Monkeypox is a zoonotic infection previously endemic in Africa which has been spreading to other continents in recent months. Transmission occurs through direct contact with damaged skin, mucous membranes, respiratory droplets and through contaminated fomites. The most common clinical manifestations include fever, skin lesions and lymphadenopathy. It should be suspected in a patient with compatible lesions and epidemiological risk factors, and confirmed by detection of viral DNA by polymerase chain reaction (PCR).1 The main management is based on supportive measures and symptomatic treatment. However, antivirals such as tecovirimat can be used in selected patients. We report here on a kidney transplant patient diagnosed with a severe case of monkeypox infection.
The patient was a 26-year-old male, recipient of a kidney transplant in January 2021, with a baseline creatinine of 2.6 mg/dl and no proteinuria. His underlying nephropathy was secondary to atypical haemolytic uraemic syndrome (aHUS), and he was being treated with eculizumab. Since transplantation, the patient has had two episodes of acute humoral rejection in the first and sixth month, due to poor adherence to treatment. He is currently being treated with tacrolimus, mycophenolate mofetil and prednisone.
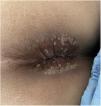
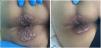
He came to the emergency department with a 4-day history of fever, pain in the anal region and diarrhoea with no blood or mucus. He reported having had anal intercourse without using a condom. Papular lesions were visible in the perianal region (Fig. 1) and on his trunk and abdomen. He had no palpable lymphadenopathy or abdominal pain. Analyses showed leucocytosis with neutrophilia and elevated acute phase reactants (APR): C-reactive protein 29.7 mg/dl and procalcitonin 5.7 mg/dl. PCR for monkeypox in skin exudate and blood was requested and both were positive. We decided to halve the daily dose of mycophenolate and admitted the patient, but he self-discharged 48 h later.
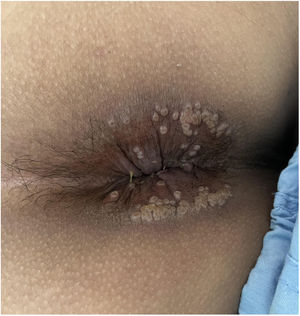
After 4 weeks, he returned to the emergency department with diarrhoea and severe rectal bleeding. He had exudative ulcerated lesions on the entire perianal margin (Fig. 2), while the lesions in the other locations were in the process of healing. Given the poor clinical progress and the suspicion of proctosigmoiditis, an application was made to the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency for Medicines and Medical Devices] to start treatment with tecovirimat, which was approved.
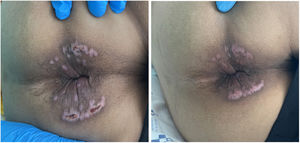
After seven days of treatment, a colonoscopy was performed showing two ecchymotic lesions and fibrin-covered ulcers in the anal canal in the process of healing. The lesions in the perianal region were resolving (Fig. 2) and the rectal bleeding had subsided. A new monkeypox PCR was performed on the samples obtained and was negative. Given the good progress and the lack of availability of the drug, as it is a foreign medication, the patient completed seven days of treatment with tecovirimat instead of the usual 14 days recommended and was discharged from the hospital.
We describe here the first case in our centre of a kidney transplant patient with monkeypox infection, having found no other cases published in the literature to date. In view of the patient's poor clinical progress, treatment with tecovirimat was administered after approval of the application by the AEMPS. Tecovirimat is the drug of choice for the treatment of monkeypox.2 Despite the lack of clinical trials to determine efficacy in humans, studies in primates have shown increased survival in those who received tecovirimat compared to those who received placebo, even when the drug was administered after the onset of severe complications. The most common side effects are headache, nausea and abdominal pain. It was administered to approximately 360 human volunteers in an extended safety trial, which found an adverse effect profile similar to that of placebo.3 It cannot be ruled out that tacrolimus levels may decrease, as it acts as an inducer of the CYP3A4 cytochrome, although in our patient's case blood levels remained stable during treatment.
Cidofovir is a second-line drug. Although it has in vitro activity against monkeypox and has been shown to be effective in animal models, there are no clinical data on its efficacy in humans at present and its use may be associated with significant adverse events, such as nephrotoxicity and/or hepatotoxicity.4 Brincidofovir is a modification which has also shown activity in vitro and in primate trials. Its advantages lie in the fact that it is administered orally and causes less nephrotoxicity.5
In the case described here, the patient had a satisfactory clinical outcome with no side effects and no interactions with tacrolimus throughout the treatment.