INTRODUCTION
Quality is one of the strategic elements on which the transformation and improvement of modern health systems is based. The effort made in recent years towards quality assurance in this field, and in particular in nephrology, entails a recognition of the need for access to objective and normalised measurement tools for health activities: “Quality is not just about good intentions”.1-3 The Spanish Society of Nephrology (SEN), sensitive to this need, has promoted the creation of a series of clinical guides that give orientation on how things should be done and what quality is, as in the case of haemodialysis (HD) centres.4 One of the most important tasks that must be developed in quality management systems is the periodical follow-up of indicators. This allows us to learn about our own situation, as well as that of other centres, and gives us the opportunity to improve certain aspects of the care we offer our patients. The systematic and planned revision of all those parameters (indicators), which we consider necessary for adequate follow-up, forms part of the tasks which must be developed in any activity which is intended to improve outcomes. These indicators, which are associated with a previously determined objective or standard, allow the introduction of improvement activities and give ongoing verification that they are effective. The intention is to identify the existence of problem situations for evaluation or intervention. It is basically, therefore, an internal tool which permits us to make comparisons among ourselves; at the same time, the pooling of results from different centres will enable us to know which are the true quality standards in peritoneal dialysis (PD) in the Spanish population.
It is important to establish across-the-board quality criteria, some patterns of reference with which the results of the health care process can be compared between centres since, in certain aspects, the population data that would help us to define them is not available. The idea is to establish the desired quality as an objective, to measure results by comparing reality to that objective, to correct defects and to measure the effects of the changes introduced. It has already been shown that the periodical measurement of quality indicators, and having standards and establishing objectives, helps to improve the control and outcomes of the HD process5,6 and contributes to improving outcomes in terms of patient morbidity and mortality.7,8 Access to software supports which facilitate data management becomes a priority. There are several software applications which are generally used in Spanish PD and HD centres (Renalsoft®, Nefrolink®, Nefrosoft®, etc.) and several of them are developing quality indicator modules that allow fast, automatic calculation.
In order to introduce innovation and ongoing improvement, we must maintain a self-critical attitude. Obtaining results alone is not sufficient, we must also question ways in which they can be improved by way of research and training.9 The survey conducted by the quality group in 2003 brought to light the low implementation of quality systems in the PD area.10 Given the existing experience in HD as far as the definition of indicators and quality standards11 and their monitoring,12 it became crucial to unify criteria and define useful indicators and standards in PD that would serve to evaluate the activity being performed while allowing the situation of some centres to be seen in relation to others. Along these lines, a group of PD experts, with the support of the Nephrology Quality Management Group of the SEN, has designed a proposal for the definition of quality indicators and standards that can be understood and used by all members of the nephrology community involved in PD, and which serve as a point of reference for areas of future improvement, given the leap between the production and subsequent monitoring of guidelines.
The aim of this study has been to outline a PD monitoring proposal agreed among PD expert nephrologists from different centres around Spain for generalised implementation.
1. MATERIAL AND METHODS
An initial meeting was held between the project coordinators and the SEN Quality Management Group to agree on the methodology that would be followed to define the indicators and standards. The aspects of renal replacement therapy in PD that were considered a priority due to their relevance, the level of scientific evidence to support them and the possibility of accurately measuring their degree of implementation, were chosen. Some aspects with a direct impact on patients’ lives but not compiled in the guides were also included. Four area coordinators were established, one or two for each group of indicators, and various people were assigned to each group. Between them, all these people developed the corresponding indicators and the coordinator was responsible for drawing up the final version from each corresponding group. The general coordinators reviewed the definitive version which was sent to all those involved and the authors for their suggestions. The document has been exhibited on the SEN Web page so that all nephrologists can provide feedback.
From the selected recommendations, the quality indicators were drawn up according to a format which included: definition, criterion, formula, units, periodicity, standard, bibliographical references and comments. The methodologies of the Joint Comission,13,14 and the Standing Committee of the Hospitals of the European Union15 were followed for health care process monitoring systems and the specific HD methodology that is followed by the American ESRD Special Project and implemented by the Centres for Medicare and Medicaid Services (CMS) such as the ESRD Clinical Performance Measures (CPM) Project.16 Initially, quality criteria were selected from each recommendation for performance measurement. The indicator is a quantitative measurement to evaluate a criterion. The “standard” was set for each indicator (required degree of performance to ensure an acceptable quality level) based on scientific evidence or, in its absence, by consensus. On many occasions, sufficient scientific evidence has not been available, but experience derived from the follow-up of indicators in Spain will help us define them in the future. Furthermore, ongoing improvement objectives, independently of those defined in this document, must be established by each unit according to its outcomes.
In principle, several indicators have been established: each unit was to use those they considered useful in their daily routine. The indicators are expected to be updated according to adaptations to new editions of guidelines and availability of new therapeutic tools and to the results of indicator monitoring in daily clinical practice.
2. GLOBAL INDICATORS
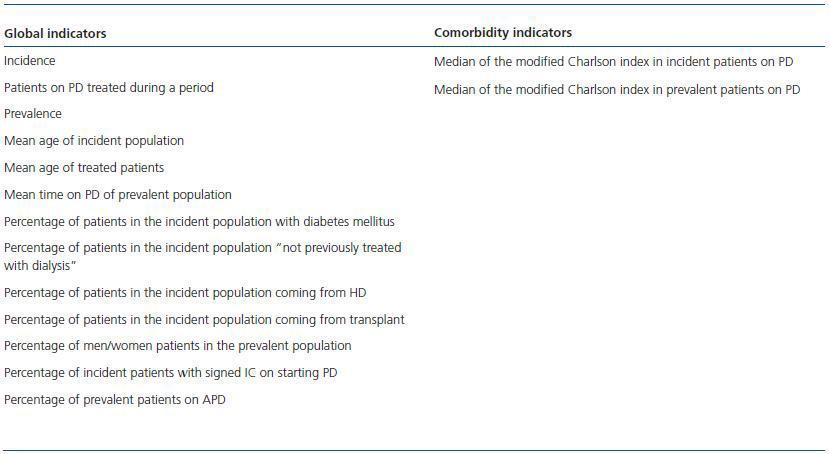
These are not quality indicators but terms of reference which enable us to learn certain patient characteristics and the PD units (PDU) that may influence outcomes.17-28 It is interesting to see how their evolution pans out over time. Table 1 shows the general indicators of the study population, and also includes those of comorbidity.
2.1. Period incidence of peritoneal dialysis
- Definition: Number of new patients incorporated into the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Units: Absolute value.
- Periodicity: Yearly.
- Basis: PD outcomes are influenced by experience in this treatment at the centre.
- Interpretation and underlying factors: It assesses the process of offering alternative treatments in advanced chronic kidney disease (ACKD) and the activity of the PDU.
2.2. Patients on peritoneal dialysis treated during the period
- Definition: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Formula: Sum of prevalent patients at 31 December of previous period with highest PD rates during the study year.
- Standard: Not defined.
- Units: Number of patients/year.
- Periodicity: Yearly.
- Basis, interpretation and underlying factors: Similar to paragraph 2.1.
2.3. Prevalence
- Definition: Number of prevalent patients in the PDU at 31 December of the study year.
- Standard: Not defined.
- Units: Absolute value.
- Periodicity: Yearly.
- Basis, interpretation and underlying factors: Similar to paragraph 2.1.
2.4. Mean age of incident population
- Definition: The arithmetic mean of the ages of new patients who were incorporated into the PDU between 1 January and 31 December of that year.
- Formula:
Numerator: Sum of the ages of the incident patients of the PDU between 1 January and 31 December of that year.
Denominator: Number of incident patients in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Units: Years.
- Periodicity: Yearly.
- Basis: It assesses the degree of population bias.
- Interpretation and underlying factors: A PDU with patients of very advanced age has a greater risk of failure and one comprising individuals with a mean age of under 55 years should respond to higher expectations (transplant, work related, etc.).
2.5. MEAN AGE OF TREATED PATIENTS
- Definition: Arithmetic mean age of all patients treated in the PDU (taken from the indicator obtained in paragraph 2.2) between 1 January and 31 December of that year.
- Formula:
Numerator: Sum of the ages of prevalent PDU patients between 1 January and 31 December of that year.
Denominator: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Units: Years.
- Periodicity: Yearly.
- Basis, interpretation and underlying factors: Similar to paragraph 2.4.
2.6. Mean time on peritoneal dialysis of prevalent population
- Definition: Mean length of stay on PD of prevalent patients at the end of each year. It is the arithmetic mean of the months on PD of patients subjected to dialysis in the PDU at 31 December of that year.
- Formula:
Numerator: Sum of the months of prevalent patients in the PDU at 31 December of that year.
Denominator: Number of prevalent patients in the PDU at 31 December of that year.
- Standard: Not defined.
- Units: Months.
- Periodicity: Yearly.
- Basis: PD patients should be assured stability in dialysis time that is only minimally altered by complications.
- Interpretation and underlying factors: It assesses the capacity of the PDU to keep patients in treatment for a reasonable time. Only a reduction of this time in anticipation of renal transplant (RT) should be evaluated positively.
2.7. Percentage of incident patients with diabetes mellitus
- Definition: New PD patients with diabetes mellitus included by year in relation to total new PD patients.
- Formula:
Numerator: 100 x sum of incident patients in the PDU between 1 January and 31 December of that year who have diabetes mellitus.
Denominator: Number of new patients who began PD that year.
- Standard: Not defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses degree of population bias and treatment options in ACKD.
- Interpretation and underlying factors: High presence of diabetes may represent a health care burden for the PDU and add to morbidity and mortality.
2.8. Percentage of patients from incident population “not previously treated with dialysis”
- Definition: Patients new to kidney replacement treatment included in PD in the year, in relation to total new PD patients.
- Formula:
Numerator: 100 x sum of incident patients in the PDU, not previously treated with other kidney replacement treatment techniques, between 1 January and 31 December of that year.
Denominator: Number of new patients who began PD that year.
- Standard: Not defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses degree of population bias and treatment options in ACKD.
- Interpretation and underlying factors: Patients who are totally new to dialysis represent the original state of the patient with ACKD-5, without comorbidities induced by other replacement treatments. It assesses the treatment options in ACKD, patient participation in treatment selection and patient referral time to nephrology services.
2.9. Percentage of patients from the incident population coming from haemodialysis
- Definition: New PD patients coming from HD included in the year in relation to total new PD patients.
- Formula:
Numerator: 100 x sum of incident patients in the PDU coming from HD between 1 January and 31 December of that year.
Denominator: Number of new patients who began PD in that year.
- Standard: Not defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses the degree of population bias. Patients treated with HD should also have the knowledge and opportunity to switch to PD.
- Interpretation and underlying factors: It assesses the opportunity of HD patients to change treatment, whether by necessity or choice.
2.10. Percentage of incident population patients coming from transplant
- Definition: New PD patients included in the year coming from transplant in relation to total new PD patients.
- Formula:
Numerator: 100 x sum of previously transplanted incident patients in the PDU between 1 January and 31 December of that year.
Denominator: Number of new patients who began PD in that year.
- Standard: Not defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses the degree of population bias. Patients who lose their kidney graft should have the same opportunities as new patients to evaluate their dialysis options. An extensive transplant program inevitably generates a number of patients annually who need to return to dialysis.
- Interpretation and underlying factors: It assesses the process of offering alternative treatments to transplant patients requiring dialysis and their participation in treatment selection. It may explain certain variations in the results caused by the type of population attended to, due to a possible greater comorbidity of this group.
2.11. Percentage of men/women patients of the prevalent population
- Definition: Percentage of presence by gender of prevalent population in the PDU in relation to total patients subjected to dialysis in the study period.
- Formula:
Numerator: Sum of the men/women patients subjected to dialysis in the PDU at 31 December of that year.
Denominator: Number of patients subjected to dialysis in the PDU at 31 December of that year.
- Standard: Not defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses the degree of population bias. Similar to that of the new global population in dialysis. A tendency of 10% greater presence of men is expected.
- Interpretation and underlying factors: A PDU whose percentage differs from that expected represents some population bias.
2.12. Percentage of incident patients with signed informed consent at start of peritoneal dialysis
- Definition: Percentage of patients who have signed informed consent (IC) for PD on starting the treatment in relation to total dialysed patients in the study period.
- Formula:
Numerator: Sum of incident patients who have signed the IC at the start of the technique.
Denominator: Number of incident patients in the PDU in that year.
- Standard: 100%.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: For everyone’s benefit, all medical action should be covered by a well presented and explained IC.
- Interpretation and underlying factors: It assesses the security given to every PDU by having evaluated with each patient the arguments for and against the treatment implemented. It should be considered a quality indicator.
2.13. Percentage of prevalent patients on automatic peritoneal dialysis (APD)
- Definition: Percentage of prevalent patients who are treated with automatic peritoneal dialysis (APD) with respect to total.
- Formula:
Numerator: Sum of patients who are treated with APD at 31 December of the study year.
Denominator: Number of prevalent patients in the PDU at 31 December of the study year.
- Standard: To be defined.
- Units: Percentage.
- Periodicity: Yearly.
- Basis: It assesses the use of a very convenient treatment option which improves the independence of active patients who are capable of learning how to use it.
- Interpretation and underlying factors: A PDU should offer APD to a number of its patients. These techniques are more beneficial than manual techniques for some patients.
3. COMORBIDITY INDICATORS
3.1. Median Charlson comorbidity index in incident peritoneal dialysis patients
- Definition: Median of the modified Charlson index of all incident patients on PD in that period.
- Formula: Median and interquartile range (percentile 50 and percentiles 25 and 75), calculated in the first month of PD, of all patients incorporated into the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Units: Numerical value.
- Periodicity: Yearly.
- Basis: Patients who begin renal replacement treatment (RRT) are increasingly older and have more associated pathologies. Both factors have a significant impact on their morbidity and mortality and quality of life. The use of the Beddhu29 modification of the Charlson index has been proposed, given its simplicity, extensive use in dialysis patients and validity to adjust the results in terms of state of health as well as morbidity and mortality.
- Interpretation, underlying factors and bibliography: Appendix 1.
3.2. Median of the Charlson comorbidity index in prevalent peritoneal dialysis patients
- Definition: Median of the modified Charlson index of all prevalent patients on PD at 31 December.
- Formula: Median and interquartile range (percentile 50 and percentiles 25 and 75) of all patients who are in the PDU at 31 December of that year.
- Standard: Not defined.
- Units: Numerical value.
- Periodicity: Yearly.
- Basis, interpretation, underlying factors and bibliography: Similar to paragraph 3.1.
4. OUTCOME INDICATORS (HOSPITALISATION)
Table 2 shows all indicators of clinical outcomes.
4.1. Number of hospital admissions
- Definition: Number of admissions by patient and year.
- Formula:
Numerator: Number of patient admissions to the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: Hospital admission has a negative effect on patients’ quality of life, elevates costs and increases certain risks to the affected population. It may indicate certain rectifiable deficiencies in ambulatory treatment.
- Interpretation and underlying factors: There are important factors that may influence hospitalisation, such as higher comorbidity of a determined population or social characteristics of a certain health area (geographical dispersion, displacement costs, etc.)
- Observations: It could refer to a specific process or diagnosis, allowing comparisons of the same pathologies between different centres.
4.2. Percentage of total patients on PD admitted in a period
- Definition: Percentage of patients treated who were admitted between 1 January and 31 December of that year.
- Formula:
Numerator: Number of patients admitted in the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: Similar to paragraph 4.1.
- Interpretation, underlying factors and observations: Similar to paragraph 4.1.
4.3. Mean stay in hospital admissions
- Definition: Mean stay of admitted patients.
- Formula:
Numerator: Number of days of PDU patients between 1 January and 31 December of that year.
Denominator: Number of hospital stays of PDU patients between 1 January and 31 December of that year.
- Standard: To be defined.
- Basis: It assesses the agility and functioning of the hospital.
- Interpretation and underlying factors: The mean stay should be reduced as much as possible to avoid prolonging admissions unnecessarily.
5. OUTCOME AREA INDICATORS (PATIENTS WHO LEAVE PERITONEAL DIALYSIS)
Periodicity: Yearly.20,30-36
5.1. Number of patients who leave peritoneal dialysis treatment for any reason
- Definition: Percentage of patients who leave the PDU for any reason in the year.
- Formula:
Numerator: 100 x sum of patients who leave the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: Directly related to the length of time the patient stays in treatment. Deviations in these results with respect to other centres may indicate situations that need study and possible improvement.
- Interpretation and underlying factors: Measures the capacity of a certain centre to maintain PD treatment for an adequate length of time and the relative weight of each of the reasons for abandonment. Leaving dialysis for transplant should be valued positively.
5.2. Number of patients who leave peritoneal dialysis treatment due to transfer to haemodialysis
- Definition: Percentage of patients who leave the PDU due to transfer to HD.
- Formula:
Numerator: 100 x sum of patients who leave the PDU due to transfer to HD between 1 January and 31 December of that year.
Denominator: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: It represents the true technique survival. Currently, survival of patients on HD and PD is very similar with an initial advantage for PD. However, technique survival is less in PD although in recent years it has improved substantially, reflecting greater experience of dialysis units, technological advances, dialysis schedules which allow a better quality of life (APD) and enhanced biocompatibility of PD solutions. All these aspects could be measured by the different causes for transfer to HD and the relative weight of each one.
- Interpretation and underlying factors: It enables the evaluation of differences in evolution over time and comparison of the results with other centres. In the event of higher frequency, potentially rectifiable causes should be studied. Higher transfer does not always indicate a greater failure rate of the technique; care should be taken with transfers not made “on time”, which may be increasing abandonment due to death.
5.3. Number of patients who leave peritoneal dialysis due to death
- Definition: Percentage of patients who leave the PDU due to death.
- Formula:
Numerator: 100 x sum of patients who leave the PDU due to death between 1 January and 31 December of that year.
Denominator: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: To be defined.
- Basis: Knowing the mortality rate enables improvement of candidate selection criteria and follow-up.
- Interpretation and underlying factors: Knowing the mortality rate enables better detection of complications. Adjustments for age and principal comorbidity factors are necessary.
6. TRANSPLANT INDICATORS37-40
6.1. Inclusion rate on the renal transplant waiting list
- Definition: Number of patients included on the waiting list for renal transplant (RT) in relation to the total number of patients on PD.
- Formula:
Numerator: 100 x sum of patients included on the list for RT in the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: To know the percentage of patients on PD included on the waiting list in relation to the total patients on PD in the period of one year. Patients treated in different centres should have the same RT opportunities.
- Interpretation and underlying factors: It assesses the process of offering alternative treatments. Once adjusted to the characteristics of the different populations, it analyses the variability that may exist between different centres and equal opportunity. It assesses quality in the RT candidate selection process.
6.2. Time to inclusion on the renal transplant waiting list
- Definition: Mean time to inclusion on the waiting list.
- Formula: Mean time in days from start of PD to inclusion on the waiting list.
- Standard: Not defined. Proportion of patients included in less or equal time to that established as adequate (to be defined).
- Basis: It assesses the speed of inclusion and, as a result, the quality of functioning of the overall service and its coordination.
- Interpretation and underlying factors: Inclusion on the waiting list should be as efficient as possible. It should, in fact, no longer be a job done exclusively by dialysis units and be done in ACKD consultations.
6.3. Number of patients subjected to transplant in the peritoneal dialysis unit
- Definition: Percentage of patients on the waiting list who are subjected to transplant.
- Formula:
Numerator: 100 x number of patients subjected to transplant in the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients included on the waiting list who are being or have been treated in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: To know the transplant activity of different areas and equity in the access to transplant of the dialysis population. Due to the variability which may exist between different PD centres as a consequence of patient hetereogeneity, this indicator should be adjusted to population characteristics.
- Interpretation and underlying factors: Useful to evaluate whether the number of transplants performed on our patients agrees with the rest of the population, there being neither positive nor negative discrimination. Equal opportunity for RT.
6.4. Time on dialysis prior to renal transplant
- Definition: Mean time on PD before transplant.
- Formula: Mean time from start of PD to transplant.
- Standard: Not defined.
- Basis: It is an indirect indicator of the transplant activity of referral centres and is useful in monitoring the evolution of the number of transplants, in relation to those included on the waiting list in PDUs.
- Interpretation and underlying factors: It evaluates whether transplant is delayed in patients on PD and it enables the investigation of its causes.
6.5. Time until removal of peritoneal catheter after renal transplant
- Definition: Mean time until removal of peritoneal catheter after RT.
- Formula: Mean time in months between transplant and removal of catheter.
Numerator: Sum of elapsed months between performing the transplant and the removal of the catheter in patients subjected to transplant in the PDU between 1 January and 31 December of that year.
Denominator: Total number of patients subjected to transplant in the PDU between 1 January and 31 December of that year.
- Standard: Not defined.
- Basis: The most appropriate time to remove the catheter has not been established, but it appears that it should be carried out as soon as there is a reasonable guarantee of the viability of the graft, generally between the second and third months after transplant. Early removal (including during the RT surgery) has been advised for paediatric recipients.
- Interpretation and underlying factors: Presence of the catheter may favour complications (peritonitis, infections of the exit site, injury to hollow viscera, etc.). It is an indicator of continuous care until final completion of PD.
6.6. Percentage of patients whose peritoneal catheter is removed within 3 months after renal transplant
- Definition: Percentage of patients whose peritoneal catheter is removed within 3 months after renal transplant.
- Formula:
Numerator: Number of patients subjected to transplant in the PDU between 1 January and 31 December of that year whose catheter is removed within 3 months after transplant.
Denominator: Total number of patients subjected to transplant in the PDU between 1 January and 31 December of that year.
- Standard: 75% within 3 months.
- Basis, interpretation and underlying factors: Similar to paragraph 6.5.
7. INFECTION INDICATORS41-63
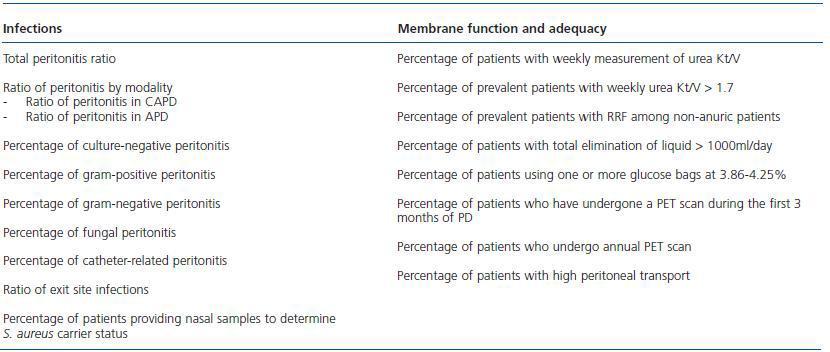
Table 3 shows the indicators related specifically to the technique and includes infections, adequacy of dialysis and peritoneal membrane function.
7.1. Total peritonitis ratio (patient/month)
- Definition: Annual peritonitis incidence in the PDU expressed in terms of number of patients and time of exposure.
- Formula:
Numerator: Sum of months of exposure to risk of each patient treated during the year.
Denominator: Number of peritonitis episodes.
- Units: One episode every x months-patient.
- Periodicity: Yearly.
- Standard: Less than one episode every 24 patient-months.
- Basis: Based on indices obtained by Y-systems that are referred to in randomised studies (evidence level B).
- Interpretation and underlying factors: A PDU should estimate this value annually and take the indicated value as reference. Where frequency is higher, potentially rectifiable causes should be studied. Recurrences of peritonitis count as new episodes, but relapses do not.
7.2. Ratio of peritonitis by modality
- Definition: Annual incidence of peritonitis for each modality of the PDU expressed in terms of number of patients and time of exposure.
7.2.1. Ratio of peritonitis in continuous ambulatory peritoneal dialysis
- Definition: Annual incidence of peritonitis in continuous ambulatory peritoneal dialysis (CAPD) of the PDU expressed in terms of number of patients and time of exposure.
- Formula:
Numerator: Sum of months of exposure to risk of each patient treated during the year with CAPD.
Denominator: Number of peritonitis episodes in CAPD modality.
- Units: One episode every x months-patient.
- Periodicity: Yearly.
- Standard: Less than one episode every 24 patient-months.
- Basis, interpretation and underlying factors: Similar to paragraph 7.1.
7.2.2. Ratio of peritonitis in automatic peritoneal dialysis
- Definition: Annual incidence of peritonitis in APD in the PDU expressed in terms of number of patients and time of exposure.
- Formula:
Numerator: Sum of months of exposure to risk of each patient treated during the year with APD.
Denominator: Number of peritonitis episodes in APD modality.
- Units: One episode every x months-patient.
- Periodicity: Yearly.
- Standard: Less than one episode every 24 patient-months.
- Basis, interpretation and underlying factors: Similar to paragraph 7.1. Comparative studies which have shown no essential or definitive differences between CAPD and APD.
7.3. Percentage of culture-negative peritonitis
- Definition: Percentage of all correctly taken peritonitis cultures (without previous general or intraperitoneal antibiotics) that are culture-negative.
- Formula:
Numerator: Number of culture-negative peritonitis episodes x 100.
Denominator: Total number of peritonitis episodes with correctly taken samples (without previous general or intraperitoneal antibiotics).
- Units: Percentage.
- Periodicity: Yearly.
- Standard: Less than 20% of all correctly performed cultures.
- Basis: Bacterial growth in peritonitis samples is possible to a high degree and it is necessary for adequate management of the clinical situation.
- Interpretation and underlying factors: A value higher than the standard represents a questionable sample management methodology and should require a revision of the collection and culture method. At least 10 valid episodes should be counted for the result to be representative.
7.4. Percentage of gram-positive peritonitis
- Definition: Percentage of total peritonitis episodes caused by gram-positive germs.
- Formula:
Numerator: Number of peritonitis episodes caused by gram-positive germ x 100.
Denominator: Total number of peritonitis episodes.
- Units: Percentage.
- Periodicity: Yearly.
- Standard: Approximately 60-70%.
- Basis: A PDU should know the annual figure of germs causing peritonitis in its patients in order to establish the corresponding empiric treatment protocols.
- Interpretation and underlying factors: A high percentage may require reconsidering the patient training process in the technique. This percentage is important as much as it grows, since a decrease should lead to the revision of the other germs.
7.5. Percentage of gram-negative peritonitis
- Definition: Percentage of all peritonitis episodes caused by gram-negative germs.
- Formula:
Numerator: Number of peritonitis episodes caused by gram-negative germ x 100.
Denominator: Total number of peritonitis episodes.
- Units: Percentage.
- Periodicity: Yearly.
- Standard: 10-30%.
- Basis: Similar to paragraph 7.4.
- Interpretation and underlying factors: The mean age of the PDU may be a determinant factor of a greater percentage of these infections. By expressing the indicator as a percentage of all peritonitis episodes, units which are able to reduce gram-positive peritonitis can be penalised. Even so, it is difficult to accept a gram-negative percentage higher than 30%, in which case analysis would be advisable.
7.6. Percentage of fungal peritonitis
- Definition: Percentage of all peritonitis episodes caused by fungi.
- Formula:
Numerator: Number of fungal peritonitis episodes x 100.
Denominator: Total number of peritonitis episodes.
- Units: Percentage.
- Periodicity: Yearly.
- Standard: Less than 5%.
- Basis: Similar to paragraph 7.4.
- Interpretation and underlying factors: An elevated percentage is an indication that the general policy for use of antibiotics should be reconsidered and the more frequent use of antifungal prophylaxis evaluated.
7.7. Percentage of catheter-related peritonitis
- Definition: Percentage of peritonitis episodes in which the peritoneal catheter can be considered the cause and in which the same germ is isolated in the peritoneal liquid and at the exit site or subcutaneous tunnel.
- Formula:
Numerator: Number of peritoneal catheter-related peritonitis episodes x 100.
Denominator: Total number of peritonitis episodes.
- Units: Percentage.
- Periodicity: Yearly.
- Standard: 10-25%.
- Basis: A PDU should know the annual number of peritonitis episodes in which the peritoneal catheter is clearly implicated.
- Interpretation and underlying factors: An elevated percentage should require exit site care protocol and interpretation of data to be reevaluated.
7.8. Ratio of exit site infections
- Definition: Annual incidence of exit site infections in the PDU expressed in terms of number of patients and time of exposure.
- Formula:
Numerator: Sum of months of exposure to risk of each patient treated during the year.
Denominator: Number of exit site infection episodes.
- Units: One episode every x patient/month.
- Periodicity: Yearly.
- Standard: Less than one episode every 24 patient-months.
- Basis: Exit site care and the diagnosis and treatment of infections that affect the exit site are fundamental for catheter survival and the prevention of peritonitis.
- Interpretation and underlying factors: An elevated percentage should require the exit site care protocol to be reevaluated. As there is no consistency in the diagnostic criteria for catheter infections, the indicator is valuable for comparison within the unit itself using the same criteria. The rates of catheter infection described vary between 0.05 and 1.02 episodes/patient-year and the continual application of antibiotics to the exit site can reduce incidence.
7.9. Percentage of patients providing nasal samples to determine Staphylococcus aureus carrier status
- Definition: Percentage of patients who have provided at least one annual sample to determine Staphylococcus aureus nasal carrier status.
- Formula:
Numerator: Number of patients providing nasal sample x 100.
Denominator: Total number of treated patients.
- Units: Percentage.
- Periodicity: Yearly.
- Standard: 100% on at least one occasion.
- Basis: S. aureus nasal carrier status has been related to peritonitis and exit site infections caused by this germ. Its eradication seems to be associated with a lower incidence of these complications.
- Interpretation and underlying factors: The repeated monitoring of this status in all unit patients is highly recommended.
8. MEMBRANE FUNCTION AND ADEQUACY INDICATORS64-89
8.1. Percentage of patients with weekly urea Kt/V measurement
- Definition: Percentage of PDU patients who have at least one half-yearly measurement of urea Kt/V.
- Formula:
Numerator: Number of prevalent patients with half-yearly determination of urea Kt/V x 100.
Denominator: Total number of prevalent patients in the PDU at the end of the semester.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: 90%.
- Basis: It assesses the quality of the PDU in relation to the calculation of dialysis dose.
- Interpretation and underlying factors: There may be cases of patients who have difficulty collecting the samples. Patients with less than 3 months on PD may still not have determined a Kt/V.
- Observations: Weekly urea Kt/V is the total Kt/V and peritoneal Kt/V in patients with renal function.
8.2. Percentage of prevalent patients with weekly urea Kt/V below 1.7
- Definition: Percentage of all prevalent PDU patients with half-yearly urea Kt/V whose weekly urea Kt/V >1.7.
- Formula:
Numerator: Number of prevalent patients with weekly urea Kt/V > 1.7 x 100 during the six months of study.
Denominator: Total number of prevalent patients with half-yearly determination of urea Kt/V.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 90%.
- Basis: It assesses the percentage of PDU patients who meet certain minimum objectives of prescribed dialysis dose. Those objectives are related to PD patient survival.
- Interpretation and underlying factors: A high percentage of patients with Kt/V below 1.7 would indicate poor medical care. There may be a number of patients not reaching these objectives, for whom modification of the dialysis schedule or transfer to another technique is not considered due to different reasons.
8.3. Percentage of prevalent patients with determination of residual renal function among non-anuric patients
- Definition: Percentage of PDU patients who have, at least, one half-yearly measurement of RRF (adjusted to body surface area).
- Formula:
Numerator: Number of prevalent patients with half-yearly determination of RRF x 100.
Denominator: Total number of prevalent patients in the PDU with RRF during those six months.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: 95%.
- Basis: It assesses the quality of the PDU in relation to RRF determination as information which contributes to dialysis dose. RRF has been associated with PD survival.
- Interpretation and underlying factors: It may be difficult to obtain samples from certain patients.
- Observations: RRF is measured as the mean urea and creatinine clearance divided by two and corrected to body surface area. Its units are ml/min/1.73m2.
8.4. Percentage of patients with total liquid elimination over 1,000ml/day
- Definition: Percentage of total prevalent PDU patients with total liquid elimination > 1,000ml/day (sum of diuresis and peritoneal ultrafiltration).
- Formula:
Numerator: Number of prevalent patients at the end of the six month period with total liquid elimination > 1,000ml/day x 100.
Denominator: Total number of prevalent PDU patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 90%.
- Basis: It assesses the percentage of PDU patients who meet some minimum objectives of daily liquid elimination, a fact associated with PD survival.
- Interpretation and underlying factors: There may be patients with a lower daily elimination of liquids who are in a state of euvolaemia.
8.5. Percentage of patients using one or more glucose bags at 3.86-4.25%
- Definition: Percentage of total prevalent PDU patients who use at least one glucose bag at 3.86-4.25% daily.
- Formula:
Numerator: Number of prevalent patients who use one or more glucose bags/day at 3.86-4.25% x 100.
Denominator: Total number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Below 20%.
- Basis: It assesses the use of hypertonic glucose solutions in the PDU.
- Interpretation and underlying factors: The use and abuse of hypertonic glucose solutions has been related to deterioration of the peritoneum and to systemic effects associated with daily glucose absorption and, therefore, its use should be restricted. The use of APD and alternative solutions such as icodextrin may reduce the use of hypertonic solutions. A high percentage of anuric patients and the lack of available treatment alternatives may explain the higher percentage of use.
- Observations: Regular use of these solutions will be considered, not the sporadic use on specific occasions (volume overload, etc.).
8.6. Percentage of patients who undergo peritoneal equilibration test during the first three months on peritoneal dialysis
- Definition: Percentage of total PDU patients who undergo a peritoneal equilibration test (PET) in the first 3 months on PD.
- Formula:
Numerator: Number of new PD patients subjected to PET in the first 3 months on PD x 100.
Denominator: Total number of new PD patients.
- Units: Percentage.
- Periodicity: Annual.
- Standard: Above 90%.
- Basis: It assesses those studies allowing the evaluation of peritoneal membrane function at the start of PD treatment.
- Interpretation and underlying factors: It is helpful for gaining knowledge on the peritoneal membrane and for prescribing the most adequate dialysis schedule for each patient.
- Observations: Only patients with at least 3 months on PD treatment will be considered.
8.7. Percentage of patients who undergo an annual peritoneal equilibration test
- Definition: Percentage of prevalent PDU patients who undergo an annual PET.
- Formula:
Numerator: Number of prevalent patients with annual PET x 100.
Denominator: Total number of prevalent patients.
- Units: Percentage.
- Periodicity: Annual.
- Standard: Above 90%.
- Basis: It assesses those periodical studies evaluating peritoneal membrane function and the changes which occur over time on dialysis.
- Interpretation and underlying factors: It is helpful for gaining knowledge on the peritoneal membrane and for prescribing the most adequate dialysis schedule for each patient at all times.
8.8. Percentage of patients with high peritoneal transport
- Definition: Percentage of patients with creatinine dialysate/plasma (D/P) ratio at 4 hours equal to or above 0.81 in the annual follow-up PET.
- Formula:
Numerator: Number of prevalent patients in whom the annual PET shows a creatinine D/P equal to or above 0.81 x 100.
Denominator: Number of prevalent patients who have undergone the annual PET.
- Units: Percentage.
- Periodicity: Annual.
- Standard: Below 15%.
- Basis: It assesses those patients whose peritoneal function is, or could be, altered, and allows patients with ultrafiltration (UF) failure to be detected. It allows patients at risk of abandoning the technique in the short-medium term to be recorded. The state of high transporter has been associated with higher mortality and technique failure in PD patients.
- Interpretation and underlying factors: It is helpful in recognising patients with present or future deterioration in peritoneal membrane function, helping to prescribe the most adequate dialysis for those patients. A value above the standard may be due to some patients having difficulty accessing other renal replacement treatment options.
9. ANALYTIC INDICATORS90-95
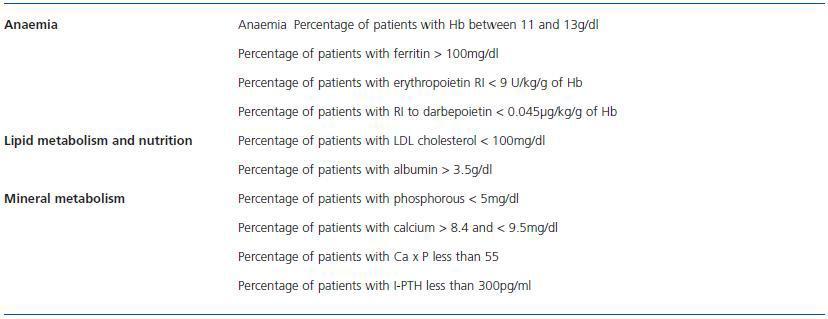
Table 4 shows these indicators.
9.1. Percentage of patients with target haemoglobin
- Definition: Percentage of prevalent patients with mean haemoglobin (Hb) between 11 and 13g/dl in the study period.
- Formula:
Numerator: Number of prevalent patients with mean Hb > 11g/dl and < 13g/dl x 100.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: 80%.
- Basis: It assesses the degree of correction of anaemia in the PDU. Hb levels above 11g/dl are thought to be associated with a reduction in morbidity and mortality, as well as levels above 13g/dl.
- Interpretation and underlying factors: Up to 20% of patients are thought not to reach the target due mainly to associated comorbidity. It is important to carry out an evaluation with individual EPO dose, since patients not reaching the target should receive high doses of erythropoietic-stimulating factors (ESF) and be evaluated to look for causes. Otherwise, this would indicate poor medical care.
9.2. Percentage of patients with ferritin above 100mg/dl
- Definition: Percentage of prevalent patients with mean ferritin above 100mg/dl in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean ferritin > 100mg/dl.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: It assesses the degree of iron deficiency in PD patients. Patients should have sufficient iron deposits to be able to reach the target Hb and accommodate the use of ESF.
- Interpretation and underlying factors: The use of ESF and Hb levels reached should be evaluated jointly. “False” elevations of ferritin are included in the 20% margin allowed for these cases.
9.3. Percentage of patients with an index of resistance to erythropoietin below 9U/kg/g of haemoglobin
- Definition: Percentage of prevalent patients with EPO resistance index (ERI) below 9U/kg/g of Hb in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean ERI < 9U/kg/g of Hb.
Denominator: Number of prevalent patients treated with EPO. RI = EPO dose (U/kg/week)/haemoglobin (g/dl).
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: ERI measures efficacy in anaemia management. Alterations may warn of poor anaemia control due to the existence of iron deficiency, insufficient EPO dose, associated comorbidity, infradialysis, etc.
- Interpretation and underlying factors: An ERI below 9 would be 6,000U/week for 60kg and Hb = 11g/dl.
9.4. Percentage of patients with darbepoetin resistance index below 0.045µg/kg/g of haemoglobin
- Definition: Percentage of prevalent patients with darbepoetin resistance index (DRI) below 0.045mg/kg/g of Hb in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean DRI < 0.045mg/kg/g of Hb.
Denominator: Number of prevalent patients treated with darbepoetin (DA). RI = DA dose (mg/kg/week)/Hb (g/dl).
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: Similar to 9.3, but for DA.
- Interpretation and underlying factors: A DRI below 0.045mg/kg/g of Hb would be 30mg/week for 60kg and Hb = 11.
9.5. Percentage of patients with LDL cholesterol below 100mg/dl
- Definition: Percentage of prevalent patients with mean LDL cholesterol < 100mg/dl in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean LDL cholesterol < 100mg/dl.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: It assesses the risk factor associated with morbidity and mortality in PD patients.
- Interpretation and underlying factors: Patients who do not meet this parameter should be treated with hypolipidaemic drugs. Failure to do so would indicate disregard for the associated comorbidity. There may be a percentage of patients in which this objective cannot be reached in spite of receiving adequate treatment, or because they are contraindicated for the use of hypolipidaemic drugs. Some guides suggest a target level below 75mg/dl in patients with high CV risk (PD patients are possibly in this group). No evidence to that effect has been obtained from PD patients.
9.6. Percentage of patients with albumin above 3.5g/dl
- Definition: Percentage of prevalent patients with mean albumin > 3.5g/dl in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean albumin > 3.5g/dl.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: This parameter is correlated with survival, although it is too multifactorial to achieve adequate feedback.
- Interpretation and underlying factors: It is a nutritional parameter, but it also assesses haemodilution and peritoneal and renal protein loss. The laboratory method used to determine this parameter changes its values and should be taken into account.
9.7. Percentage of patients with phosphorus below 5mg/dl
- Definition: Percentage of prevalent patients with mean phosphorus < 5mg/dl in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean phosphorus < 5.5mg/dl.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: Some adequate phosphorus levels depend on the dialysis dose, the recommended diet and the use of binding agents. Its control is correlated with lower cardiovascular comorbidity. Hypophosphataemia is an indicator of increased mortality in relation to malnutrition.
- Interpretation and underlying factors: A unit with poor phosphorus controls may entail insufficient PD dose, poor attention to dietary advice or unreliable prescription compliance by patients.
9.8. Percentage of patients with calcium above 8.4 and below 9.5mg/dl
- Definition: Percentage of prevalent patients with mean calcium > 8.4 and < 9.5mg/dl in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean calcium > 8.4 and < 9.5mg/dl.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 80%.
- Basis: It assesses the adequate control of calcium levels. Inadequate control of calcaemia and calcium-phosphorus product has been associated with morbidity and mortality in dialysis.
- Interpretation and underlying factors: Calcium control is quite independent of dialysis dose, although the kind of solutions used may influence its levels. The use of calcium binders, vitamin D derivatives and calcimimetics should be taken into account when analysing its values.
- Observations: Calcium levels should be corrected to albumin. The formula normally used is: corrected Ca = measured Ca (mg/dl) + (4 – Alb [g/dl] x 0.8).
9.9. Percentage of patients with calcium x phosphorus below 55
- Definition: Percentage of prevalent patients with a mean calcium-phosphorus product below 55 in the study period.
- Formula:
Numerator: 100 x number of denominator patients with mean calcium x phosphorus product below 55.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 70%.
- Basis: It assesses control of Ca/P metabolism, which is an important factor of morbidity and mortality in dialysis patients. It is also related to the appearance of vascular calcification.
- Interpretation and underlying factors: The dialysis solutions used, dialysis dose, recommended diet and use of different drugs (phosphorus binders, vitamin D derivatives and calcimimetics) should be taken into account when analysing its values. The recent inclusion of non-calcium phosphate binders enables this objective to be reached in a greater number of patients.
9.10. Percentage of patients with intact parathyroid hormone below 300pg/ml
- Definition: Percentage of prevalent patients with a mean intact parathyroid hormone (iPTH) below 300pg/ml in the study period.
- Formula:
Numerator: 100 x number of denominator patients with iPTH below 300pg/ml.
Denominator: Number of prevalent patients.
- Units: Percentage.
- Periodicity: Half-yearly.
- Standard: Above 70%.
- Basis: Target iPTH values recommended by clinical guides vary between 150 and 300pg/ml.
- Interpretation and underlying factors: The iPTH values may be changed depending on the laboratory method used for their determination. Levels below 150pg/ml are, in many cases, indicative of low bone remodelling.
Table 1. General indicators of the study population
Table 2. Clinical outcome indicators
Table 3. Technique-specific indicators
Table 4. Analytic indicators