EXAMINING PROBLEMS ASSOCIATED WITH MINERAL AND BONE DISORDER
Definitions
Renal osteodystrophy is a term that has traditionally been used to refer to chronic kidney disease (CKD)-mineral and bone disorders. Recently, the KDIGO (Kidney Disease: Improving Global Outcomes) has proposed new definitions and a more integrated classification system, thus diluting the importance of the traditional renal osteodystrophy definition.1-5
Renal Osteodystrophy (ROD). This term should be exclusively used to define bone morphology and architecture disorders in CKD patients (Figure 1). It should be diagnosed by bone biopsy.
Chronic Kidney Disease-Mineral Bone Disorder. This term refers to all biochemical, skeletal and extraskeletal calcification alterations caused by systemic CKD-mineral bone disorders (Figure 2). It is manifested by one or a combination of the following:
1. Abnormalities of calcium (Ca), phosphorus (P), parathyroid hormone (PTH) and vitamin D metabolism.
2. Abnormalities in bone turnover, mineralisation, volume, linear growth or strength.
3. Vascular or other soft-tissue calcifications.
Physiopathology
The different metabolic alterations are secondary to progressive loss of renal mass and function.
It is understood that moderate decreases in creatinine clearance (less than approximately 70ml/min) may cause an increase in phosphataemia following phosphorus overload, together with a potential decrease in calcaemia. It may lead on isolated occasions to early CKD evolution.6
Serum phosphorus values increase in patients with CKD stages 4 and 5, although phosphate retention is produced much earlier and is not detected in serum measurements. An early increase in plasma fibroblast growth factor-23 (FGF-23), as well as increased urinary phosphorus excretion rates, may reveal phosphate retention.
The common slight, but significant, decrease in calcitriol (1,25[OH]D3) in early stages of CKD is secondary to:
1. Loss of renal mass, provoking 1-alpha-hydroxylase to be less available.
2. Decrease in the glomerular filtration rate (GFR), leading to a decrease in tubular 25(OH)D3 25(OH)D3 should be filtered by the glomerulus in order to reach the proximal convoluted tubule where it is engulfed by cellular endocytosis (mediated by megalin, an endocytic receptor located in the apical membrane).
3. Phosphate retention, which decreases renal calcitriol synthesis, either directly or indirectly, via an increase in FGF-23.
The deficit of the calcitriol synthesis decreases intestinal calcium absorption. This is an early phenomenon in patients with CKD stages 2 and 3.
Phosphate retention, as well as the calcitriol deficit and the kidney disease per se, are also responsible for skeletal resistance to PTH.5
An increase in PTH levels is especially observed when GFR are less than 60ml/min/1.73m2.
An increase in FGF-23 and molecules produced by the very kidney tissue may also occur prematurely, alongside phosphate retention, calcitriol deficit and hypocalcaemia when secondary hyperparathyroidism (HPT2) develops. A decrease or lack of affinity or underregulation of receptors such as megalin, or other correlation factors may also be involved.
Low 25(OH)D3 levels (lack of consumption or insufficient solar exposure) in CKD can also, as in the general population, lead to HPT2 and metabolic bone disease, especially during early CKD stages.1
There are at least two classic receptors in the parathyroid glands, which channel the effects of molecules and hormones, modifying PTH synthesis and secretion, such as gland size, and which are important when assessing therapeutic options to HPT2.
The two parathyroid gland receptors are:
1. Vitamin D receptor (VDR) a) Vitamin D action onto PTH is mediated by this receptor, which is a cytosolic receptor. b) As CKD progresses, the number of VDR decreases, the very uraemic state can reduce VDR mRNA stability, causing the receptor’s protein levels to decrease. Furthermore, “uraemic toxins” reduce vitamin D-VDR complex translocation to the nucleus and its binding to DNA-response elements.7 c) VDR deficit produces resistance to vitamin D’s inhibitory action on PTH synthesis. d) Hyperplasia of parathyroid glands is accompanied by a decrease in VDR density. VDR decrease is very significant in advanced hyperplasia stages, i.e. “nodular hyperplasia”.
2. Calcium-sensing receptor (CaR). Minimal changes in serum calcium levels are detected by CaR, which is located on the surface of the parathyroid cells. When the serum calcium level decreases, there is not enough calcium bound to the calcium receptors and PTH secretion inhibition stops.
a) Deficiency in CaR produces resistance to the Ca action on the parathyroid gland.
b) Progressive development of parathyroid hyperplasia secondary to CKD is linked to a decrease in the number of calcium receptors in the parathyroid cells. Recently, the FGF-23 receptor (FGF-R) and its “co-receptor”, the protein Klotho, was found in parathyroid glands, allowing FGF 23 to exert an inhibitory action on the parathyroid gland. A decrease in FGF-R and Klotho was found in uraemic animal models and hyperplastic parathyroid samples of uraemic patients, which causes hyperplastic parathyroid glands to become resistant to FGF-23 inhibitory action.
Effect of molecules and hormones on receptors
1. A decrease in extracellular calcium is detected by the calcium receptor in the plasma membrane, stimulating PTH production.
2. Phosphate retention stimulates PTH synthesis and secretion. Furthermore, it induces parathyroid hyperplasia, which in turn reduces CaR and VDR expression, promoting PTH synthesis and secretion.
3. Vitamin D acts on the VDR, suppressing PTH synthesis and secretion. Vitamin D deficiency reduces this effect.
4. Calcitriol deficiency causes VDR mRNA expression to be underregulated. A decrease in calcium also underregulates CaR and VDR expression. In contrast, calcitriol is able to overregulate its own receptor in different tissues. Also, there are differences between different vitamin D analogues (selective vitamin D receptor activators (sVDRA) and it also seems that calcimimetics could increase VDR expression in the parathyroid gland.8
5. Calcitriol can also increase CaR expression. This effect weakens with hypocalcaemia present and is more important when calcium levels are normal or high, or when calcimimetics are administered.9
Related lesions
As a result, these alterations will produce injury to target tissues. The skeletal and cardiovascular systems are the main tissues affected. Soft tissues calcification and calciphylaxis are also important complications, as they are associated with a significant morbidity and mortality increase in CKD patients.
Vascular calcifications are not a passive process. An increase in phosphorus, calcium, inflammatory mediator and uraemia levels have been observed to promote smooth muscle cells transforming into osteogenic lineage cells. These cell produce collagen matrix, which is later mineralised. Acidosis reduces calcifications.10
Other age-related processes, such as bone fragility, muscular weakness, falling more often, and arteriosclerosis, are not considered to be directly related to CKD, but they can coexist alongside it. Moreover, they influence the diagnosis, treatment and prognosis of the effects that CKD has on target organs.
It is likely that these fatal effects are not only due to the bone disorders. Hyperphosphataemia has been associated with an increase in intima-media thickness, stiffness and vascular calcifications, myocardial hypertrophy and mortality,11 among others, as well as with CKD progression. The parathyroid hormone (PTH) has traditionally been considered as a uraemic toxin and has been associated with different systemic effects.5 More recently, vitamin D deficiency has become more important, also in the general population, as it is linked to alterations in immunoregulatory mechanisms, inflammatory response, cellular proliferation regulation, insulin secretion and renin production. Furthermore, 25(OH)D (calcidiol) has a direct action on bone metabolism and is a substrate for calcitriol generation. As a consequence of these effects, mineral and bone disorders have proven to be independent mortality predictors in the uraemic population, especially cardiovascular mortality.12-21
DIAGNOSTIC STRATEGIES
The objective is to define the ideal diagnosis methods for assessing and treating mineral disorders. The data presented here is the result of recommendations extracted from clinical guidelines, such as the KDOQI guides, the recently published KDIGO guidelines and the “KDOQI US Commentary of the 2009 KDIGO Clinical Guideline”. It is also based on expert opinion obtained from the references and the authors’ own opinions.1,2,4,22,23
Although the biochemical test intervals have been established in the S.E.N quality guidelines,24 more detailed recommendations will be provided below.
Biochemical parameters (Tables 1-3)
As is the case for other international guides, we recommend that laboratories inform clinicians of the measurement method used, and report any change in the methodology or sample nature (serum or plasma). Samples should be handled correctly to ensure that all biochemical results can be interpreted correctly (1B).
Calcium and phosphorus
Calcium and phosphorus levels have little predictive value for underlying bone diseases and are often normal because PTH levels increase. However, calcium and phosphorus, as well as PTH measurements should be taken regularly (1C).
Ideally, ionic calcium should be used, but there are processing and cost-related problems for its systematic use. When using total calcium, we recommend correcting the albumin levels (or plasmatic proteins) for cases of hypoalbuminaemia and hypoproteinaemia.
Corrected total Ca (mg/dl) = total Ca (mg/dl) +0.8[4 – albumin (g/dl)]
We should however consider that using albumin-corrected calcium and ionised calcium is not strongly recommended, probably due to variations in albumin, pH, and blood concentration problems, among others, which are found in dialysis or CKD patients.25,26
Correction formulae have even been developed which also consider plasma phosphate, as well as albumin.27
It is also important to remember that extracellular calcium concentration does not always correlate with the calcium balance (which could be positive or negative with the normal plasma calcium).
We find the KDIGO-recommended monitoring intervals reasonable: a) stage 3: Ca and P every 6-12 months; b) stage 4: Ca and P every 3-6 months; c) stage 5: Ca and P every 1-3 months. However, we think that monthly assessments would be better for stage 5D rather than every 1-3 months as proposed by KDIGO (BC).
Tests may have to be performed more frequently in patients under calcimimetic or vitamin D derivative treatments (active metabolites, analogues, sVDRA), especially during dose-titration phases (NG).
Calcium and phosphorus tests for haemodialysis patients should be standardised and performed over the longer period, i.e. at the beginning of the week (BC).
The calcium-phosphate product provides useful data for dialysis patients, but should never be considered in isolation (i.e. without calcium and phosphorus levels). It is the least useful parameter for detecting mineral bone disorder in pre-dialysis patients (2D).
Parathyroid hormone (PTH)
Serum iPTH values (intact PTH, normal range 10-65pg/ml with obsolete Nichols’ Allegro assay) measured by immunoradiometric or immunochemiluminescent assays are the biochemical parameters that best correlate with the histological HPT2 lesions, especially with osteoblastic activity.
Therefore, PTH levels (compared to calcium and phosphorus levels) are a good marker (at least the best available) for the underlying bone disease, meaning that diagnostic bone biopsy can be avoided in most situations (2B).
It is likely that new HPT2 treatments or PTH measuring techniques modify the PTH levels considered as adequate in the near future.
iPTH levels >450-500pg/ml (or equivalent) indicate high-turnover bone disease, more specifically osteitis fibrosa or mixed bone disease, and practically exclude low-turnover disease with a high specificity (1B).
iPTH levels <100-120pg/ml (or equivalent) are associated with low-turnover bone disease (adynamic or osteomalacia), with a predictive value close to 90% (1B).
No correlation has been made between PTH levels and cardiovascular lesions. Relatively high or low PTH levels have been correlated with greater risk of mortality, especially cardiovascular, although no definitive range has been established. However, low-bone turnover seems to be associated with a higher degree of vascular calcifications (2D).
PTH levels should be measured every 6-12 months for stage 3-4 depending on the baseline value and CKD progression rate. Even when treatment is not to be modified, it is convenient to know the PTH increase speed to take measurements in extremely serious cases. For stage 5 (including 5D), KDIGO recommends that patients are monitored every 3-6 months (NG).
Check-ups may have to be performed more frequently in patients undergoing treatment, especially during dose-titration phases, so as to analyse treatment efficacy and adverse effects, as well as detecting or establishing trends (this is applicable to CKD 3-4). In this respect, evolutionary trends should be taken into account rather than the individual calcium, phosphorus and isolated PTH values, which can be contradictory on occasions. They should also be considered alongside other complex CKD-MBD parameters (vascular calcification, etc.). (1C).
We currently have many problems given that there is no standardisation between the different PTH monitoring standards, with a lack of good correlation coefficients, which hinders correct interpretation of laboratory results. The S.E.N. created a document which attempts to clarify how to interpret these different methods29 and correction formulae of intact PTH at the “equivalent” reference value in accordance with the classic Allegro assay used. Almost all the original information from this PTH was obtained from this document.
Measuring “whole” PTH (Scantibodies) or “bio-PTH” (Nichols Institute), which theoretically only measure 1-84 PTH, as well as calculating the quotient between different PTH fragments,30 is not recommended in clinical practice, although the two former methods are increasingly used (NG).
25-(OH)-Vitamin D
We recommend measuring vitamin D levels (calcidiol) to prevent and treat frequent 25-(OH)-Vitamin D insufficiency and deficiency.
It is traditionally defined, both in the general public and CKD sufferers, as vitamin “insufficiency” when serum calcidiol values are <30ng/l, and as “deficiency” when serum values are <15ng/l. There are no other studies on the general public showing that values above 40ng/ml are beneficial. However, studies do show that values above 200ng/ml are toxic31 (2B).
For CKD patients with low values, a nutritional or supplement intake of native vitamin D is suggested, as is also recommended for the general population1 (2C).
The relative importance of different assays available on the market, or the best time of the year to take these measurements is still unknown.
25-(OH)-Vitamin D is being used more frequently, for the general population and patients undergoing dialysis, due to the potential pleiotropic effects of vitamin D on aspects other than bones and the prevention of falls.32 In experimental trials, co-administration of calcidiol with paracetamol seems to provide the best anti-inflammatory and antifibrotic outcomes.33
However, no study has reported an improvement in dialysis patient survival when native vitamin D is used, although different pleiotropic effects have been described.
Low serum 25-OH-vitamin D values have been associated with greater mortality rates in incident haemodialysis patients and active vitamin D derivatives seems to eliminate this effect.35
One question that has yet to be resolved is why the calcidiol levels are much lower in CKD patients than in the general population. A recent study shows that the liver’s ability to produce 25 (OH) is reduced in uraemic rats, and that parathyroidectomy improves the liver’s ability to produce 25(OH).36
Alkaline phosphatase
Total alkaline phosphatase can be useful alongside PTH, as a predictor of bone turnover. Bone alkaline phosphatase probably has marginal advantages, but they do not justify their additional cost (2C).
It is also currently considered a mortality risk marker in haemodialysis patients.37 The independent association of >120 IU/l alkaline phosphatase levels with coronary calcification has also recently been reported.38
1,25-(OH)-Vitamin D
There is no evidence suggesting that regular serum 1.25-(OH)-vitamin D measurements are useful for monitoring patients with kidney disease, although they can be used for research purposes or for differential diagnosis in some hypercalcaemia cases. When patients have high PTH levels, it is still unknown as to how much 25-(OH)-vitamin D would be considered normal or recommendable.
Calciuria-phosphaturia
Patients may experience reduced urinary calcium or phosphate excretion as kidney disease develops. We recommend measuring serum calcium levels regularly to monitor the potential calcium overload in CKD patients. Furthermore, the excretion fraction of phosphorus can be used an early phosphorus overload marker (NG).
A decrease in GFR is the main determining factor as to whether the excretion fraction of phosphorus has increased, as occurs with many other solutes. The distribution of the same filtered solute load among less functioning units, causes the absorption fraction to be lower, and, therefore, the excretion fraction to be higher.
When the phosphorus load is greater (in relation to the GFR), the excretion fraction will increase, which is mainly regulated by phosphatonins and PTH. But this excretion fraction has a maximum limit (approximately 50%-55% in patients without proximal tubular disorders); therefore, given a certain phosphorus load, a critical GF reduction can not deal with the excretion, and as such a positive phosphorus balance is produced.
Other bone turnover markers
Other bone turnover markers, such as osteocalcin, free serum pyridinoline, and C-terminal telopeptide of collagen, show good correlations with bone histology, but do not improve the PTH predictive power, meaning that its systematic use is not justified. (2C).
Fibroblast Growth Factor 23
This phosphatonin is acquiring more importance as a physiopathology HPT2 mediator and because of its association with CKD patient survival. It is also used as an early marker of phosphorus overload, or a prognostic indicator in treating patients with HPT2. However, measuring FGF-23 is still not recommended in the clinical field (NG).
Imaging techniques. Bone radiology (Tables 4-6)
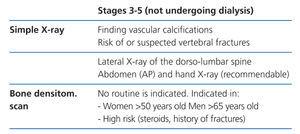
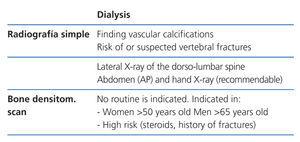
Radiology is useful as a first step into detecting vascular calcifications and amyloidosis associated with beta-2-microglobulin deposits. Simple abdomen and hand X-rays can identify vascular calcifications. A lateral dorso-lumbar spine X-ray is indicated for symptomatic or at risk patients for detecting vertebral fractures (2C).
Bone imaging techniques are, in general, of little diagnostic value, given that the biochemical changes precede the radiological ones. At present, systematic bone imaging in asymptomatic patients is not justified (NG).
Subperiosteal resorption in the radial side of the phalanges is the earliest and most specific sign of osteitis fibrosa. Other classical lesions are acroosteolysis, “salt and pepper” skull, and “rugger-jersey-spine”, supporting osteitis fibrosa diagnosis, and looser lines, owing to osteomalacia. All of which are delayed signs of underlying bone disease.
Direct correlations have been described between the presence of vascular calcifications observed in simple X-rays and cardiovascular risk in haemodialysis patients.39,40 Therefore, vascular calcifications could determine later therapeutic choices. Patients with vascular or valvular calcifications should be considered in the highest cardiovascular risk group (2A).
Lateral abdomen X-rays (dorso-lumbar spine), and echocardiograms, can be used to detect vascular or valvular calcifications as reasonable computerised tomography alternatives. Both have a low, negative predictive power (if they are negative, we are unable to ensure the absence of calcifications) (2C).
Bone densitometry (Tables 4-6)
Dual X-ray (DXA) absorptiometry is currently the standard method for determining bone mineral density (BMD) in the general population. This method is used because it provides detailed images of clinically important parts of the body and ensures minimal radiation exposure. The femoral neck and the spine are usually examined (antero-posterior and lateral projections).
It provides information of the changes in bone mineral content, but not the type of underlying disease, which is especially important in CKD patients.
The relationship between BMD and risk of fractures in the CKD population is inconsistent. However, several studies have shown that measuring BMD in the distal radius can predict risk of fracture41 and it is (negatively) correlated with PTH in haemodialysis patients.42 As a result, the distal radius is the preferred place to measure BMD in CKD patients, avoiding the arteriovenous fistula in use.43 This is in accord with the International Society for Clinical Densitometry 2005 recommendations (www.iscd.org/Visitors/positions) (2B).
There are some concerns that BMD hip or lumbar results could be misinterpreted and lead to an inadequate administration of antiresorptive drugs.
We do not recommend systematically measuring BMD in patients with CKD stage 3-5D. However, it has been found to be extremely useful to monitor post-transplant bone mass or to examine changes in bone mass (2B).
Histology
Iliac crest bone biopsy with double tetracycline labelling and a histomorphometric study is the most precise method for diagnosing an underlying bone lesion and the gold standard for examining the predictive value of other less invasive diagnostic techniques (1A).
Application of histomorphometric criteria has made it possible to standardise ROD classifications. This term is currently only applied to define CKD-associated morphological bone alterations and includes bone turnover parameters, mineralisation rates, and bone volume (bone quantity per unit of total bone tissue volume). Attempts are being made to introduce the TMV classification so as to assess bone histomorphometry (T=Turnover, M=Mineralisation, V=Bone Volume).
Traditionally, bone lesions were classified (Figure 1) as high turnover (HT) or low turnover (LT). HT is most characteristic in patients with osteitis fibrosa (OF) and its only cause is HPT2. LT is subdivided depending on the mineralisation rate. If they occur with normal mineralisation, this is called adynamic bone disease (ABD) and if they occur with abnormal mineralisation (with the consequent increase of osteoid tissue), it is osteomalacia (OM). Morphological alterations could also occur with variable bone mass.
The KDIGO group has classified ROD forms in accordance with turnover type, mineralisation rate, and bone volume:
Indicating bone biopsy
A better understanding of the disease and the predictive value of the biochemical parameters have meant that bone biopsy has become an exceptional indication.
Transiliac bone biopsy indication is currently included but not limited to the following situations: (NG):
1. Unexplainable hypercalcaemia and hypophosphataemia.
2. Pathological fractures without or due to a minimal trauma.
3. Symptomatic patients (e.g. unexplainable bone pain) with unrelated clinical parameters. A characteristic case is the presence of unexplainable hypercalcaemia due to a pharmacological cause or systemic disease, with inconclusive iPTH serum values (between 120 and 450 [100-500]pg/ml as a guideline range).
4. Patients with suspected aluminium-induced bone disease (history of aluminium exposure, with PTH<100-120pg/ml, and eventually, positive deferoxamine test) before deciding upon a chelation treatment with deferoxamine.
5. Pre-parathyroidectomy, if there has been an important exposure to aluminium in the past, or if the biochemical HPT2 parameters are not evident.
6. Before starting treatment with bisphosphonates in patients with a much reduced GFR, especially if the iPTH level is very low.
Other imaging techniques. Assessing extraskeletal calcifications
There are currently no clinical guidelines that have been agreed upon by consensus for assessing and monitoring extra-bone calcifications in CKD patients.
Simple X-ray
It is a simple and inexpensive diagnostic tool for detecting vascular calcifications in adults. Some studies show that, by means of a simple score, hand, pelvis and lateral lumbar spine radiology can help us to calculate the severity of vascular calcifications and it is of prognostic importance.40 Therefore, baseline simple X-ray (antero-posterior X-ray of the abdomen, lateral dorso-lumbar spine, and if possible, hands) should be performed on all patients with CKD so as to assess vascular calcifications (2C).
Other instruments for imaging diagnosis are employed in accordance with their availability in the workplace, the operator’s experience and the type of study design to be performed.
Echocardiogram
It is useful for assessing valvular calcifications, as well as geometry and cardiac function. As has been previously mentioned, it has a low, negative predictive power.
Carotid ultrasound
It identifies calcifications on atheromatous plaques and measures the carotid vessels’ intima-media thickness. It can also reveal atheromatous plaque calcification or even the presence of calcifications involving only the internal elastic lamina.
Carotid-femoral pulse wave velocity (PWV)
It is used to measure arterial stiffness (or loss of distensibility). It is an easy-to-perform non-invasive method with high reproducibility, which is harmless to the patient. There is a correlation between pulse wave velocity and level of vascular calcification.
Angiotomography techniques
This method is less invasive than arteriography, and provides good quality images to assess the morphology of the vascular tree.
Helicoidal CT scan or multidetector CT
It is useful for assessing coronary calcifications.
Electron-beam computed tomography (EBCT)
This is the best validated technique for detecting coronary calcifications, but it is extraordinarily expensive.
RECOMMENDED BIOCHEMICAL VALUES IN ACCORDANCE WITH DIFFERENT STAGES
The recommended serum values, according to KDOQI, KDIGO and the literature review, are shown in Table 7.
There is insufficient evidence, especially for pre-dialysis patients (2D) to determine biochemical values, especially for PTH, whose specificity is particularly low for intermediate PTH values.
Some authors recommend standardising PTH values, others suggest starting treatment when PTH increases with regard to a baseline value if there is one available (even within the normal range) and others consider that a certain degree of hyperparathyroidism is necessary to maintain a normal bone turnover rate, in cases with evident renal failure.
In either case, recommendations on biochemical parameters are based on observational studies that, actually, can only discuss associations.
As a result, we recommend maintaining calcium (2D) and phosphorus (2C) levels within the normal laboratory range as long as the measures to achieve them are reasonable.
Furthermore, therapeutic objectives for mineral and bone disorders should be adapted to the clinical characteristics and overall therapeutic objectives of each patient, i.e. not trying to reach certain figures or plasma concentrations.
Furthermore, mineral and bone disorder treatments may sometimes cause adverse effects, which in some cases are serious, and are more common the more aggressive the treatment is.
THERAPEUTIC OPTIONS
Stage 1-2
Both the KDOQI and KDIGO guidelines are yet to recommend treatment for CKD stages 1-2. However, if we wish to be consistent with data obtained from several studies, we would be able to at least start up prevention mechanisms, if not treatment.
Diet
It is understood that patients with slight deterioration in renal function already have phosphorus retention, with undetectable decreases in total calcium and increases in PTH if subjected to a phosphorus overload. This means that patients should start reducing the amount of phosphorus in the diet.
On the other hand, patients are more receptive to diet changes when kidney disease is first diagnosed than after a long period of time. Patients should start by consuming 1g of protein/ideal body weight/day. This would benefit the patient in two ways: on one hand, it would reduce the phosphorus intake, and on the other, reduce the harmful effects of glomerular hyperfiltration44,49 (2D).
25(OH)D3 (calcidiol)
Adequate levels of 25(OH)D3 are especially important, given that it is the substrate that produces 1-25(OH)2 D. Furthermore, a deficit in 25(OH)D3 worsens HPT2. In these stages, a deficit in 25(OH)D3 may be the only cause of HPT2, and as such, it seems that it should be measured (2C). We also suggest 25(OH)D3 supplementation (2C), to ensure the pleiotropic effects of vitamin D beyond the HPT2 control. It is well known that VDR is present and is expressed in many tissues other than the most commonly recognised ones.
We also suggest measuring calcidiol levels in patients with CKD stage 3 onwards, including dialysis patients (2C). Repeating measurements will depend on the baseline values and therapeutic interventions decided upon.
Vitamin D deficiency or insufficiency should be addressed following the strategies recommended for the general population (2C).
There are no well-defined dosages (clinical trials in the general population have generally used a dose between 300-800IU/day) considering a maximum of 2000IU/day (approximately 60 000IU/month),50,51 although a recent review concluded that the maximum dose for adults (without CKD) should be up to 10 000IU/day.52 The US Institute of Medicine committee which revises the dietary calcium and vitamin D references, considers that a safe daily dose is up to 4000IU/day.31
In Spain, there is no pharmacopoeia available for vitamin D2 (ergocalciferol) except in multivitamin preparations. Meanwhile, we have vitamin D3 available in liquid format (bottles of 10ml=20 000IU/bottle=2000IU/ml=30 drops) and various preparations containing 200-800IU of vitamin D3 + different quantities of calcium. We recommend being cautious with these combinations, especially for patients with vascular calcifications or at risk of developing them.
Another, more convenient indication is calcifediol (or calcidiol) (ampoules of 226µg=16 000IU). Fortnightly or monthly administration of calcifediol, monitoring calcidiol levels, is a convenient alternative for adjusting the nutritional intake in CKD patients. It should however also be accompanied by occasional calcium and phosphorus plasma controls.
Calcium
Patients must have a sufficient intake of calcium to reduce PTH stimulation. Between 15mg/kg/day and 20mg/kg/day in the diet would be sufficient for ensuring that calcium needs are covered. The daily recommended quantity in the general population varies depending on age and sex between 1000mg/day and 1300mg/day, considering the maximum tolerable limit as 2000mg/day (2B). It is unknown as to whether these quantities should only be limited to patients with vascular calcifications.
Bisphosphonates
There are currently very few data on this type of patient, which can be considered paradoxical if we were to consider that CKD patients have a greater risk of non-vertebral fractures than the general population.
Various studies performed on the general public have included patients with deteriorated renal function, and have observed an improvement in BMD and in reducing the risk of fractures, independently of kidney function. Furthermore, the few studies published on dialysis patients have also observed an improvement in BMD, especially when patients had elevated PTH.53
It is unknown as to whether the accumulation of bisphosphonates increases as renal function deteriorates. In these patients the accumulation could cause a decrease in bone turnover, making it difficult to repair microfractures and bone quality deterioration. However, this has not been proven, given that there are few data on bone biopsy, and those available are contradictory.
The WHO recommends that patients with CKD stages 1-2, osteoporosis, and/or at high risk of fractures, use the same treatment as the normal population, as is also suggested for stage 3 patients with normal PTH, osteoporosis, and/or at high risk of fractures (1A). Additional tests (biopsy) are suggested for patients with CKD stages 4-5D who have significantly reduced BMD and/or stress fractures, before using antiresorptive agents (NG).
We could summarise by stating that patients with advanced CKD or undergoing dialysis must only be indicated bisphosphonates when they have osteoporosis and evident high bone turnover (meaning that a previous bone biopsy is probably recommendable) (NG).
Caution must be taken, including mandatory measures such as a bone biopsy, when considering indicating bisphosphonates for patients suspected to have ABD.
When intravenous bisphosphonate administration is indicated it is important to maintain or even extend infusion time, so as to avoid secondary effects, even though very few negative effects have been reported for the kidney (NG). Oral bisphosphonate administration does not seem to alter renal function54 (2B).
Mandibular osteonecrosis has recently been related with bisphosphonate treatment, although the incidence is very low. This lesion is defined as the presence of one or several ulcerated lesions with bone exposure in the upper maxilla and/or mandible having evolved over more than 8 weeks. It is very rare for it to develop with oral bisphosphonates. The risk factors include administering intravenous bisphosphonates during a long period of time, neoplasias, chemotherapy, radiotherapy, high-dose steroids, alcohol and/or tobacco abuse and, above all, local factors such as periodontal disease, dental extraction and maxillofacial surgery.
Although no osteonecrosis has been reported to date in CKD patients, it is still important to consider preventing it.
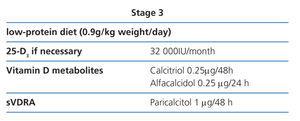
Stage 3 (Table 8)
A clear increase in the PTH levels is observed during this period which starts to depict an exponential growth.
Recommendations therefore change from the previous stages.
Diet
Protein restriction is slightly more severe (0.9g/kg weight/day), so as to avoid phosphorus intake and hyperfiltration. The patient will easily tolerate the change if their diet had already been modified in previous stages (2D).
Some authors only limit products with a phosphorus content that is out of proportion with the protein content (lactose abuse, sodas, processed products) as many patients spontaneously lose their appetite for proteins as kidney disease progresses.
25(OH)D3 (calcidiol)
We recommend that 25(OH)D3 values are still monitored to ensure that they are at normal levels (2C).
Phosphate binders
With this degree of renal function and diet, it is not difficult to maintain a normal phosphataemia. If an irregular phosphataemia is found, the patient could start taking calcium-rich phosphate binders at meals, although this indication should be used with caution for patients with vascular calcifications.
Calcium acetate’s binding power is similar to that of calcium carbonate, but with less calcium overload, meaning that it would have certain advantages, as well as a greater effect at different pH ranges (2C). Calcium carbonate is, on the other hand, the least expensive binder.
At present, lanthanum carbonate and sevelamer carbonate are already indicated in pre-dialysis patients (although the summary of product characteristics specifies phosphate values of >1.78mmol/l [5.5mg/dl]).
Active vitamin D metabolites and analogues
As mentioned above, if PTH increases progressively above normal values despite correcting modifiable factors (hyperphosphataemia, hypocalcaemia, vitamin D deficit), we suggest indicating active vitamin D metabolites or selective vitamin D receptor activators (sVDRA) (2C).
Low doses of active metabolites do not seem to cause hypercalcaemia or hyperphosphataemia and have shown, in experimental trials, that they could also reduce CKD progress.54
Meanwhile, calcitriol controls its metabolism, and prevents vitamin D receptors (VDR) from being lost, meaning that the parathyroid gland continues to respond to calcitriol. The density of the megalin receptor located in the renal tubular cells is also maintained.
Furthermore, the number of Ca receptors (CaR) in the parathyroid gland is also maintained.
Both the use of calcitriol and alfacalcidol have been associated with a better survival for pre-dialysis patients in observational studies.55-57
We recommend an initial calcitriol dose of 0.25µg every 48 hours and alfacalcidol 0.25µg every 24 hours.58 These doses should be modified with regular biochemical tests.
Selective vitamin D receptor activators (sVDRA)
Only paricalcitol has been marketed in Spain. It is based on 19-nor-vitamin D2 which has different affinities with VDR, presenting a higher affinity in parathyroid cells than in osteoblasts, intestinal wall cells and vascular smooth muscle cells. It therefore seems to cause less hypercalcaemia and hyperphosphataemia than calcitriol, as has been shown in different published studies.
It use has not only been associated with HPT2 control, but also with other pleiotropic effects of vitamin D, such as a decrease in proteinuria in 24-hour urine in diabetic patients, which is likely to be related to its antirenin effect. Results from these studies are still preliminary.
On the other hand, it seems to exert, by unclear mechanisms, a positive effect on dialysis patient survival.59,60
In experimental studies, paricalcitol causes fewer vascular calcifications compared with equipotent doses of calcitriol.61 We recommend a paricalcitol dosage of 1µg every 24/48 hours or 2µg three times a week at this CKD stage. These doses should be modified with regular biochemical tests.
Bisphosphonates
If appropriate, for patients using bisphosphonates and normal renal function, who have a GFR less than 30ml/min/1.72m2, the dose should be reduced to half the recommended amount (NG).53
Stage 4 (Table 9)
In this phase PTH value increase is much more severe and rapid, meaning that a stricter diet and treatment should be indicated.
Diet
Protein restriction will be slightly more severe (0.8g/kg weight/day). Protein intake greater than 0.8mg/kg/day assures an adequate nutrition (2D).
25(OH)D3 (calcidiol)
We recommend that 25(OH)D3 values continue to be monitored to ensure that they are normal (2C).
Phosphate binders
At this stage, it starts to become difficult to maintain normal phosphataemia despite diet modifications. If this were not the case, a greater dose of phosphate binders can be used at meals. If they are calcium-rich binders, 1500mg/day would be the maximum amount (NG).
If this were not sufficient, aluminium hydroxide could be used during a short period of time, only administering it in foods whose phosphorus content justifies it (BC).
The safe quantity of aluminium binders is not known. Furthermore, several conditions could promote their intestinal absorption, such as diabetes mellitus, HPT2, state of vitamin D, citrate intake, and state of iron deposits.
Serum aluminium should be measured twice a year in those patients who receive aluminium phosphate binders (BC). Baseline serum aluminium values of <20µg/l show a likely absence of aluminium overload. Repeated values between 20µg/l and 60µg/l are difficult to interpret. Repeated values >60µg/l show an aluminium overload (this does not always imply aluminium-induced bone disease).
The risk of tissue incorporation of aluminium is greater in patients with iron deficiency; therefore, much lower values than those already mentioned can be pathological.
At present, lanthanum carbonate and sevelamer carbonate are also indicated.
Active vitamin D metabolites and analogues
In this stage, depending on the PTH levels, we recommend that the initial calcitriol dosage is 0.25-0.50µg every 24-48 hours and alfacalcidol 0.25µg every 24 hours (2D). These doses should be modified with regular biochemical tests.
sVDRA
The lower effect of sVDRA on phosphorus absorption, could recommended sVDRA for this stage (NG).
The initial recommended paricalcitol dose for this stage is 1µg every 24 hours (or 2µg every 48 hours) (2D). These doses should be modified with regular biochemical tests.
Stage 5 (Table 9)
HPT2 control is most difficult at this stage. Renal function is severely deteriorated and even its excretion and endocrine functions are deficient. The biochemical variability is important and the situation can change in very little time, meaning that it is more difficult to standardise treatment.
If the patient has been monitored since the early stages, then the regimen used for the previous stage is generally sufficient.
Diet
Protein restriction will be slightly more severe (0.8g/kg weight/day), taking care to ensure that adequate nutrition is not compromised (2D).
25(OH)D3 (calcidiol)
We recommend that 25(OH)D3 levels are still monitored to ensure that they are normal (2C).
Phosphate binders
It is very difficult to maintain phosphataemia at a normal level without administering phosphate binders at meals. The regimen is the same as the previous stage, usually with a higher dosage (NG).
Active vitamin D metabolites and analogues
At this stage, the recommended doses are the same as in the earlier stage, although they can be modified in accordance with the calcaemia, phosphataemia and PTH serum values (2D).
sVDRA
The initial recommended paricalcitol dose for this stage, as a guideline, is 1µg every 24 hours if the PTH is less than 500pg/ml and 2µg every 24 hours if the iPTH is more than 500pg/ml, which can also be modified in accordance with calcaemia, phosphataemia and PTH serum values (2D).
Stage 5D (dialysis) Phosphorus control
The increase in serum phosphorus is one of the main problems found in CKD patients undergoing dialysis. Preventing hyperphosphataemia has two objectives: firstly, to achieve an adequate control of bone mineral metabolism, mainly to prevent HPT2 developing and its associated complications; and secondly, to reduce cardiovascular risk and high morbidity and mortality in these patients. Hyperphosphataemia has independently been associated with mortality, and it has been shown by retrospectively analysing several extensive databases.
Therefore, the main objective in this stage is to maintain a normal level of phosphorus. In this instance, treating hyperphosphataemia has three main objectives:
1. Limiting the intake of high phosphorus content foods without compromising the basic protein intake.
2. Modifying the dialysis characteristics and regimen to optimise elimination of this solute.
3. Administering phosphate binders. In most cases a combination of these three therapeutic options is required.
Diet
Haemodialysis patients require more protein than is recommended for the general population, given the catabolic nature of the technique and the disease. Logically, it is also greater than is recommended for uraemic pre-dialysis patients.
Firstly, an adequate calorie, protein and mineral intake must be guaranteed. A presumably adequate diet must never result in insufficient nutrition. Common sense sets the standards for a balanced diet: patients should eat at least four well-balanced meals, including organic compounds (carbohydrates, fats and proteins).
The optimum protein intake must be 1-1.2g/kg/day (of which 50% must be of high biological value, i.e. animal proteins) and have a caloric value of 30-35kcal/kg weight (35 for <65 years and 30 for >65 years). The recommendation is even greater for patients undergoing peritoneal dialysis (1.2-1.3g/kg weight/day) (2D).
This greater freedom with regards protein intake could have an adverse effect on phosphorus entry: i.e. a greater dose of intestinal phosphate binders are needed to ensure the minimum protein requirements.
Dialysis
The ideal dialysis duration is a very controversial subject. At present, dialysis should be personalised in accordance with each patient’s requirements. Although there is no clear evidence showing that dialysis duration has an effect on phosphorus control,62-70 at present, dialysis is recommended to last a minimum of 4 hours, three times a week, except for patients with an elevated residual renal function.
In general, increasing dialysis duration and frequency improves solute elimination (2B).
The dialysis session duration could be a determining factor in eliminating small solutes that are mainly located in the intracellular space, as is the case for phosphorus.
There are no prospective, controlled, randomised studies confirming that an increase in dialysis duration has an effect on controlling hyperphosphataemia.
However, most of the studies published report that increasing haemodialysis duration is beneficial to phosphorus elimination.
Increasing the frequency of haemodialysis sessions is another alternative. There are no adequate studies that assess the effect of increasing the frequency on phosphorus clearance. Most of these studies are observational and with a small sample of patients that are monitored over a short period.
To achieve a significant reduction in serum phosphorus levels, the session needs to last more than 2 hours. At present, there is a tendency to increase haemodialysis duration to 2.5-3.0 hours 5-6 times a week. In patients with elevated phosphorus and haemodialysis every other day, sessions should last a minimum of 4 hours.
As mentioned above, increasing both time and frequency, could be effective at treating refractory hyperphosphataemia. With daily long night dialysis (5-6 sessions of 6-10 hours) hyperphosphataemia is significantly decreased and the dose of phosphate binders in reduced, even with increased daily phosphorus intake.
Techniques using high convective transport
Its use should be considered as a therapeutic alternative of hyperphosphataemia.
High-flux membranes have a higher phosphorus elimination capacity than low-flux membranes. Meanwhile, several randomised studies have confirmed that haemodiafiltration (diffusion and convention) with high convective transport increases clearance in a wide spectrum of solutes, specifically phosphorus, when compared with haemodialysis with low-and high-flux membranes. However, at present, there is no clear evidence on the potential advantages of high-flux membranes or haemodiafiltration.
Phosphate binders
As it has already been mentioned in the section above, most haemodialysis patients have a positive phosphorus balance, meaning that they require additional treatment with intestinal phosphate binders to prevent hyperphosphataemia.
Potential limitations to calcium binders and aluminium hydroxide are the same as for earlier stages. There is extensive experience with both sevelamer carbonate and lanthanum carbonate.71,72
Sevelamer is a phosphate binder that does not contain calcium or aluminium. It is a polymer that binds phosphate in the intestine and prevents its absorption. Several prospective studies show that it is capable of slowing coronary and aortic calcification progression, and also reduces lipid levels, among other multiple pleiotropic effects shown in experiments. However, a study performed on a population undergoing haemodialysis with greater cardiovascular risk did not show that sevelamer was better at reducing vascular calcification progress in comparison with calcium acetate, if trying to maintain LDL levels <70mg/dl with statins. It is not clear as to why sevelamer did not reduce calcification progression in this study.73,74
A disadvantage related to sevelamer is that many patients have to take high numbers of tablets, which could be poorly tolerated. At present, sevelamer carbonate is available in powder for oral suspension. The concentration of sevelamer carbonate in sachets is triple that of tablets, this will allow to reduce the number of tablets to take.
A study shows an improvement in survival of sevelamer-treated incident patients on dialysis, compared with calcium binders.75 However, another prospective study limits survival improvement to certain sub-populations (above 65 years old).76
Lanthanum carbonate is a strong aluminium- and calcium-free phosphate binder. It can improve serum phosphorus control without any significant secondary effects, as has been shown in several studies.77,78 Preliminary observational studies also seem to show that lanthanum carbonate has beneficial effects on reducing the vascular calcification progression and survival, as compared with calcium binders.79
The calcium acetate and magnesium carbonate combination has recently been marketed in Spain for dialysis patients. The results seem promising and they have not been associated with possible hypermagnesaemia-associated problems, and also reduce PTH levels.80 Potential anti-calcifying effects of magnesium have also been described, both in experimental trials and preliminary clinical studies.81 We recommend monitoring serum magnesium and ECG in digitalis-treated patients.
To date, there are no other studies that convincingly demonstrate which binder should be of first choice. Hyperphosphataemia is often treated with a combination of several of these binders, although there is no evidence showing that combined treatments are more effective.
As we have mentioned previously, the CKD stage, presence of other CKD-MBD components (i.e. vascular calcification), concomitant therapies and secondary effects profile should be taken into consideration when selecting a binder for pre-dialysis patients (NG). Furthermore, restricting calcium-based binders in presence of persistent or recurrent hypercalcaemia is recommended, (1B) and suggested in presence of arterial calcification and/or ABD and/or if serum PTH levels are persistently low (2C).
Calcium control
Calcium should be maintained within a normal range, recommending that these values remain between 8.4 and 9.5mg/dl in dialysis patients (2D). Before the advent of calcimimetics, an increase in mortality had been reported, with high calcium levels in extensive retrospective database studies, although these results have not always been confirmed. High calcium levels should especially be avoided with low PTH, as well as high calcium and phosphorus levels. These combinations have been related to increases in mortality in patients on dialysis or with vascular calcifications.
Diet
The proportional increase in calcium with the increase in protein recommendations would vary depending on the amount of dairy products consumed. As a guideline, a diet of 1-1.2g/kg/day of proteins contains between 550mg and 950mg of calcium (NG).
The total daily calcium intake should not exceed 2g, including calcium in the diet and that included in phosphate binders or ion-exchange resins (e.g. calcium-resin).
Dialysis
Calcium concentration adjustments in the dialysate could contribute to optimising the calcium balance in these patients.
There is no consensus on the recommended calcium content in the dialysate. Values of 1.25mM (2.5mEq/l; 5mg/dl) have been associated with a negative calcium balance and the tendency for PTH to increase. Furthermore, with 1.25mM there is worse haemodynamic tolerance to ultrafiltration, which is strengthened if the magnesium content is not adequate. Higher levels, 1.75mM (3.5mEq/l; 7mg/dl) slow down PTH secretion, but cause a positive calcium balance.
Where possible, calcium content in the dialysate should be personalised in accordance with each patient’s characteristics. A persistent increase of calcium in the dialysate does not seem a recommendable response to calcimimetic-induced hypocalcaemia. On the other hand, for patients with low PTH levels, dialysate with 1.25mM/l of Ca (even 1mM/l) can be indicated to stimulate PTH secretion and prevent ABD. However, low calcium concentration in the solution could predispose patients to arrhythmias and haemodynamic instability.
The recommendable concentration for normocalcaemia and controlled PTH would be 1.5mmol/l (3mEq/l; 6mg/dl) (NG), although the KDIGO guidelines suggest using dialysate between 1.25mmol/l and 1.50mmol/l (2.5-3mEq/l) (2D). The calcium balance according to dialysate in patients with hypocalcaemia secondary to calcimimetics is still unknown. All of these considerations are applicable to peritoneal dialysis.
PTH control
We suggest maintaining iPTH between 150 and 300pg/ml corrected for the assay used. (2C). Figures out of this PTH range have been related to an increase in morbidity and mortality in haemodialysis patients, advising that values lower than 100pg/ml and above 500pg/ml should especially be avoided, as has already been mentioned in previous guides (2C). These last values coincide approximately with values considered as “values at the extremes” as expressed in KDIGO guides (values <2 and >9 times the upper normal limit of the iPTH assay).
The work group believes that establishing the range <2 and >9 times the upper limit of the assay as single quality control margin (KDIGO guidelines), is likely to be inappropriate. That would inevitably cause a significant number of patients to be outside of these ranges due to the Gaussian distribution in populations. Establishing a stricter therapeutic objective, such as the one suggested, would ensure that more patients would be within the security margin (BC).
To maintain this PTH range, controlling the serum calcium, phosphorus and possibly calcidiol levels must be a priority (NG). If once this objective is achieved, high and growing PTH values persist, we suggest using active vitamin D metabolites, calcimimetics or both to reduce PTH (NG).
Here, we also suggest basing initial therapy on calcium and phosphorus levels, as well as on other CKD-MBD aspects (e.g. vascular calcification) and adjusting the phosphate binder dosage so that PTH-controlling treatments do not compromise calcium and phosphorus levels (NG).
25(OH)D3 (calcidiol)
There is evidence that adequate levels of 25(OH)D3 must also be maintained in this stage. This could be useful for controlling HPT2, but especially other pleiotropic effects of vitamin D (2D).
Active vitamin D metabolites and analogues
Treatment with active metabolites (calcitriol or alfacalcidol) reduces PTH values, but an inadequate use could cause an increase in phosphorus, calcium, and calcium x phosphate product. In retrospective studies, all active metabolites have been associated to increases in dialysis patient survival.
Active vitamin D metabolite treatment should be reduced or suspended if high calcium and/or phosphorus values are observed or if the equivalent PTH is less than 100pg/ml (or approximately <2 times the upper limit of the assay used (1B).
sVDRA
Given that sVDRA has a lesser effect on calcium and phosphorus absorption, it could be recommended for this stage.
Retrospective studies show that paricalcitol has survival advantages in dialysis patients when compared to calcitriol.
As with vitamin D metabolites, sVDRA treatment should be reduced or suspended if high calcium and/or phosphorus figures are observed or if the equivalent PTH is less than 100pg/ml (or approximately <2 times the upper limit of the assay used) (1B).
Furthermore, some authors believe that a minimum weekly dosage of 1-5µg of paricalcitol should be maintained to ensure that the vitamin D receptors are activated (NG).
Both active metabolites and sVDRA can be administered orally or intravenously, at a dose which depends on serum PTH levels, provided that calcium and phosphorus levels are controlled (e.g. <9.5mg/dl and <5mg/dl, respectively) (NG).
According to our experience, the initial paricalcitol dosages calculated according to the iPTH value should be less than those recommended in the summary of product characteristics (iPTH/80). We suggest lower doses (maximum 1µg for iPTH/120) for an adequate HPT2 control and when combined with calcimimetics (BC).
Calcimimetics
Cinacalcet is a calcimimetic agent that binds to the calcium receptor of the parathyroid gland and allosterically modifies it so that it becomes more sensitive to extracellular calcium actions.
Cinacalcet significantly reduces PTH levels, and as a consequence, reduces the serum calcium values, and eventually, the phosphorus values too.82-88 Different pleiotropic effects have also been reported, among which, its positive effect on vascular calcification is highlighted.89
Retrospective clinical studies have shown that its use significantly reduces the amount of parathyroidectomies, fractures and vascular-related hospital admissions. The possible reduction in the parathyroid gland size and vascularisation has recently been described.
Reduction in PTH with cinacalcet is rapid and transitory, with a maximum effect after 4 hours, followed by a slow increase towards previous levels, without reaching baseline levels. After weeks or months, PTH levels are stabilised, although daily fluctuations persist.
Gastrointestinal intolerance is among the secondary effects, which in some cases has lead to drug suspension and hypocalcaemia. Gastrointestinal intolerance improves when the drug is administered after eating or at divided doses.
No interaction between cinacalcet and proton-pump inhibitors or phosphate binders has been shown.
Cinacalcet is metabolised via cytochrome P450, meaning that drugs influencing this enzyme complex could increase cinacalcet levels (ketoconazole, itraconazole, cimetidine, clarithromycin, ritonavir, grapefruit juice) or reduce these levels (barbiturates, phenytoin, carbamazepine, dexamethasone, rifampicin).
Adjusting CYP2D6-metabolised drugs (with narrow therapeutic ranges) should also be assessed (flecainide, quinidine, tricyclic antidepressants, vinblastine, thioridazine, propafenone, metoprolol, etc.) Caution must be taken with patients with a history of epilepsy.
Regular calcium controls should be taken at the start of treatment in patients receiving cinacalcet, adjusting phosphate binders and combining treatment with metabolites or sVDRA if necessary.
Recommendations
1. Cinacalcet treatment should not be started for patients with serum calcium concentrations (albumin-corrected) below the lower normal limit (<8.4mg/dl). Treatment can be maintained below this value if the clinical situation and safety allow so (2D).
2. For patients undergoing dialysis, cinacalcet can be used if the iPTH is less than 300pg/ml, provided that the Ca x P product is high (NG).
3. The initial recommended dose for adults is 30mg/day, which should be adjusted every 2 to 4 weeks, but not exceeding the maximum dose of 180mg/day. If lower doses are needed, drug administration every 48 hours has been used, although no data is documented for this matter. Other intermediate doses are achieved with administration of different doses (e.g. 60-30mg every other day would correspond to a dose of 45mg/day) (NG).
4. PTH levels must be assessed at least 12 hours after drug intake.
5. Serum calcium should be measured weekly with each dose adjustment. If hypocalcaemia were to develop (i.e. lower than 7.5mg/dl), we suggest combining or increasing vitamin D doses (metabolites or sVDRA) reducing cinacalcet dosage or interrupting administration (2D).
Combining cinacalcet and vitamin D metabolites or sVDRA
It is possible that the combination of vitamin D metabolites or sVDRA and cinacalcet could be additive and/or synergistic on the HPT2 control or provide other beneficial effects (e.g. on vascular calcification). Use of calcimimetics has been associated with a decrease in the need for vitamin D metabolites or sVDRA and vice versa.
Figures 3 and 4 show a guideline control algorithm of the biochemical bone mineral metabolism alterations for stage 5D patients, depending on iPTH or serum phosphorus.
Stage 5T (kidney transplant)
Persistence of hyperparathyroidism is frequent following kidney transplant, most recipients suffer low- or high-turnover bone disease and immunosuppressive drugs, mainly steroids, have harmful effects on bones. Furthermore, we must consider persistence to different grades of renal failure, occasionally acidosis, and the negative effect on the calcium balance from using loop diuretics. All of this mainly leads to the following problems:
1. Persistent secondary hyperparathyroidism
2. Bone mass loss and fractures.
Secondary hyperparathyroidism
Between 15% and 50% of patients develop persistent secondary HPT during the first month after transplantation. Patients who have the most elevated serum PTH and calcium values at the time of the transplant will have greater HPT persistence in the long term.90
At present, since cinacalcet has been introduced, the percentage of patients on dialysis which access transplant with controlled PTH has increased notably. This has led to a new problem, deciding whether cinacalcet should be suspended or not at the time of transplant.
HPT persistence is a risk factor of:
1. Bone mass loss and an increase in fracture risk.
2. Hypercalcaemia. Its incidence would vary greatly depending on the value considered and the post-transplant period considered. Among other negative cardiovascular effects, it has also been considered as one of the multiple factors responsible for medium-term graft failure.91
3. Hypophosphataemia. It is also likely to be secondary to persistently elevated FGF-23 values.92
4. Renal function deterioration and tubulo-interstitial calcifications.93
To control these alterations, the most cautious option would be to wait for it to evolve, maintaining a close monitoring of the serum calcium, phosphorus and PTH values.
There are two alternative treatments:
1. Parathyroidectomy. Together with calcaemia control it would also improve BMD, although short-term renal function deterioration has also been described following parathyroidectomy.94 Since the use of cinacalcet has been extended, we believe that parathyroidectomy could be reserved for patients that do not respond to cinacalcet treatment (BC).
2. Calcimimetics (cinacalcet). They have been proven effective in normalising hypercalcaemia and hypophosphataemia secondary to persistent hyperparathyroidism. No negative effects on renal function or interactions with immunosuppressive drugs (anti-calcineurin agents, MMF or mTOR inhibitors) have been reported.95-99 Furthermore, they could have a beneficial effect on BMD,100 although more studies need to be conducted to confirm this fact.
In patients with calcaemia above 10.5mg/dl and iPTH>100pg/ml it would be recommendable to start treatment with 30mg/day, and modify doses in accordance with the response (BC).
Bone mass loss and fractures.
Different prospective studies have shown that a rapid loss of BMD (between 7%-10%) occurs during the first 6 months following kidney transplant, which mainly affects the trabecular bone. This loss is 1.5% per month in the lumbar spine. Significant bone loss is also described in the proximal femur. This BMD reduction stabilises and is usually recovered after 12 months.101-104
These findings highlight the importance of starting prophylactic measures from the time of transplantation.
There are discrepancies in the studies with regards long-term bone loss. Some show a bone loss rate of 1%-2% per year in the lumbar spine, while others do not show any changes and even report a slight increase in BMD. These discrepancies could be due to the use of different corticoid maintenance doses.
Rapid bone loss which occurs after transplant determines an elevated prevalence (7%-20%) and incidence (3%-4% per year) of fractures.
Fractures often occur in the late post-transplant period, and although bone loss is mainly in the trabecular bone, most affect the appendicular skeleton, particularly the feet and ankles.105
Given that vertebral fractures are a strong risk factor for developing future fractures, it is important that they are detected as it lends the opportunity to intervene in secondary prevention. An imaging test can therefore be used to detect asymptomatic vertebral deformities in transplant patients with a greater risk of fractures.
A subject that is currently under debate is whether BMD measured by DEXA has the same predictive value of fractures in kidney transplant patients as in the general population.106 A recent, prospective study in transplant patients revealed that the existence of osteopaenia or osteoporosis in the hip increased the subsequent risk of fractures 2.7 and 3.5 times, respectively.107
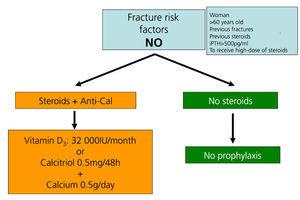
Recommendations for preventing bone mass loss and post-kidney transplant fractures (Figures 5 to 7)
Preventing bone loss and fractures with the transplantation.
Immunosuppressive drugs
The first measure is to reduce corticosteroid dose and suspend immunosuppressive treatment as soon as it is safe to do so.
Vitamin D and calcium supplements
Administering oral calcium supplements (0.5g/day) and vitamin D (vitamin D: 400-800IU/day or calcitriol 0.5µg/48 hours) prevents bone mass loss during the first few months after transplantation108-111 (2B).
We recommend measuring 25(OH)D3 levels regularly (every 6 or 12 months) after transplantation. They should be normalised by administering vitamin D or 25(OH)D3 (cholecalciferol 32 000IU/month) (2C).
Bisphosphonates
Bisphosphonates, in combination with calcium and vitamin D supplements, have also proven to be effective in preventing post-transplant bone loss, and in treating osteoporosis.112-121 All bisphosphonates have been reported as being equally effective i.e. pamidronate IV, ibandronate (monthly oral or IV), risedronate (oral weekly) or alendronate (weekly) (2B).
Universal use of bisphosphonates during the post-transplant period should not be recommended.
We recommend performing a bone densitometry scan and a lateral thoracic and lumbar spine X-ray during the post-transplant hospital stay in recipients at high risk of fractures (Figure 5). Instead of X-ray, DEXA-based vertebral morphometry test could be useful. We will assess initial treatment with bisphosphonates in accordance with BMD and PTH situation.
We recommend repeating examination after a year in patients at risk (2C). If the bone densitometry has worsened after a year, or new fractures have appeared, standard treatment should be bisphosphonates (alendronate, risedronate or ibandronate orally) (2C).
DEXA can later be performed every two years.
In principle, co-administration with calcimimetics and bisphosphonates is still not recommended given the lack of evidence.
Parathyroid hormone
The synthetic parathyroid hormone (teriparatide or PTH 1-84) have not been proven effective in preventing rapid bone loss which occurs during the first few months after transplantation.122,123
If ABD is suspected in patients with low or normal PTH levels, who are >60 years old or diabetic, synthetic PTH treatment for a year should be considered (teriparatide: 20µg/day s.c. or PTH 1-84: 100µg/day s.c.). Synthetic PTH is not indicated when HPT2 is present (NG).
In more complex cases, previous bone biopsy following tetracycline labelling could be used with the aim of managing treatment in accordance with the state of turnover and bone mineralisation.
PARATHYROIDECTOMY
Parathyroidectomy should be considered if all of the measures above are ineffective in controlling PTH. Currently, with the introduction of new drug treatment alternatives, indications could be reduced to (2B):
1. HPT2 with non-iatrogenic hypercalcaemia (tertiary hyperparathyroidism) resistant to drug alternatives (mainly calcimimetics).
2. Primary hyperparathyroidism in CKD patients (especially young patients).
3. Patients with calciphylaxis and iPTH above 500pg/ml, which do not respond rapidly to calcimimetic treatment. It could also be recommended for those who have associated complications such as tendon rupture, severe bone pain or refractory anaemia.
We recommend performing imagining tests before the parathyroidectomy to examine the gland size, location and especially to rule out the presence of ectopic glands.124 The association of MIBI scan and cervical ultrasound shows great sensibility and specificity (BC).
There is much controversy on which removal technique is the most adequate: total or subtotal parathyroidectomy or total with autologous transplant. The most used at present is subtotal parathyroidectomy, because it has the lowest post-parathyroidectomy recurrence rate, although this depends greatly on the surgical team’s experience and ability. A gland that appears on the bone scan should never be left as residual tissue (in subtotal parathyroidectomy or to be used in autologous transplantation).
CALCIPHYLAXIS (CALCIFIC URAEMIC ARTERIOLOPATHY)
Calciphylaxis is a severe rare vascular alteration, with a high rate of morbidity and mortality, which is characterised by a progressive vascular calcification with ischaemic necrosis of soft tissues and skin. It almost exclusivey affects patients with chronic renal failure. Its diagnosis is essentially clinical.
Calciphylaxis can often be found in patients undergoing haemodialysis or kidney transplant recipients, and it is extremely rare in patients undergoing peritoneal dialysis or at pre-dialysis stages.125-127
The most important risk factors for calciphylaxis development are:
1. Elevated serum calcium and phosphorus concentration.
2. HPT2.
3. Hypoalbuminaemia.
4. Obesity.
5. High doses of active vitamin D metabolites.
6. Warfarin.
The most sensitive X-ray procedure for detecting calciphylaxis is a mammogram.
Bone scans have proven to be a very sensitive diagnostic technique, showing a subcutaneous increased uptake of (technetium-99) isotope, characteristic of calciphylaxis corresponding to calcified plaques.
If it is possible, cutaneous lesion biopsy should be avoided, given that it may cause it to ulcerate and subsequently become infected, with a further risk to septicaemia. Furthermore, the histopathological findings can be inconclusive. Diagnosis can be based on previously described characteristic clinical findings.
Therapeutic measures that have been proven effective but irregular include (Table 10):
1. Normalising calcaemia, phosphataemia, and phosphate-calcium product. Mainly using non-calcium binders, low calcium concentrations in the dialysate, and avoiding active vitamin D metabolites.
2. HPT2 control, with calcimimetics128 or parathyroidectomy if there is a slow response to calcimimetics.
3. Topically treating ulcers with antiseptics, without debridement. Sterile larvae available on the market have come to be used.
4. Hyperbaric oxygen chamber, with the aim of increasing the tissue PO2 and therefore produce fibroblasts and collagen, which could promote angiogenesis.129-131
5. Bisphosphonates. Bisphosphonates possess a powerful inhibitory effect on osteoclast activity and bone resorption. Inhibition of bone resorption reduces calcium concentration in the blood, which would reduce the tendency of the mineral nuclei to form and grow on the arterial walls. On the other hand, bisphosphonates could inhibit pro-inflammatory cytokine secretion in the vascular wall and therefore contribute to improving symptoms.132-135
Oral or intravenous bisphosphonates can be used. Oral bisphosphonates have the advantage that they can be administered in smaller, more constant and homogeneous doses than intravenous bisphosphonates. As such, bisphosphonates would probably have less effect on bones and greater effect on vascular tissue.
We recommend the following doses: ibandronate 6mg/i.v. or pamidronate 90mg/i.v. followed by any other oral bisphosphonate: ibandronate, 150mg once a month, alendronate, 70mg orally once a week or risedronate at 35mg once a week (1C).
6. Intravenous sodium thiosulphate is proven to be effective. The mechanism of action is unknown, although it is probably related to the dissolution of calcium salts deposited in vessels. It is administered as sodium thiosulphate solution at 25%, 25g/1.73m2 for an hour at the end of each haemodialysis session. It should be administered until all symptoms are resolved 136,137 (1C).
KEY CONCEPTS
1. The concept “renal osteodystrophy” is no longer relevant as it used to be and we should now consider “Chronic Kidney Disease-Mineral Bone Disorder”, conceived as a systemic syndrome.
2. The acronym CKD-MBD (Chronic Kidney Disease-Mineral and Bone Disorder) is used to refer to the bone-mineral disorder associated with chronic renal disease.
3. Chronic kidney disease-mineral and bone disorder initiates prematurely while kidney disease progresses.
4. We should also add fibroblast growth factor-23 and its co-factor Klotho to vitamin D receptors and calcium-sensing receptor, given their physiopathological importance in developing secondary hyperparathyroidism and parathyroid hyperplasia.
5. The cardiovascular system, as well as the skeleton and others, is one of the most important tissues that is secondarily affected by CKD-MBD.
6. Vascular calcifications are not only a passive phenomenon making calcium and phosphorus available, as they promote uraemia due to different factors, actively transforming vascular smooth muscle cell into chondrogenic or osteoblast cells.
7. The clinical laboratories should inform clinicians of the measuring methods employed (especially for PTH and calcidiol levels).
8. Serum calcium and phosphorus values are not sufficient to control patients with mineral and bone disorders, and calcium and phosphorus plasma concentrations have little relevance to the potential balance-overload of both elements.
9. Calcium, phosphorus, PTH and alkaline phosphatase must be monitored regularly in accordance with the periods recommended. Monitoring calcidiol levels is also of utmost importance, given the extremely high incidence-prevalence of vitamin D insufficiency or deficiency in chronic kidney disease patients.
10. PTH is a good marker for underlying bone disease, but intermediate levels are less specific. iPTH levels >450-500pg/ml (or equivalent) indicate high-turnover bone disease. iPTH<100-120pg/ml (or equivalent) are associated with low-turnover bone disease (adynamic or osteomalacia).
11. It is important to treat evolutionary PTH trends, not isolated data, meaning that the two should be considered together and alongside other CKD-MBD parameters.
12. We suggest using PTH correction formulae at the equivalent reference of the classic Allegro assay used (Nichol’s Institute), but this approach is only valid for dialysis patients.
13. Alterations in serum calcium, phosphorus, PTH, alkaline phosphatase and vitamin D, among others, have been associated with an increase in mortality in patients with CKD and/or undergoing dialysis. However, there is still no definitive proof of this causality.
14. Measuring calciuria and phosphaturia (fraction of phosphorus excretion) could be useful for evaluating potential overload of these elements.
15. FGF-23 measurements are still recommended in clinical practice, although it is considered to be the parameter of the future.
16. With a high degree of evidence, patients with vascular or valvular calcifications should be considered to be in the highest risk cardiovascular group.
17. Although measuring bone-mineral density routinely in non-transplant patients with CKD is not recommended, given that the findings do not correlate with underlying bone disease, to some extent it could be useful in patients at risk of developing osteoporosis or history of stress fractures.
18. Using bone biopsy is extended to patients with advanced stages of CKD and those undergoing dialysis before starting bisphosphonate treatment, especially if parathyroid hormone is not very high.
19. We suggest maintaining normal calcium and phosphorus levels in pre-dialysis CKD patients.
20. Regular calcium and phosphorus plasma tests should accompany calcifediol use (Hidroferol®) in CKD patients.
21. Detecting an increase in PTH in patients with CKD firstly leads to a correction of modifiable factors (hypocalcaemia, hyperphosphataemia, vitamin D deficiency.)
22. In most of the studies published (retrospective), active vitamin D derivatives and selective vitamin D receptor activators have been associated with beneficial effects (including higher survival rates) beyond the biochemical control parameters, both in pre-dialysis and dialysis patients.
23. There are experimental differences between different active vitamin D derivatives and the selective vitamin D receptor activators
24. Given that there are numerous alternatives available and the inability of determining a safe dosage, a prolonged use of aluminium-based phosphate binders should be avoided.
25. Sevelamer carbonate and lanthanum carbonate are calcium-free binder alternatives for pre-dialysis patients.
26. We recommend maintaining phosphorus as close to the normal values as possible in dialysis patients, given that it is associated with high a mortality in CKD patients.
27. CKD stage, presence of other CKD-MBD components (i.e. vascular calcification), concomitant therapies and secondary effects profile should be taken into consideration when selecting a binder.
28. Restricting calcium-based binders for persistent or recurrent hypercalcaemia is recommended, and suggested for arterial calcification and/or adynamic bone disease and/or if serum PTH levels are persistently low.
29. Combining calcium acetate with magnesium carbonate as a phosphate binder for patients undergoing dialysis has yielded promising results, with a lower calcium intake.
30. We suggest maintaining iPTH between 150 and 300pg/ml, corrected for the assay used, as a therapeutic objective. Values at extremes should be especially avoided (e.g. below 100pg/ml and above 500pg/ml).
31. It is reasonable to base initial therapy on calcium and phosphorus levels, as well as other CKD-MBD aspects (e.g. vascular calcification).
32. Cinacalcet significantly reduces PTH levels, simultaneously reducing serum calcium values, and, eventually, phosphorus values too.
33. Calcimimetics and active vitamin D derivatives can be combined, meaning that an additive or synergic action is possible.
34. Administration of oral bisphosphonates does not seem to alter renal function (with GFR>30ml/min/1.73m2).
35. When intravenous bisphosphonate administration is indicated, it is important to maintain or even prolong infusion time, so as to avoid secondary effects (even though very few negative effects have been reported).
36. If bisphosphonates are used for patients with a GFR less that 30ml/min/1.73m2, then the dosage should be reduced to half the amount recommended for patients with normal renal function.
37. Persistence of HPT following kidney transplantation is a risk factor for hypercalcaemia, hypophosphataemia, bone mass loss, an increase in fracture risk and renal function deterioration.
38. After kidney transplantation, we recommend treatment with calcimimetics if calcaemia is higher than 10.5mg/dl.
39. To prevent post-transplant bone mass loss, steroid dose should be reduced and vitamin D, vitamin D analogues or bisphosphonates can be employed.
40. We do not recommend universal use of bisphosphonates during the post-transplant period and they should only be used in patients at risk.
41. If the kidney transplant patient is suspected to have adynamic bone disorder with very low serum parathyroid hormone values, synthetic PTH treatment should be considered.
Table 1. Biochemical test intervals
Table 2. Biochemical test intervals
Table 3. Biochemical test intervals
Table 4. Diagnostic strategies. Imaging techniques:
Table 5. Diagnostic strategies. Imaging techniques:
Table 6. Diagnostic strategies. Imaging techniques:
Table 7. Recommended biochemical values
Table 8. Alternative therapies
Table 9. Alternative therapies
Table 10. Alternative therapies. Calciphylaxis
Figure 1. Renal osteodystrophy
Figure 2. ROD classification
Figure 3. Treatment algorithm (guideline)
Figure 4. Treatment algorithm (guideline)
Figure 5. Kidney transplant treatment algorithm (guideline)
Figure 6. Kidney transplant treatment algorithm (guideline)
Figure 7. Kidney transplant treatment algorithm (guideline)