronym in English as CKD-MBD (chronic kidney disease, mineral and bone disorders).4,5
In order to prevent these outcomes, the Kidney Disease: Improving Global Outcomes5 (KDIGO) and the Spanish Society of Nephrology (SEN)6 guidelines recommend to maintain serumphosphorus levels within the normal range. The results of the COSMOS study indicate that the values associated with lower mortality are between 2.5 and 4.5mg/dL.7 There are 3 main therapeutic approaches to achieveg these objectives: a low phosphorus content in the diet, phosphate removal by dialysis, and the use of phosphorus-binding drugs. In more than 80% of dialysis patients, dietary restrictions and dialysis are not enough, so the use of phosphorus binders is required.8
There is currently a wide variety of phosphorus binders with different active compounds: based on calcium, on metals such as magnesium, iron or aluminum, or polymers.8,9 Among its differential characteristics are the binding capacity for phosphorus in the pH range of the gastrointestinal tract, the required number of tablets per day, and the tolerability profile.10 Despite the proven efficacy of phosphate binders necessary to obtain significant benefits, a European study showed that only 26.7% of patients who received this treatment achieved the KDIGO11 therapeutic target.
Sucroferric oxyhydroxide (OHS, Velphoro®; Vifor Fresenius Medical Care Renal Pharmaa, Glattbrugg, Switzerland) is a novel iron-based phosphorus binder with potent phosphorus-binding capacity over a wide pH range, allowing the use of a reduced number of tablets, an advantage that has been shown to improve compliance.12 The OHS was marketed in the United States and Europe and is used in adult patients with CKD receiving chronic hemodialysis or peritoneal dialysis treatment.13,14 The efficacy of OHS as well as its non-inferiority as compared to sevelamer carbonate in lowering serum phosphorus were demonstrated in a phase III STUDY and its subsequent extension in 1059 CKD patients on dialysis.15,16 Most of the adverse events were gastrointestinal (diarrhea) and were generally mild/moderate in intensity.15,16 Subsequently, other observational studies have also demonstrated the effectiveness of OHS even using a reduced number of tablets.17–19 Compared with other phosphorus binders (calcium carbonate, calcium acetate, lanthanum, sevelamer carbonate, and ferric citrate), one study ranked OHS as the most potent binder with the highest phosphorus-binding capacity.20 Until the publication of the VERIFIE study (Velphoro Evaluation of Real-lIfe safety, effectIveness and adherence), a prospective, multicenter, multinational study carried out in 7 countries and 172 centers and which included 1406 patients, there were no post-authorization safety data on long term.21 In order to find out the safety and effectiveness profile of Spanish patients, this sub-analysis shows the results of the Spanish centers that participated in the VERIFIE study.
MethodsStudy design and patients populationVERIFIE (NCT02687594) is a prospective, multicenter, non-interventional study carried out in 7 European countries. The oresent document shows the results obtained in the group of patients recruited in the 34 Spanish centers. The study was conducted in accordance with the principles of the Declaration of Helsinki. All participants gave their written informed consent. The international study protocol was approved by the Clinical Research Ethics Committee of each participating center.
The objective of the VERIFIE study was to evaluate the long term (up to 36 months) safety and effectiveness of OHS.21 To do this, demographic, clinical, and comorbidity information was collected, as well as data related to previous and concomitant treatment with other phosphorus binders or with intravenous or oral iron supplements. The included patients had clinical information during the preceding 6 months before starting the treatment with OHS. The observation periods corresponding to the prospective and retrospective phases of the study are detailed in the publication of the VERIFIE study.21
The study included adult patients (≥18 years) on hemodialysis and peritoneal dialysis for at least 6 months, who gave their consent to participate in the study, and had clinical indication for the treatment with OHS according to their technical data sheet; patients could have been previously treated with OHS or have not received previous treatment (naïve) with phosphorus binders. Patients who discontinued treatment and those who had participated in another interventional study or who had been recruited in another study with OHS were excluded.21
Study variablesThe primary safety endpoints were the incidence and proportion of patients with adverse drug reactions (ADRs) and medical events of special interest (MESIs), such as diarrhoea, the potential risk (physician-assessed) of masking gastrointestinal bleeding caused by OHS-associated stool discoloration and parameters related to iron metabolism (ferritin, transferrin, transferrin saturation index, and hemoglobin). ADRs are defined as any adverse event that presents a reasonable causal relationship with the study treatment. MESIs include gastrointestinal bleeding, iron overload, and diarrhea regardless of their relationship to OHS. The effectiveness of the OHS was evaluated by masuring the change in serum phosphorus levels, as well as the percentage of patients achieving control of serum phosphorus levels (≤5.5 and ≤4.5mg/dL).
As secondary objectives, other laboratory parameters related to bone and mineral metabolism such as calcium, vitamin D, and intact parathyroid hormone (iPTH) were evaluated. Mean daily OHS dose and changes in OHS dose were also determined.
The study variables were collected before the initiation of treatment and throughout the observation period (months 1, 3, 6, 12, 18 and 24). In those patients who left the study prematurely, the data of the last completed observation were collected in the variable “last visit”. The information was collected in an aggregate manner for patients on hemodialysis (216; 76.6%) and peritoneal dialysis (66; 23.4%) to maintain sample size.
Statistical analysisTo calculate the sample size of the international study, it was taken into account a 5% incidence of gastrointestinal bleeding as previously reported in the DOPPS study (Dialysis Outcomes and Patient Patterns Study).22 Calculations were performed using the one-sample Chi-square test, with a two-sided significance level of 0.05. It was established that 1000 patients would be sufficient to achieve the study objective of an exposure of 900–1000 patient-years.
The results were analyzed in an exploratory and descriptive manner. ADRs, MESIs, fatal events, and other safety variables were classified by organ system and preferred term according to MedDRA (Medical Dictionary for Regulatory Activities) terminology version 22.0.
The results of the primary variables were expressed by number of patients and percentage and 95% confidence interval for the frequency of ADRs, MESI, and fatal events. The exposure-adjusted incidence rate per patient/year was calculated as the number of patients with a specific event divided by the total follow-up time of all patients. All safety variables were analyzed in the safety analysis set, which include those patients who received at least one dose of OHS and with at least one available safety assessment.
The effectiveness variables were expressed as the mean and standard deviation, the change from the baseline value and the proportion of patients who reached target (≤5.5mg/dL) and optimal levels (≤4.5mg/ dL) of phosphorus. P values were calculated using an exploratory post hoc analysis. Analysis of phosphorus levels was performed on the full analysis set, which includes those patients who received at least one dose of OHS, with at least one baseline and at least one subsequent effectiveness assessment.
Statistical analyzes were performed with the SAS statistical analysis package (SAS Institute Inc., Cary, NC, United States), version 9.4 TS1M3. A p<0.05 was considered statistically significant.
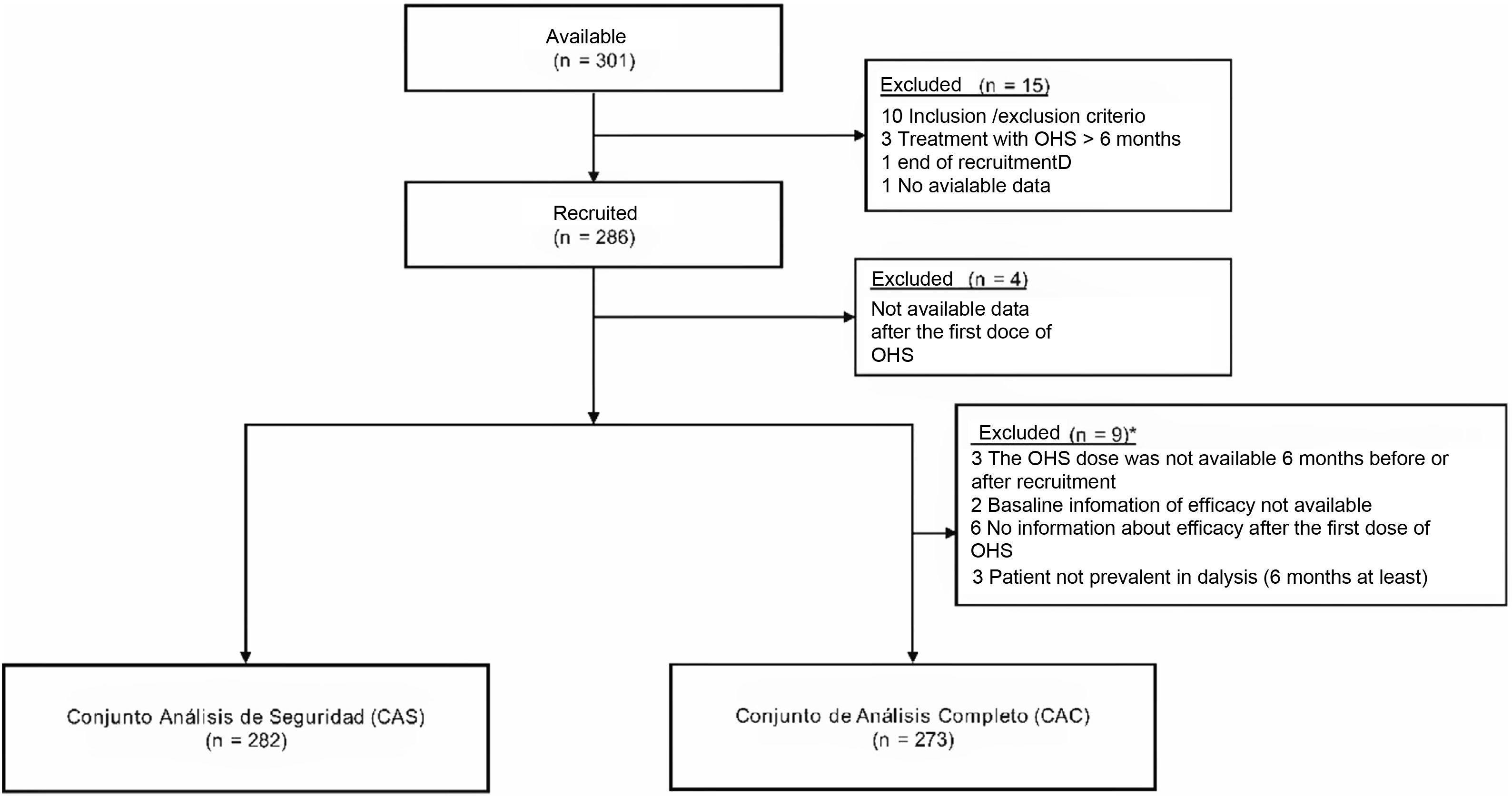
ResultsStudy populationIn this sub-analysis of the international study, thre were included 286 patients from 34 Spanish. Four patients had no follow-up data after the first dose of OHS and were excluded from the analysis (282 patients were part of the safety analysis set and 273 were part of the full analysis set) (Fig. 1).
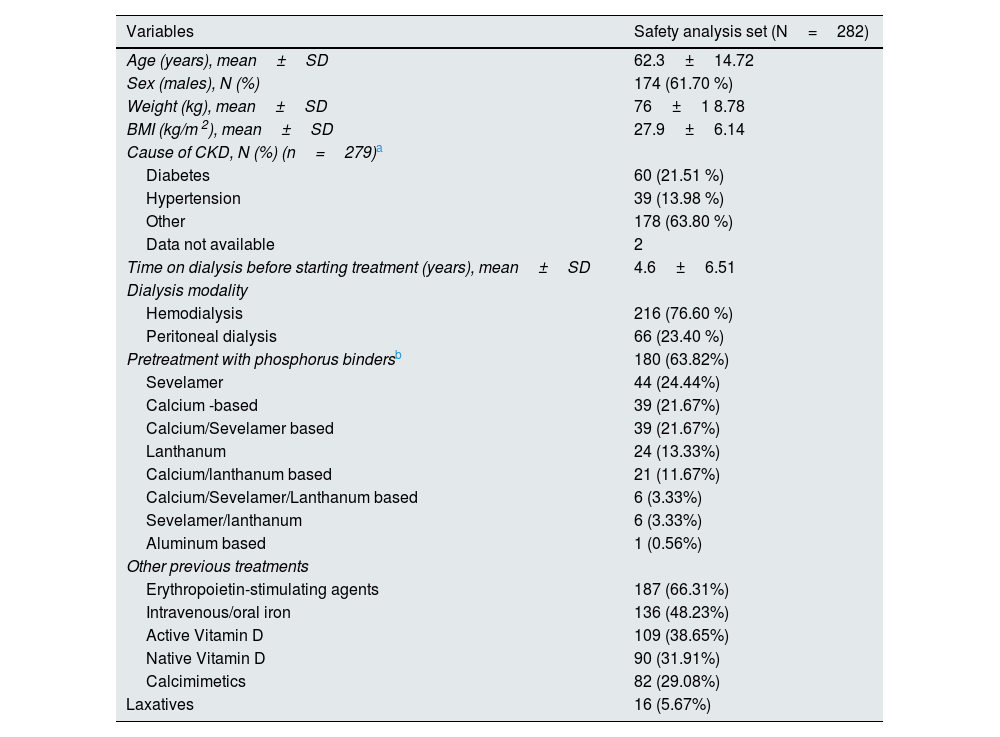
The baseline characteristics of the subjects included in the safety population are shown in Table 1. There was a higher proportion of men (174; 61.7%) and a predominance of patients on hemodialysis (216; 76.6%) as compared with patients on peritoneal dialysis (66; 23.4%). Most patients had received previous treatment with other phosphorus binders (63.8%): sevelamer (24.4%), calcium-based (21.7%), calcium/sevelamer-based (21.7%) and lanthanum 13.33% (Table 1). During study follow-up, a total of 148 patients (52.5%) received concomitant treatment with other phosphate binders (Appendix B Supplementary Table S1).
Baseline demographic and clinical characteristics of the patients.
| Variables | Safety analysis set (N=282) |
|---|---|
| Age (years), mean±SD | 62.3±14.72 |
| Sex (males), N (%) | 174 (61.70 %) |
| Weight (kg), mean±SD | 76±1 8.78 |
| BMI (kg/m 2), mean±SD | 27.9±6.14 |
| Cause of CKD, N (%) (n=279)a | |
| Diabetes | 60 (21.51 %) |
| Hypertension | 39 (13.98 %) |
| Other | 178 (63.80 %) |
| Data not available | 2 |
| Time on dialysis before starting treatment (years), mean±SD | 4.6±6.51 |
| Dialysis modality | |
| Hemodialysis | 216 (76.60 %) |
| Peritoneal dialysis | 66 (23.40 %) |
| Pretreatment with phosphorus bindersb | 180 (63.82%) |
| Sevelamer | 44 (24.44%) |
| Calcium -based | 39 (21.67%) |
| Calcium/Sevelamer based | 39 (21.67%) |
| Lanthanum | 24 (13.33%) |
| Calcium/lanthanum based | 21 (11.67%) |
| Calcium/Sevelamer/Lanthanum based | 6 (3.33%) |
| Sevelamer/lanthanum | 6 (3.33%) |
| Aluminum based | 1 (0.56%) |
| Other previous treatments | |
| Erythropoietin-stimulating agents | 187 (66.31%) |
| Intravenous/oral iron | 136 (48.23%) |
| Active Vitamin D | 109 (38.65%) |
| Native Vitamin D | 90 (31.91%) |
| Calcimimetics | 82 (29.08%) |
| Laxatives | 16 (5.67%) |
SD, standard deviation; CKD, chronic kidney disease; BMI, body mass index.
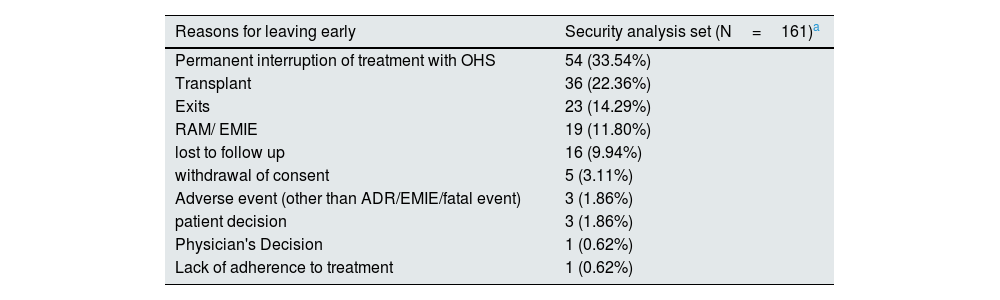
A total of 161 (57.1%) patients left the study prematurely. The main causes of premature abandonment were permanent interruption of OHS treatment (33.5%), transplantation (22.4%), death (14.3%) or the occurrence of ADR/MESI (11.8%) (Table 2). The main causes of discontinuation of OHS were the occurrence of ADR/MESI/toxicity/fatal events (48.9%) and the patient's decision (27.1%).
Reasons for leaving the study early.
| Reasons for leaving early | Security analysis set (N=161)a |
|---|---|
| Permanent interruption of treatment with OHS | 54 (33.54%) |
| Transplant | 36 (22.36%) |
| Exits | 23 (14.29%) |
| RAM/ EMIE | 19 (11.80%) |
| lost to follow up | 16 (9.94%) |
| withdrawal of consent | 5 (3.11%) |
| Adverse event (other than ADR/EMIE/fatal event) | 3 (1.86%) |
| patient decision | 3 (1.86%) |
| Physician's Decision | 1 (0.62%) |
| Lack of adherence to treatment | 1 (0.62%) |
EMIE, medical events of special interest; OHS, sucroferric oxyhydroxide; ADR, adverse drug reactions.
The median duration of exposure to OHS was 54.1 weeks (range: 1.4–123.3 weeks) and the mean duration was of 58.2±30.1 weeks.
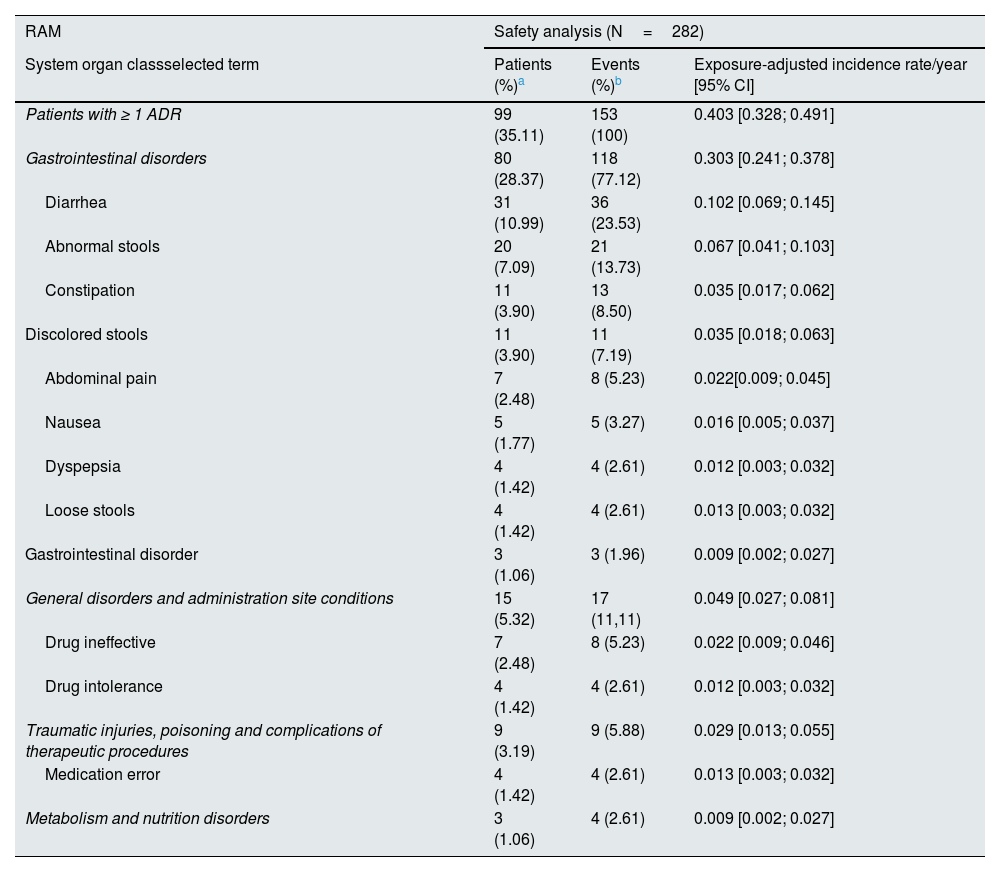
Security variablesA total of 99 patients (35.1%) reported 153 ADRs during the treatment with OHS, the most common being gastrointestinal (118 events in 80 patients) (Table 3). The ADRs were mainly classified as mild or moderate (83.7%) and in 82.4% of the cases it was suspected a relationship with the treatment. A total of 3 patients (1.1%) reported 5 serious ADRs: anemia, abnormal stools, worsening of the condition, respiratory tract infection, and polyneuropathy (Appendix B, Supplementary Table S2).
Adverse drug reactions by system organ class and selected terms.
| RAM | Safety analysis (N=282) | ||
|---|---|---|---|
| System organ classselected term | Patients (%)a | Events (%)b | Exposure-adjusted incidence rate/year [95% CI] |
| Patients with ≥ 1 ADR | 99 (35.11) | 153 (100) | 0.403 [0.328; 0.491] |
| Gastrointestinal disorders | 80 (28.37) | 118 (77.12) | 0.303 [0.241; 0.378] |
| Diarrhea | 31 (10.99) | 36 (23.53) | 0.102 [0.069; 0.145] |
| Abnormal stools | 20 (7.09) | 21 (13.73) | 0.067 [0.041; 0.103] |
| Constipation | 11 (3.90) | 13 (8.50) | 0.035 [0.017; 0.062] |
| Discolored stools | 11 (3.90) | 11 (7.19) | 0.035 [0.018; 0.063] |
| Abdominal pain | 7 (2.48) | 8 (5.23) | 0.022[0.009; 0.045] |
| Nausea | 5 (1.77) | 5 (3.27) | 0.016 [0.005; 0.037] |
| Dyspepsia | 4 (1.42) | 4 (2.61) | 0.012 [0.003; 0.032] |
| Loose stools | 4 (1.42) | 4 (2.61) | 0.013 [0.003; 0.032] |
| Gastrointestinal disorder | 3 (1.06) | 3 (1.96) | 0.009 [0.002; 0.027] |
| General disorders and administration site conditions | 15 (5.32) | 17 (11,11) | 0.049 [0.027; 0.081] |
| Drug ineffective | 7 (2.48) | 8 (5.23) | 0.022 [0.009; 0.046] |
| Drug intolerance | 4 (1.42) | 4 (2.61) | 0.012 [0.003; 0.032] |
| Traumatic injuries, poisoning and complications of therapeutic procedures | 9 (3.19) | 9 (5.88) | 0.029 [0.013; 0.055] |
| Medication error | 4 (1.42) | 4 (2.61) | 0.013 [0.003; 0.032] |
| Metabolism and nutrition disorders | 3 (1.06) | 4 (2.61) | 0.009 [0.002; 0.027] |
95% CI, 95% confidence interval; ADR, adverse drug reaction.
The exposure-adjusted incidence rate is defined as the number of patients with a specific event divided by the total follow-up time for all patients.
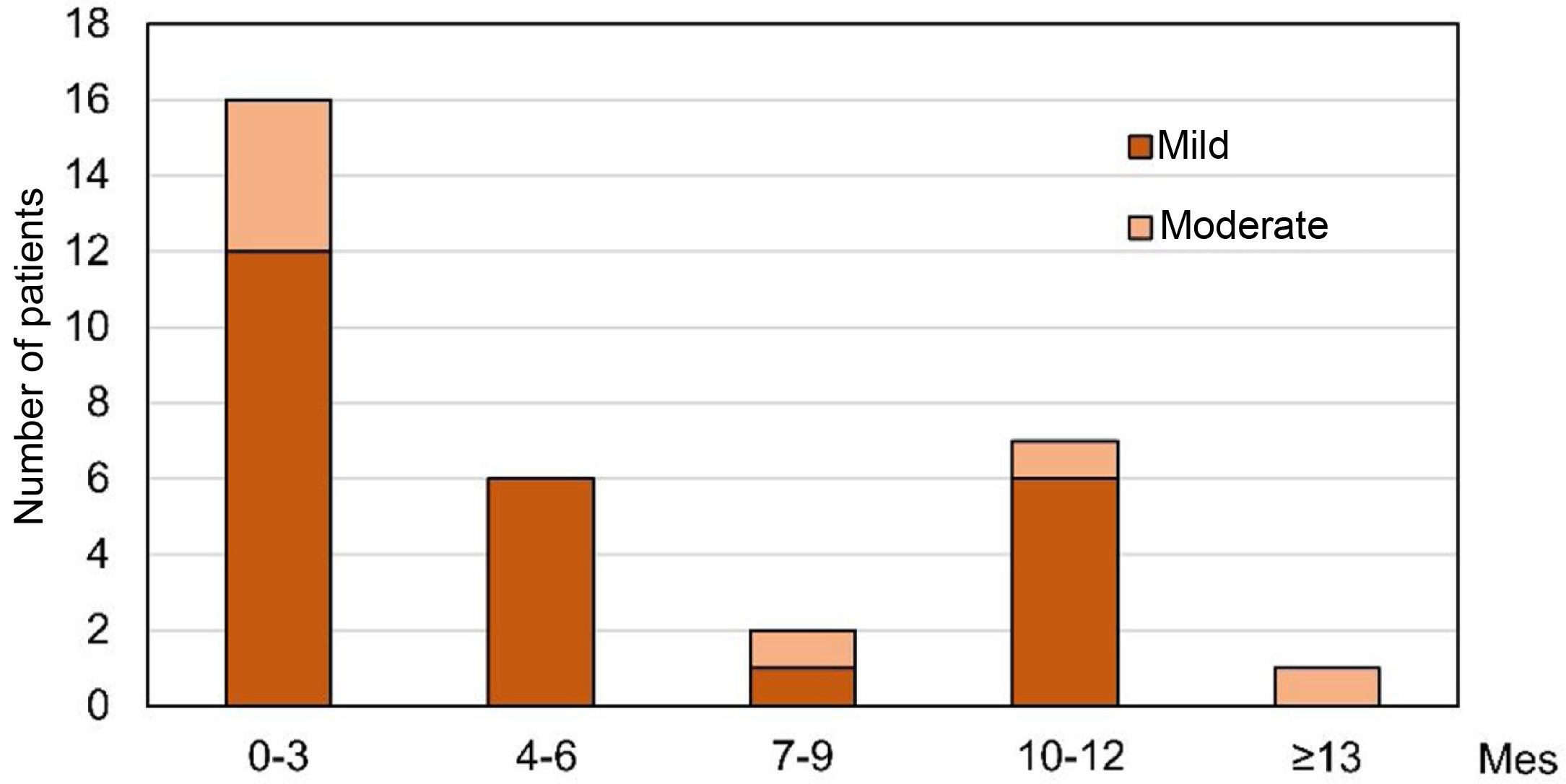
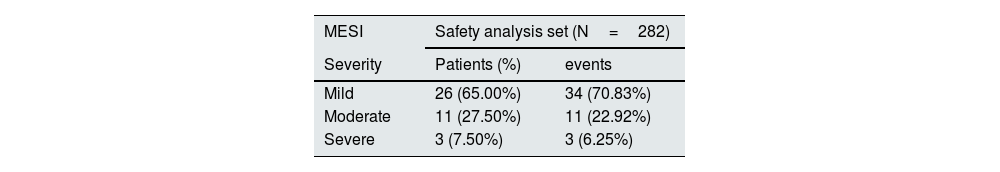
A total of 40 patients (14.2%) registered 48 MESIs, of which 34 (70.8%) were mild, 11 (22.9%) moderate, and 3 (6.3%) severe. Most were classified as gastrointestinal disorders (47 MESIs in 39 patients), with diarrhea being the most common (81.25% of events). A relationship with OHS treatment was reported in 37 MESIs (32 patients), of which one was iron overload and the rest corresponded to episodes of diarrhea (Table 4). The episodes of diarrhea were mild/moderate, and they were observed in 50% of the cases during the first 3 months (Fig. 2) and resolved during the first 2 months in 46.2% of the patients. The 8 patients with gastrointestinal bleeding had pre-existing risk factors such as medication (87.5%) or previous medical conditions/diseases (37.5%). It was not detected a significant delay in the diagnosis of gastrointestinal bleeding related to stool discoloration, nor was it found to be causally related to OHS treatment.
Medical events of special interest registered in the security population and classified by organs and systems and preferred terms.
| MESI | Safety analysis set (N=282) | |
|---|---|---|
| Severity | Patients (%) | events |
| Mild | 26 (65.00%) | 34 (70.83%) |
| Moderate | 11 (27.50%) | 11 (22.92%) |
| Severe | 3 (7.50%) | 3 (6.25%) |
| System organ class preferred term | Patients (%) | Events (%) a | Exposure-adjusted incidence rate/year [95% CI] |
|---|---|---|---|
| MESI | |||
| Patients with ≥ 1 EMID | 40 (14.18) | 48 (100) | 0.133 [0.095; 0.182] |
| Gastrointestinal disorders | 39 (13.83) | 47 (97.92) | 0.130 [0.092; 0.177] |
| Diarrhea | 32 (11.35) | 39 (81.25) | 0.106 [0.072; 0.150] |
| Gastrointestinal bleeding | 3 (1.06) | 3 (6.25) | 0.009 [0.002; 0.027] |
| Hemorrhagic gastrointestinal vascular malformation | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Hematochezia | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Melena | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Rectal bleeding | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Upper gastrointestinal bleeding | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Metabolism and nutrition disorders | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| Iron overload | 1 (0.35) | 1 (2.08) | 0.003 [0.000; 0.017] |
| MESI (serious) | |||
| Patients with ≥ 1 serious MESI | 8 (2.84) | 9 (100.00) | 0.025 [0.011; 0.049] |
| Gastrointestinal disorders | 8 (2.84) | 9 (100.00) | 0.025 [0.011; 0.049] |
| Diarrhea | 1 (0.35) | 1 (11,11) | 0.003 [0.000; 0.017] |
| Gastrointestinal bleeding | 3 (1.06) | 3 (33.33) | 0.009 [0.002; 0.027] |
| Hemorrhagic gastrointestinal vascular malformation | 1 (0.35) | 1 (11,11) | 0.003 [0.000; 0.017] |
| Hematochezia | 1 (0.35) | 1 (11,11) | 0.003 [0.000; 0.017] |
| Melena | 1 (0.35%) | 1 (11,11) | 0.003 [0.000; 0.017] |
| Rectal bleeding | 1 (0.35%) | 1 (11,11) | 0.003 [0.000; 0.017] |
| Upper gastrointestinal bleeding | 1 (0.35) | 1 (11,11) | 0.003 [0.000; 0.017] |
MESI, medical events of special interest; 95% CI, 95% confidence interval.
The exposure-adjusted incidence rate is defined as the number of patients with a specific event divided by the total follow-up time for all patients.
Time of onset of the first episode of diarrhea from the start of treatment.
The graph shows the number of patients in whom the first episode of mild and moderate diarrhea occurred during the period (months) since the start of treatment in the safety analysis set (n=282). No patient had diarrhea classified as severe. Due to the limited number of patients with diarrhoea, the results were pooled by trimester.
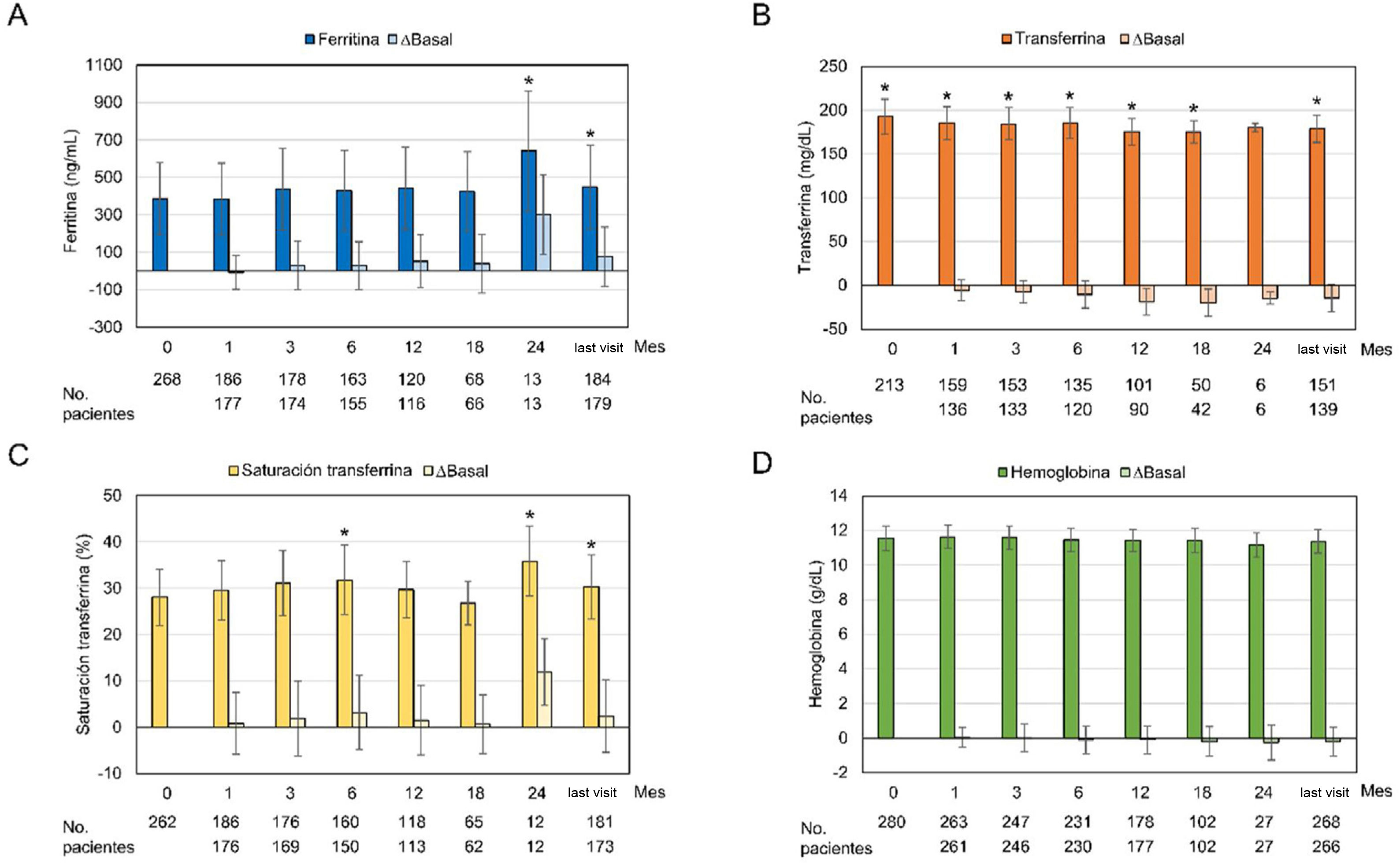
Regarding the iron-related parameters, it was observed an increase in ferritin diring OHS treatment that reached statistical significance at month 24 and at the last visit (386.66ng/mL at baseline vs 447.55ng/mL at the last visit; p=0.0013). Transferrin levels decreased significantly with OHS treatment at all visits, except at month 24 (p=0.053). The transferrin saturation index did not show a definite trend, with significant increases at months 6 and 24 and at the last visit. The hemoglobin profile remained constant, with no significant changes with respect the start of treatment (Fig. 3). Analysis of ferritin levels were analyzed based on concomitant treatment with intravenous or oral iron; it was observed that only those patients who had received concomitant treatment presented a significant increase in ferritin levels at months 3, 6, 24, and at the last month visit (362.64ng/mL at baseline and 473.99ng/mL at the last visit; p=0.0003). In contrast, patients who had not been concomitantly treated with intravenous or oral iron had decreased ferritin levels from baseline, with the exception of month 12 and the last visit (419.61ng/mL at baseline) and 413.18ng/mL at the last visit, p=0.56) (Appendix B, Supplementary Fig. S1).
Values of iron parameters and changes with respect to baseline values during the observational period.
Graphs show mean±standard deviation and changes from baseline over the observation period (months) in the safety analysis set (n=282) for: (A) ferritin, (B) transferrin, (C) transferrin saturation index and (D) hemoglobin. *p≤0.05 compared to the baseline visit.
Forty fatal events were reported in 24 (8.5%) patients: 11 suffered cardiac events (3.9%), 6 general disorders (2.1%) and alterations at the administration site, 4 (1.4%) infections and infestations and 3 (1.1%) gastrointestinal diseases (Appendix B Supplementary Table S3). None of the fatal events was considered related to treatment.
The proportion of patients with ADR and MESI was comparable in patients on hemodialysis and peritoneal dialysis, although they were slightly higher in patients on hemodialysis for MESI (15.3 vs. 10.6%) and, within these, for MESI classified as serious (21.2 vs. 14.3%). A 3.94% of the total ADR, MESI, and fatal events resulted in a dose reduction of OHS.
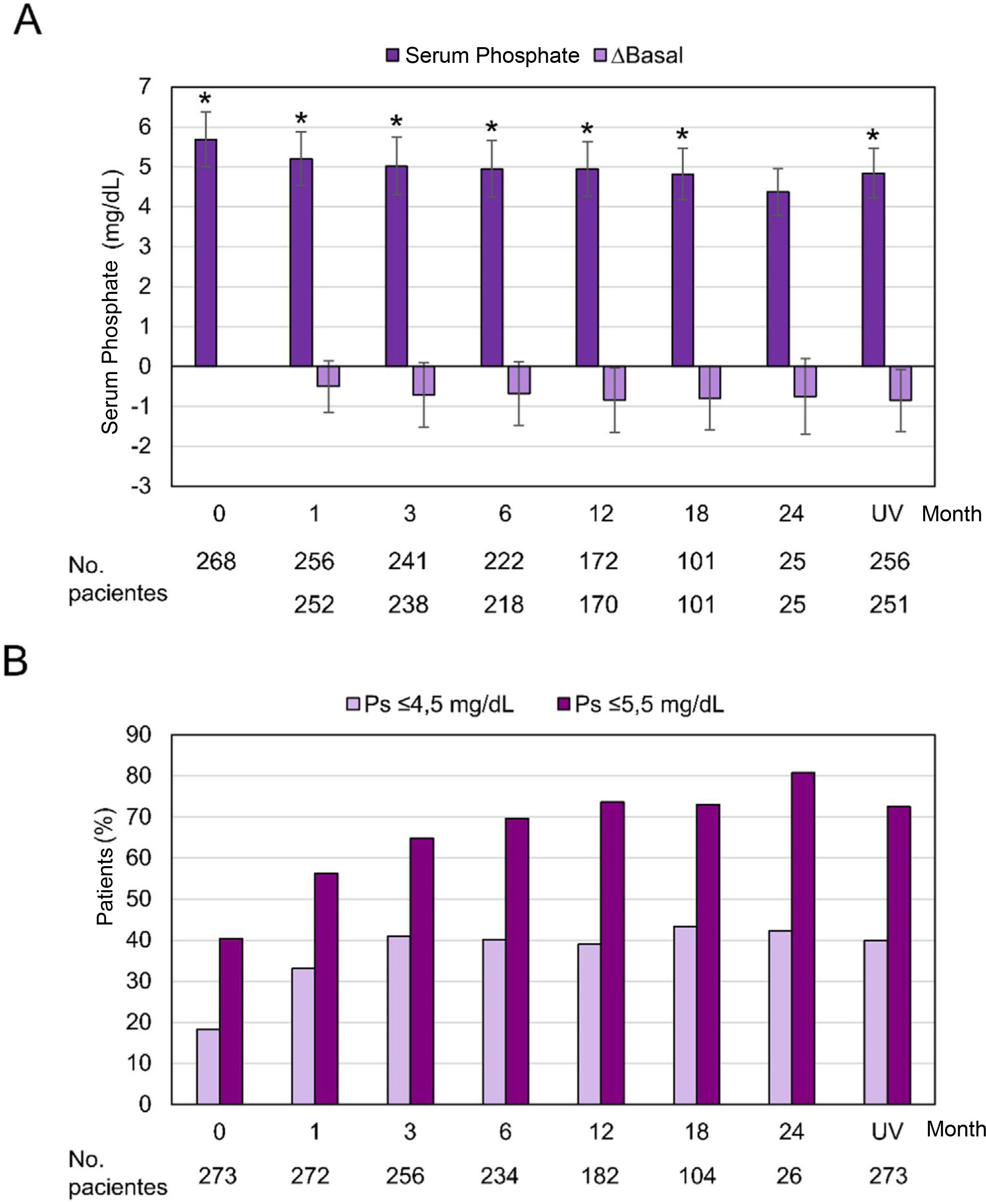
Efficiency variablesSerum phosphorus levels decreased progressively and significantly throughout the follow-up, with the exception of month 24, when the difference with respect to the baseline visit did not reach statistical significance (p=0.058). At the baseline visit, the serum phosphorus level was 5.69±1.37mg/dL and it decreased to 4.84±1.25mg/dL at the last visit (p<0.0001). A 40.3% of the patients had phosphorus levels ≤5.5mg/dL at the baseline visit, while at month 12 the proportion rose to 73.6% and 72.5% at the last visit. The proportion of patients with phosphorus levels ≤4.5mg/dL increased from 18.3% at the baseline visit to 39% at month 12 and 40.0% at the last visit (Fig. 4).
Serum phosphorus levels during the observation period.
Ps: serum phosphorus; UV, last visit
(A) Mean±standard deviation and difference from baseline in serum phosphorus throughout the observation period (months) in the full analysis set (n=273). *p≤0.05 compared to the baseline visit, p=0.058 at month 24. (B) Proportion of patients with serum phosphorus levels ≤4.5mg/dL and ≤5.5mg/dL.
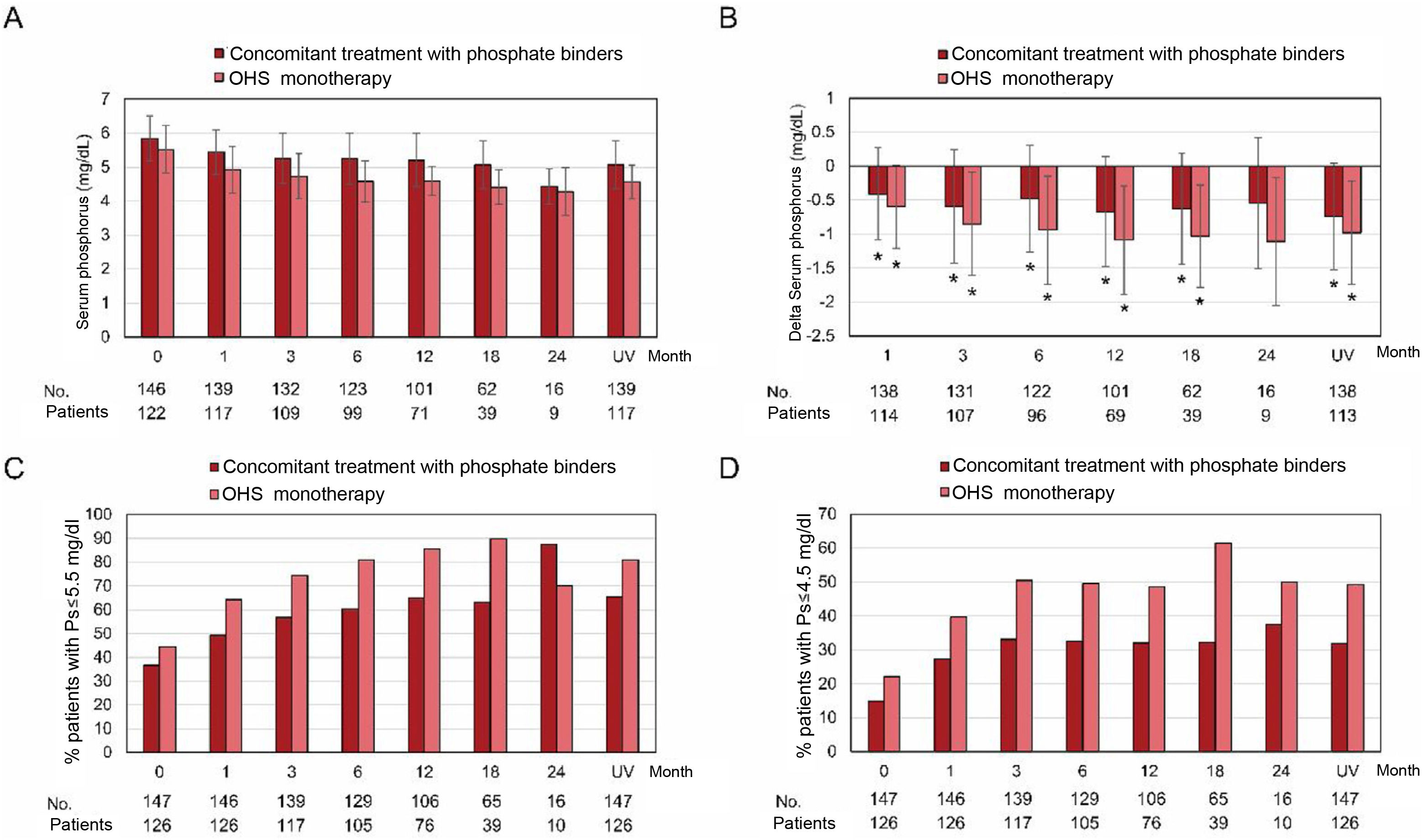
Stratified analysis based on concomitant treatment with phosphate binders during the study showed a greater reduction in absolute phosphorus values in the group of patients who did not receive concomitant treatment (OHS monotherapy). The proportion of patients who reached “target” levels of phosphorus (≤5.5mg/dL) increased in the 2 groups, being higher in the group treated with OHS monotherapy. The proportion of patients with phosphorus levels ≤4.5mg/dL at the last visit was 32% in the group that received concomitant treatment and 49.2% in those treated with OHS monotherapy (Fig. 5).
Serum phosphorus levels during the observation period as a function of concomitant treatment with phosphorus binders.
OHS: sucroferric oxyhydroxide; Ps: serum phosphorus; UV: last visit.
(A) Mean±standard deviation of serum phosphorus levels during the observation period (months) in patients receiving concomitant treatment with phosphate binders or OHS monotherapy in the full analysis set (n=273). (B) Difference from baseline in serum phosphorus levels. *p≤0.05 compared to the baseline visit. (C) Proportion of patients with serum phosphorus levels ≤5.5mg/dL and (D) ≤4.5mg/dL in patients who received concomitant treatment with phosphate binders or OHS monotherapy in the complete analysis set.
The initial daily dose of OHS was 884.6±424.94mg (1.77 tablets/day) and increased to 1002.7±520.92mg (2.01 tablets/day) in the last visit. During the study period, the mean daily dose was 988.1±448.87mg (1.98 tablets/day).
Patients who received concomitant treatment with other phosphate binders received a higher dose of OHS during follow-up (1084±450.21mg [2.17 tablets/day]) compared to those who received OHS monotherapy (876.3±422.22mg [1.75 tablets/day]).
Parameters Related to the Mineral and Bone Disorder Associated with Chronic Kidney DiseaseNo relevant changes in calcium, iPTH or 25-hydroxyvitamin D levels were observed in the safety population during follow-up (Appendix B Supplementary Fig. S2).
DiscussionThe results of the Spanish group of patients of the VERIFIE study show the effectiveness of OHS in reducing serum phosphorus, with a profile of efficacy, safety, and a reduced number of daily tablets consistent with the international study21 and with the pivotal studies.15,16
The present analysis included data from the 282 Spanish patients belonging to the international VERIFIE study,21 one of the largest studies reported to date in this population in Spain. In general terms, baseline demographic and clinical data were similar to those of the international study.21 However, some differences have been observed, such as the incidence of hypertension, which in the subgroup of Spanish patients (14%) was slightly lower than that of the international study (21%)21 and that of the phase III TRIAL (24%).15 In addition, peritoneal dialysis had a higher incidence in this analysis (23%) compared to the international study (12%)21 and the phase III STUDY (8%).15 Differences were also observed in the previous treatment with other phosphorus binders; a lower proportion of patients in the Spanish centers received sevelamer compared to the international study (24.4 vs. 35%).21 A higher percentage of treatment interruptions was also reported in this analysis (33.54%) as compared to the global study (17%).21
The long-term safety profile of OHS showed an incidence and severity of ADRs similar to that previously observed.21 The proportion of patients with ADR and MESI was slightly lower in the Spanish subgroup compared to the international study.21 As observed in previously published studies,15,16,23 the majority of ADRs were gastrointestinal and mainly diarrhea. Diarrhea was transient in most cases. The dose of OHS was reduced in 3.94% of cases due to the existence of ADRs, MESis, and also include the fatal events. Therefore, it cannot be ruled out that in some cases the improvement in diarrhea was due to the reduction of the OHS dose. A phase III STUDY and its subsequent extension in CKD patients on dialysis compared the efficacy and safety of OHS with that of sevelamer carbonate and the results showed that mild diarrhea and discolored stools were more frequent with OHS and nausea and constipation with sevelamer.15 The long-term extension of this study reported a similar incidence of gastrointestinal events between both treatments.16
Most (98%) of the MESIs reported were gastrointestinal and only 6.3% were severe. Eight gastrointestinal bleeding were recorded, but in all cases there were previous risk factors and no significant delay in the diagnosis of gastrointestinal bleeding related to stool discoloration was observed in the Spanish subgroup. A 48.2% of patients were on iron supplements (oral/intravenous) at baseline. The recorded fatal events were considered unrelated to treatment.
Despite the ferric nature of this compound, no clinically relevant alterations were observed in variables related to iron metabolism. The change in ferritin levels was slightly lower than in the international study (29.6ng/mL vs. 43.22ng/mL at 6 months)21 and also lower than in the post hoc analysis of the phase III STUDY in week 24 (119ng/mL).24 In both the international study and in the Spanish subgroup, a significant increase in ferritin levels was observed only in patients who received intravenous or oral iron. This finding is consistent with what was observed both in the phase III STUDY15,16 and the post hoc analysis24 as well as in retrospective observational studies18,19 and implies minimal iron absorption associated with OHS treatment. It should be noted that one of the 2 cases of iron overload in the international study belonged to the subgroup of Spanish patients.
The data on efficacy from our subgroup study were in line with the international study,21 reaching a significant and progressive reduction in serum phosphorus levels that remained within the range (≤5.5mg/dL) from the first month until the end of the of the follow-up. Notably, the proportion of patients with serum phosphorus levels ≤4.5mg/dL increased from 18.3% at the baseline visit to 41% at month 3 and remained around 40% during follow-up; these percentages support the rapid and sustained effect of OHS previously reported in the international study21 and are promising since, as reported in the COSMOS study, levels of 4.4mg/dL are associated with the lowest risk of mortality.7 In line with this, the SEN establishes 4.5mg/dL as the recommended upper limit for serum phosphorus.6
One of the differences with the international study is that, in the Spanish subgroup, the serum phosphorus values were systematically lower and the proportion of subjects within target levels was higher.21 After 3 months of treatment with OHS, it was observed a decrease in serum phosphorus levels of −0.71 and −0.76mg/dL in the present analysis and in the international study21 respectively, values that contrast with the greater reduction (−0.71mmol/L or −2.17mg/dL) observed after 3 moths in the phase III STUDY.15 These differences are striking but they could be explained, in addition to differences in the design of both studies, by the markedly lower baseline levels in this sub-analysis and in the international study compared to those observed in phase III STUDIES with OHS 15,25 and in other observational studies.17,19 It could also be explained by the specific characteristics of Spanish clinical practice, as well as by differences in diet. Similar to the international study,21 the proportion of patients with “target” (≤5.5mg/dL) and “optimal” (≤4.5mg/dL) phosphorus levels was higher in the subgroup treated with OHS in monotherapy.
The initial dose of OHS was 884.6±424.94mg (1.77 tablets/day) and increased to 988.1±448.87mg (1.98 tablets/day) during the observational period. This reduced number of tablets, slightly lower than that reported in the international study,21 contrasts with the average daily doses required with other phosphorus binders. A sub-analysis of the DOPPS study, reported a mean of 6 tablets/day of phosphorus binders globally and 5.1 in Spain.26 The present study also shows that the mean daily dose was higher in subjects who received concomitant treatment with other binders (2.17 tablets/day) compared to those treated with OHS monotherapy (1.75 tablets/day) and that the proportion of patients with serum phsphorus within target was higher in this patient on monotherapy. The lower number of tablets with OHS in monotherapy could be explained considering the known fact that the prescription of a higher number of tablets/day is associated with lower compliance and adherence26 and lower phosphorus control.27 In a prospective study, switching to OHS treatment resulted in decreased phosphorus levels with a less number of tablets, resulting in increased adherence to treatment.28
The small number of tablets reported with OHS is especially relevant in the dialysis population, usually polymedicated, and would help explain the high rates of patients with phosphorus levels on "target" observed in this study. However, it is important to highlight the 33.5% of permanent interruptions of OHS treatment, one of the main causes being the patient's decision (27.1%).29 In this context, the effectiveness of these agents lies not only in their ability to bind phosphorus, but also in their ability to be accepted by the patient. In turn, it has been observed that taking patient preferences into account improves patient adherence.30 The analysis by country of the DOPPS study showed that only 61% of the Spanish subjects included reported having taken the prescribed treatment with phosphorus binders.26 Similarly, in a study conducted in Spain, only 40% of the 121 patients included were compliant, showing a relationship between the number of tablets and low adherence to phosphorus binders.29
Finally, there were no substantial changes observed in variables such as calcium, iPTH or 25-hydroxyvitamin D during treatment with OHS, which is in line with what was previously reported,16 supporting the specificity of OHS towards phosphorus. It is important to highlight that modifications in the dose or the initiation of treatment with calcimimetics can modulate the levels of iPTH or phosphorus. In this study, 29.1% of the patients were receiving treatment with calcimimetics before the start of the study and 41.8% during follow-up.
The VERIFIE study was the first to show the results of the use of OHS on clinical practice prospectively at long term. This sub-analysis shows the experience with OHS in the Spanish centers participating in VERIFIE. The importance of this sub-analysis lies in the usefulness of knowing the evolution of the patients taking into account the demographic and clinical profiles and the specific clinical practice in Spain, which could help to reinforce and optimize some aspects of the clinical management of this population. One of the main strengths of the study is the acceptable sample size and the wide range of clinical variables analyzed. The results obtained are in line with those reported in the international study21 and previously published trials, but in addition it shows the specific characteristics of this subgroup.
The main limitations of the study reside in its observational nature, the lack of a control group, and the absence of data regarding patient adherence. Due to its observational nature and long-term follow-up, the sample size decreased throughout the follow-up, resulting in a limited group of patients at month 24. It should be noted that some variables, such as the period of onset of the first episode of diarrhea, had to be grouped by months due to the small number of events.
ConclusionThis sub-analysis of the VERIFIE study shows that OHS was an effective treatment for the control of hyperphosphatemia with a very small number of daily tablets relative to the usual clinical practice in Spanish patients on dialysis. The safety profile was similar to that observed in the international population with a majority of adverse events of mild/moderate intensity.
FinancingThis work has been funded by Vifor Fresenius Medical Care Renal Pharma (Glattbrugg, Switzerland).
Conflict of interestsPablo Molina has received lecture fees from Abbot, Amgen, Fresenius-Kabi, Nutricia, Sanofi, and Vifor/Fresenius-Renal Pharma, as well as consulting fees from Palex and Vifor/Fresenius-Renal Pharma. Nuria García Fernández has received training aid from Vifor Pharma and has participated in Mundipharma scientific advisory meetings. Alejandro Martin Malo has received speaking and consulting fees from Medtronic, Vifor Pharma, AstraZeneca and Astellas. Roser Peiró Jordán is an employee of Vifor Pharma. Jorge Cannata-Andia has received travel grants from Vifor Pharma. The rest of the authors declare they have no conflicts of interest.
Carla Granados from Trialance SCCL has assisted in the preparation of the manuscript.
Annex A. Researchers who are part of the Spanish VERIFIE groupProf. Mariano Rodríguez, Dr. Jesús Grande Villoria, Dr. Ana Blanco Santos, Dr. Rafael García Maset, Dr. Pilar Sánchez Pérez, Dr. Fabiola Dapena Vielba, Dr. Juan Manuel Díaz Gómez, Dr. María Cruz Cid Parra, Dr. Laura Fuentes, Dr. M. a Paz Alcaide, Dr. Jesús Calviño Varela, Dr. M. a José Fernández Reyes, Dr. Emilio Gonzáles Parra, Dr. Rosa María Ruiz Calero, Dr. Pedro Abaigar, Dr. Josefa Galán González, Dr. Alejandro Pérez Alba, Dr. Adoración Martínez Losa, Dr. Mercedes Salgueira, Dr. Delfina Yetman Almiron, Dr. Juan Manuel Buades Fuster, Dr. Meritxel Ibernon Vilaron, Dr. José María Portolés Pérez, Dr. César Remón Rodríguez and Dr. Jose Luis Lerma.