Patients with chronic kidney disease (CKD) have higher risk of developing cardiovascular disease. In CKD patients the mechanisms involved in, endothelial damage and the role of different drugs used on these patients are not completely understood. The aim of this work is to analyze the effect of statins and platelet antiaggregant (PA) on endothelial microvesicles (EMVs) and other markers of endothelial dysfunction.
Experimental approachCross-sectional study of 41 patients with CKD 3b-4 and 8 healthy volunteers. Circulating levels of EMVs, vascular endothelial growth factor (VEGF), and advance oxidized protein products (AOPPS) were quantified and the correlation with different comorbidity variables and therapeutic strategies were evaluated.
ResultsEMVs are increased in CKD patients as compared with controls (171.1 vs. 68.3/μl, p<0.001). It was observed a negative correlation between age and EMVs. Statins and PA were associated with a reduction in EMVs and VEGF levels, independently of the serum total cholesterol levels (TC). The levels of AOPPS and VEGF were not different in CKD vs. controls.
ConclusionCKD is associated with a change in EMVs, VEGF and AOPP levels. The treatment with statins and PA normalizes these values to almost the observed in controls and this effect is independently of the prevailing TC level. These findings explain the existence of the pleiotropic effects of statins and PA which deserve further studies.
Los pacientes con insuficiencia renal crónica (IRC) corren mayor riesgo de desarrollar enfermedad cardiovascular. En los pacientes con IRC, los mecanismos implicados en la disfunción endotelial y el papel de los diferentes fármacos utilizados en estos pacientes no se conocen por completo. El objetivo de este artículo es analizar el efecto de las estatinas y los antiagregantes plaquetarios (AP) sobre las microvesículas endoteliales (MVE) y otros marcadores de la disfunción endotelial.
Enfoque experimentalEstudio transversal con 41 pacientes con IRC 3b-4 y 8 voluntarios sanos. Se cuantificaron los niveles de MVE, factor de crecimiento vascular endotelial (FCVE) y productos avanzados de oxidación de proteínas (AOPP, por sus siglas en inglés) en la circulación y se evaluó la correlación con diferentes variables de comorbilidad y estrategias terapéuticas.
ResultadosLas MVE aumentaron en pacientes con IRC al comparar los niveles con los controles (171,1 frente a 68,3/μl; p<0,001). Se observó una correlación negativa entre la edad y las MVE. Las estatinas y los AP se asociaron con una reducción de los niveles de MVE y FCVE, independientemente de los niveles séricos de colesterol total (CT). Los niveles de AOPP y FCVE no fueron diferentes entre los pacientes con IRC y los controles.
ConclusiónLa IRC se asocia con un cambio de los niveles de MVE, FCVE y AOPP. El tratamiento con estatinas y AP normaliza estos valores a casi los observados en los controles y este efecto es independiente del nivel de CT predominante. Estos hallazgos explican la existencia de los efectos pleiotrópicos de las estatinas y los AP que merecen estudios adicionales.
Patients with chronic kidney disease (CKD) are more likely to develop cardiovascular disease (CVD), this is observed regardless of the rate of progression to advanced stages. It is a fact that the main cause of death in patients with CKD is cardiovascular.1 The development of CVD in uremic patients involves complex processes of inflammation and endothelial dysfunction, whose final result is the atherosclerosis. In addition, the loss of arterial wall elasticity is a finding common in CKD and has been described as an independent predictor of cardiovascular events.2
The microvesicles (MVs) are corpuscles that can be released from almost any eukaryotic cell during both activation and cellular apoptosis.3 During the recent decades it has been shown that MVs derived from the endothelium (EMVs) are useful blood markers for different pathological situations such as vascular inflammation (acute or chronic) and endothelial dysfunction.4 In addition, many studies have shown that circulating EMVs can interact a subsequent fusion with cell membrane of different cells, such as endothelial cells and leukocytes, this results in an effective delivery of components of its original cells to the target cells.3 The measurement of EMVs plasma levels may provide relevant information in both healthy and patients. Normally, the EMVs count increases after the induction of acute or chronic endothelial dysfunction.5 The mechanisms mediating the released these particles are not totally clear, and have been postulated as intermediate regulators of several growth factors and cytokines such as vascular endothelial growth factor (VEGF).6
Several studies have reported the association between CKD and circulating MVs, showing changes in serum MV levels, according to the stage of CKD or the modalities of renal replacement therapy.7 However, studies focused on circulating EMVs and CKD have led to nonuniform data.8,9 It has not been analyzed whether drugs commonly used in CKD that act on endothelial function (statins and antiplatelet agents) have some effect on the production of EMVs and other biomarkers.
The advanced oxidation protein products (AOPP) are a family of oxidized proteins that contain dithyrosine, which are generated during stress oxidative and are measurable in plasma and that are useful as an early marker of oxidative tissue damage. They have also been described as active mediators of the inflammation associated with the uremic state.10
The aim of this study was to analyze the effect of the use of statins and platelet antiaggregants (PA) on circulating EMVs and the expression of serum markers of endothelial dysfunction, oxidative stress and inflammation as AOPP and VEGF in a cohort of patients with CKD. Likewise, their relationship with other cardiovascular risk factors will be analyzed.
MethodsA cross-sectional study was conducted on 41 patients with CKD. The patients with a history of autoimmune diseases such as Lupus, Vasculitis or Glomerulonephritis with active urinary sediment were excluded. The control group included 8 healthy volunteers.
The study was approved by the Hospital Puerta de Hierro Ethics and Clinical research committee and before inclusion all participants provided written consent. Baseline demographics, clinical and laboratory data (total cholesterol level and fractions, uric acid, creatinine, etc.) and pharmacological treatment were recorded from structured electronic medical record-EMR (Nefrolink® and Selene®).
Blood collection, isolation and analysis by flow cytometry of EMVsBlood samples were taken with a 21G gauge needle after applying a light tourniquet. The first 4ml were discarded, the blood was collected in tubes with 3.2% trisodium citrate (Becton Dickinson, Plymouth, UK)]. Twenty minutes after collection, the plasma was centrifuged at 1500×g, without brake, for 20min at room temperature. The plasma was centrifuged again for 2 more minutes at 13,000×g to obtain platelet-poor plasma, according to the method developed previously with minor modifications.11 Aliquots of plasma were instantly frozen and stored at −80° C until use.
The characterization of EMVs was obtained as previously described in other studies11 using flow cytometry (Beckman Coulter Cytomic FC 500, CoulterInc, Fullerton, CA, USA) with CXP software (Beckman Coulter). According to the side scatter vs forward scatter dot-plot, MVs were considered those events gated in a size between 0.5 and 1.5μm. This system was previously established in a standardization experiment using size-calibrated fluorescent beads with sizes ranging from 0.1 to 1.9μm (SPHERO™ Flow Cytometry Nano Fluorescent Size Standard Kit, Spherotech, Lake Forest, IL USA). A triple-fluorescent labeling was performed for EMVs characterization. One hundred ml of platelet-poor plasma was incubated with fluorescein isothiocyanate-conjugated labeled monoclonal anti-CD31 (BD Pharmingen, San Diego, California, USA), Peridinin Chlorophyll Protein Complex monoclonal anti-CD42b (Abcam, Cambridge, MA), and phycoerythrin-annexin V kits (BD Pharmingen, San Diego, California, USA) in annexin V-binding buffer (10mM HEPES, 7.4 pH, 140mM NaCl, 2.5mM CaCl2) following the manufacturer's instructions of annexin V kit.
To identify the cell-specific monoclonal antibodies of MVs, these were incubated with identical concentrations of isotype-matched control antibodies to set the threshold. As both platelets and endothelial cells express CD31, but CD42b occurs only on platelets, EMVs were CD31+/CD42− events. The absolute number of EMVs per microliter was quantified according to the manufacturer specification using Flow Count Calibrator beads (Beckman Coulter Inc, Fullerton, CA, US).
AOPPs and VEGFThe plasma concentration of AOPP was measured in fasting plasma samples collected in tubes with ethylenediaminetetraacetic and kept frozen at −80°C. AOPP concentration was measured by Western Blot using the Oxy Blot™ Protein Oxidation Detection Kit, catalog no. S7150, Millipore.
THeVEGF levels were analyzed in serum by ELISA using Quantikine VEGF ELISA kit (R&D Systems, Minneapolis, MN).
Statistical analysisResults are expressed as percentage, median, range or interquartile range (IQR), unless indicated otherwise. Differences between groups were assessed by chi-square test, Fisher's exact test, Kruskal–Wallis test, Bonferroni correction or ANOVA according to the variables studied (post hoc analysis performed with Turkey's test). A linear regression analysis was performed to determine the level of EMVs according to treatment (statins or PA) adjusted for total cholesterol (TC) levels, indicating the β value and its 95% confidence interval (CI). The differences were considered significant if the p values <0.05. All tests were performed with software SSPS v 15.0 (Spss Inc., Chicago, IL, US) and Stata (StataCorp 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
ResultsThe study included 41 patients with a median age of 51.5 years IQR [42.5–72.5]. A 39% of the patients were diabetic, 85.4% hypertensive and 9.8% had a history of ischemic heart disease.
The most frequent cause of CKD was diabetic nephropathy in 39%, followed by a 19% of undiagnosed/other causes, 14.6% polycystic disease, 14.6% glomerular disease and 12.2%.vascular disease
The median estimated glomerular filtration rate (eGFR) using the CKD-EPI equation (Chronic Kidney Disease Epidemiology Collaboration) was 31.9ml/min (a 48.8% had eGFR<30ml/min).
Respect to pharmacological treatment, most patients received angiotensin converting enzyme inhibitors (ACEIs) and/or Angiotensin Receptor Blocker (ARB) (78%), statins (48.8%) and PA (14.3%).
Levels of EMVs in the ERCWe detected an increase in circulating EMVs in patients with CKD as compared with the control group (171.1 EMVs/μl vs 68.3 EMVs/μl, p<0.001), no significant correlation was observed between the eGFR and the amount of circulating EMVs.
In our CKD patients, we did not find differences in the amount of EMVs according to the treatment received, for ACEIs/ARB, diuretics, beta-blockers, or erythropoietin.
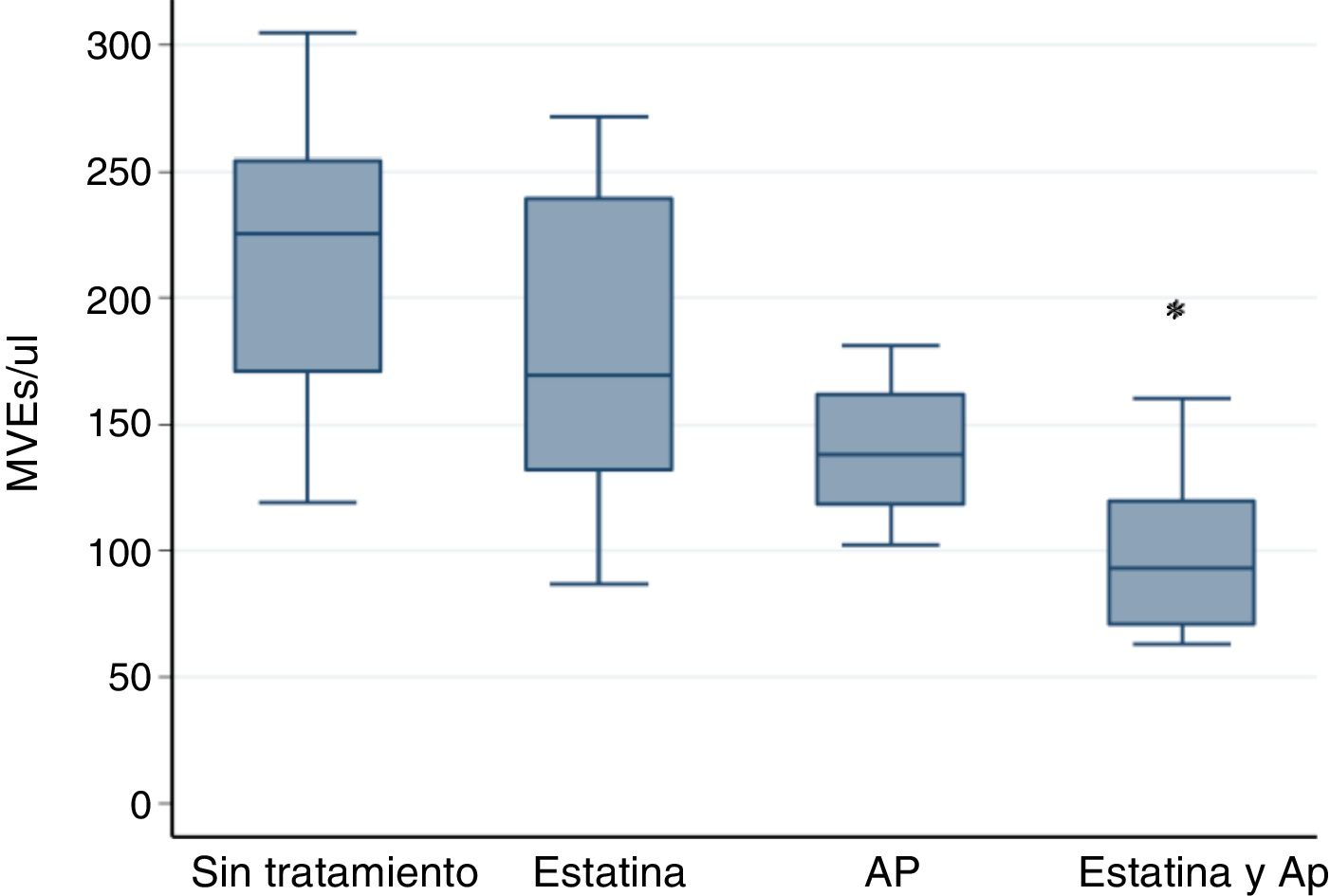
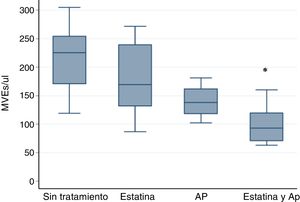
However the association of statin and PA significantly decreases the amount of EMVs. The results were: in patients without treatment, 225.55 EMVs/μl (119.11–304.88); Statin 169.33 EMVs/μl (86.9–271.56); AP 138.33 EMVs/μl (102.44–181.11); Statin plus PA 93.11 EMVs/μl (63.11–160.22) p<0.001 by Kruskall–Wallis test.
The post hoc analysis with the Bonferroni correction indicates statistically significant differences only between statin plus PA vs no treatment p<0.001 (Fig. 1).
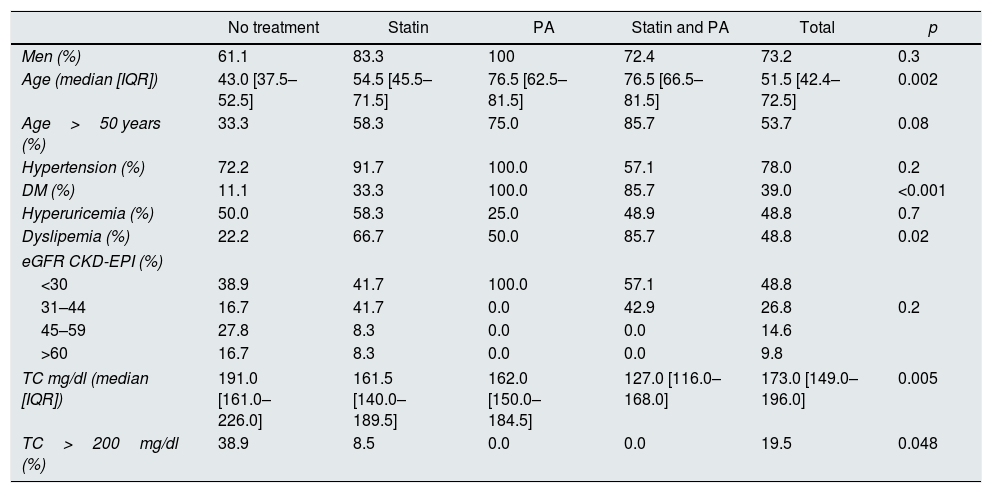
Table 1 describes the baseline characteristics according to the treatment. Patients on statins or PA were older, with higher proportion of diabetes mellitus (DM) and lower total serum cholesterol (TC) levels.
Baseline characteristics according to received treatment.
| No treatment | Statin | PA | Statin and PA | Total | p | |
|---|---|---|---|---|---|---|
| Men (%) | 61.1 | 83.3 | 100 | 72.4 | 73.2 | 0.3 |
| Age (median [IQR]) | 43.0 [37.5–52.5] | 54.5 [45.5–71.5] | 76.5 [62.5–81.5] | 76.5 [66.5–81.5] | 51.5 [42.4–72.5] | 0.002 |
| Age>50 years (%) | 33.3 | 58.3 | 75.0 | 85.7 | 53.7 | 0.08 |
| Hypertension (%) | 72.2 | 91.7 | 100.0 | 57.1 | 78.0 | 0.2 |
| DM (%) | 11.1 | 33.3 | 100.0 | 85.7 | 39.0 | <0.001 |
| Hyperuricemia (%) | 50.0 | 58.3 | 25.0 | 48.9 | 48.8 | 0.7 |
| Dyslipemia (%) | 22.2 | 66.7 | 50.0 | 85.7 | 48.8 | 0.02 |
| eGFR CKD-EPI (%) | ||||||
| <30 | 38.9 | 41.7 | 100.0 | 57.1 | 48.8 | |
| 31–44 | 16.7 | 41.7 | 0.0 | 42.9 | 26.8 | 0.2 |
| 45–59 | 27.8 | 8.3 | 0.0 | 0.0 | 14.6 | |
| >60 | 16.7 | 8.3 | 0.0 | 0.0 | 9.8 | |
| TC mg/dl (median [IQR]) | 191.0 [161.0–226.0] | 161.5 [140.0–189.5] | 162.0 [150.0–184.5] | 127.0 [116.0–168.0] | 173.0 [149.0–196.0] | 0.005 |
| TC>200mg/dl (%) | 38.9 | 8.5 | 0.0 | 0.0 | 19.5 | 0.048 |
PA: platelet antiaggregants; TC: total cholesterol; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate by CKD-EPI ml/min/1.73m2; IQR: interquartile range. Are considered significant values: p<0.05.
A subanalysis in the diabetic population could not be performed because most of them were receiving statin or PA.
We found a positive association between EMVs count and TC levels. In patients with a TC<200mg/dl treated with statins the EMVs levels were reduced (191.0 EMVs/μl vs 119.8 EMVs/μl, p=0.032). By linear regression, it was identified that patients on treatment with statins or PA had reduced circulating levels of EMVs, independently of the TC levels (β=−46.02, IC [−88.59 to −3.46]). Age correlated negatively with EMV levels (Spearman test p<0.05).
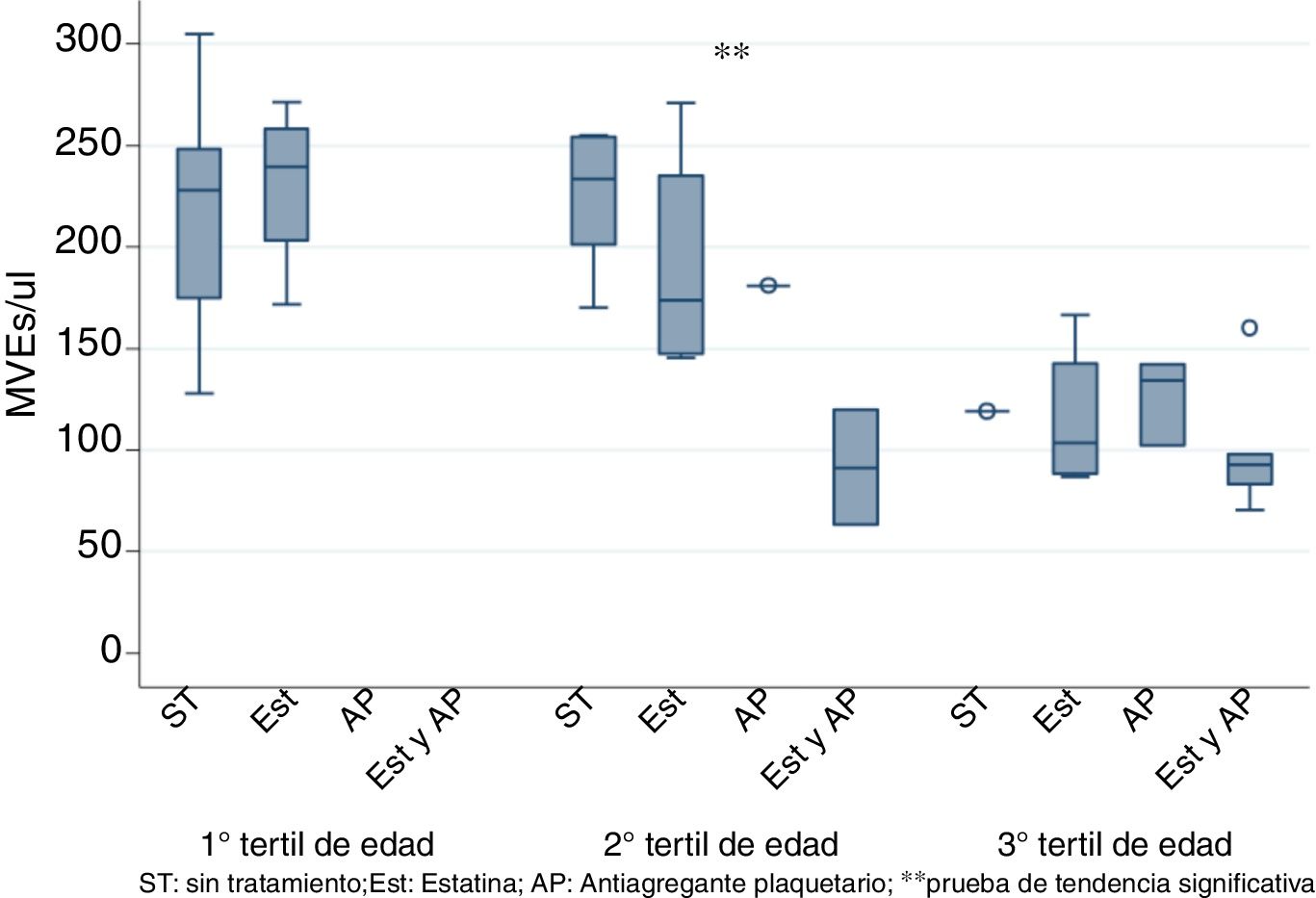
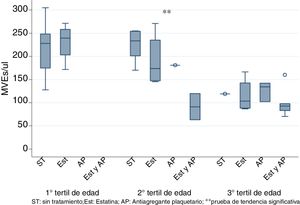
To eliminate the bias produced by age, patients were stratified by tertiles of age and it was observed the use of statins and PA together significantly decreased EMVs levels only in the 2nd tertile (50–70 years) as shown in Fig. 2 (K-tau trend test <0.05).
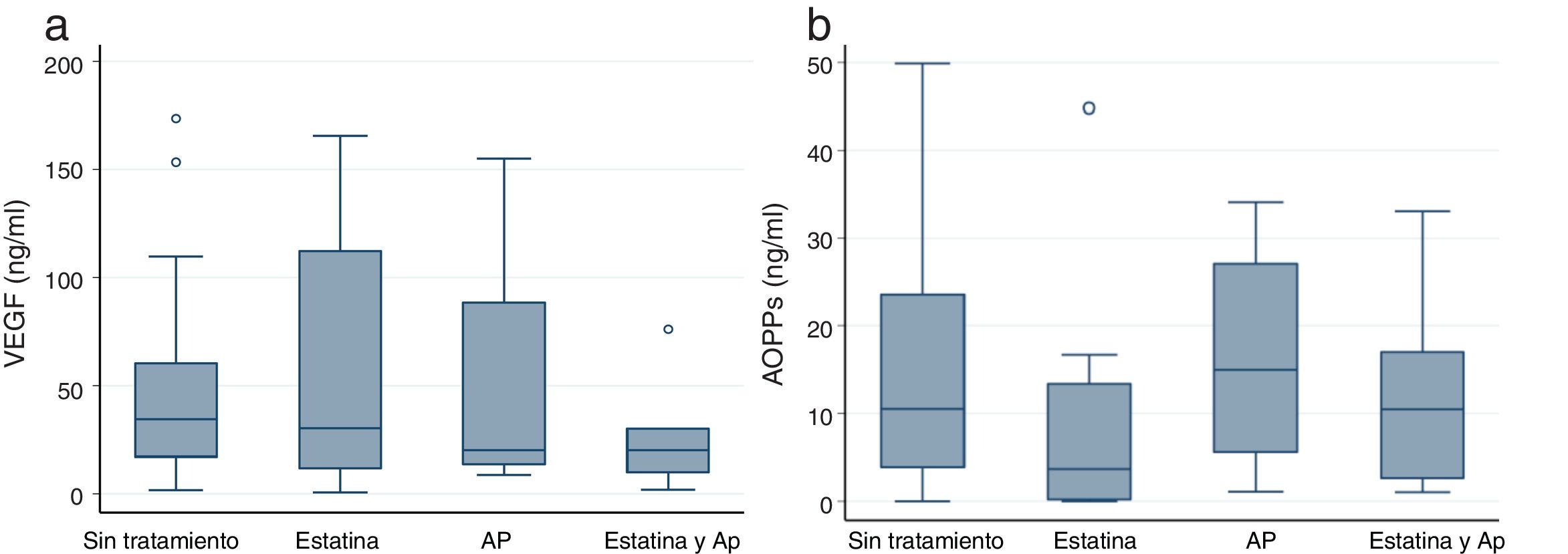
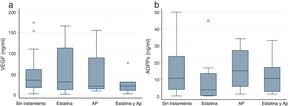
VEGF and AOPPs in patients with CKD.No differences were observed in the serum levels of VEGF of CKD vs control groups. The treatment with statins and PA had an effect on the serum levels of VEGF that was similar to the observed in circulating EMVs (Fig. 3a) although without achieving statistical significance.
AOPP (a) and VEGF (b) values according to the treatment with statin or PA. Values of p<0.05 are indicated by an asterisk (*) and outlayer values with this symbol (o). AOPP: advanced protein oxidation products; VEGF: vascular endothelial growth factor; PA: platelet antiaggregants. Box plots indicate median and interquartile range.
Serum AOPPs was increased in patients with CKD as compared with controls although without statistical significance (7.51ng/ml vs 9.91ng/ml). The pharmacological treatment showed no effect on the serum levels of AOPPs (Fig. 3b).
DiscussionOur work describes for the first time in CKD patients, an association between circulating levels of EMVs, VEGF, AOPPs and commonly used drugs in our population, specifically, the use of statins and PA. In addition, we have shown an increase in circulating levels of EMVs in patients with CKD corroborating the finding previously described by other authors.12
It is know that patients with CKD are more likely to suffer CVD, as illustrated in previous epidemiological studies1. There are several changes in the structure and functional characteristics of the artery wall that have been related to CKD, such as arterial stiffness, endothelial dysfunction and a reduction in the production of nitric oxide (NO).13,14 Although traditional risk factors such as age, hypertension and dyslipidemia are classically associated with these alterations, they do not able to fully explain the changes observed in patients with CKD. Therefore, we need additional information to sustain an integrated and more complete pathological model.
Other authors have described high levels of EMVs in patients with CKD, like in other high-risk CV patients with acute coronary syndrome or hypertension.4 We do not know the mechanisms that lead to the increase in EMVs observed in patients with CKD; several hypotheses have been proposed, including oxidative stress, cytokines and endogenous lipopolysaccharides.15
The experiments of Amabile et al.8 showed that EMVs of patients with CKD of any cause generated endothelial dysfunction decreasing generation in vitro of NO and affecting relaxation in the healthy arterial wall dependent on NO and cyclic guanosine monophosphate. For this reason, EMV could be more an effector agent than a biomarker of endothelial damage in CVD associated with CKD, in which case it becomes therapeutic target.
The role of statins in the formation of EMVs has been previously tested with non uniform results. Probably, this is due to the complexity of the pathophysiological mechanisms that involve CVD and the effects of confounding factors such as age, gender and associated comorbidity. Previous studies have described that atorvastatin decreases levels of circulating EMVs in patients with ischemic cardiomyopathy, independently of TC levels and their fractions16; this results have been confirmed in our study.
The clinical trial of cardiac and renal protection (SHARP) demonstrated that statin therapy modifies atherosclerotic events in CKD patients, regardless of the serum levels of TC17 suggesting pleiotropic effect of these drugs.
Our previous in vitro studies have shown that the addition of atorvastatin decreases the production of reactive oxygen species (ROS) induced by indoxyl sulfate in Human umbilical vein endothelial cells (HUVEC) (unpublished data) that support the previous hypothesis of the existence of pleiotropic effects of statins on endothelial cells.18,19
The results presented here, raise the possible existence of other mechanisms pleiotropics of the statin on the vascular wall, in this case mediated by EMVs.
Antiplatelet drugs have also been shown to modify the amount of EMVs circulating in various clinical and experimental scenarios.20 In this sense, Bulut et al.21 demonstrated that treatment with acetylsalicylic acid reduces the number of circulating EMVs in patients with acute coronary disease by an unknown mechanism. It is known that aspirin improves vascular endothelial function by mechanisms independent of Cyclooxygenase22 and it is debatable whether this could have additional cardioprotective effects beyond those attributed to the antithrombotic activity.
Since EMVs can directly induce endothelial dysfunction, the effect of aspirin in r reducing circulating EMVs could explain the mechanism of endothelial protection of this drug.
Inflammatory mediators such as tumor necrosis factor α, interleukin-1β and thrombin drive the generation of EMVs and it is believed that these MVs act in a paracrine fashion, inducing further vascular inflammation.23 It is known that VEGF, one of the main mediators of angiogenesis, has potent effects for mobilization in progenitor endothelial cells. However, its effect on the circulating levels of EMVs is controversial. EMVs have been shown to alter angiogenesis in different clinical situations8 and are also promoters of the generation of VEGF in some cellular subtypes such as the renal tubular cell; data described by Fernández-Martínez et al.24
In our series we have reported that treatment with antiplatelet agents and Statins decrease serum levels of VEGF, without correlation with EMVs levels. Therefore, with the results of our study we cannot clarify whether both phenomena are related or are independent pathophysiological mechanisms.
Finally, we observe an increase in the state of oxidative stress estimated by circulating concentrations of AOPP in patients with CKD. Oxidative stress is defined as an alteration of the balance between the oxidants generation and the antioxidant systems activity. Hence, this plays an important role in the development of the inflammatory syndrome associated with chronic renal failure.10 Serum levels of AOPPs have been shown to be a very good marker of oxidative stress derived from phagocytes, but they are also active mediators of the inflammatory state associated with uremia. In this sense, Arsat et al. demonstrated that the levels of AOPP are already increased in the early stages of CKD and increase with the progression of uremia.10 Experimental studies suggest that AOPP could increase oxidative stress in the kidney and be involved in the genesis of the glomerulosclerosis.25 In fact, intravenous administration of AOPPs in diabetic mice increase AOPP in the kidney, promote renal inflammation, glomerular hypertrophy and overexpression of fibronectin, and led to albuminuria.24
In our study, age is associated with decreased levels of EMVs, similar results to those previously described by Anne Forest et al. and the Erez Eitan group. The latter supports the hypothesis that MVs decrease with age because of an increase in their internalization in leukocytes26 or simply that low levels of EMVs could reflect a deceleration of the replacement due to a senescent endothelium with lower metabolism and cellular activity.27 After analyzing the effect of stratifying treatment by age, it was observed that the use of statins and PA correlates with the decrease in EMVs levels being statistically significant in the second tertile.
Another fact that should be emphasized, is the low percentage of diabetics without treatment (statin and AP). The decrease in circulating EMVs that we observe in our diabetic population is not in agreement with previously reported analysis of an association between Diabetes Mellitus and micro/macrovascular complications with an increase in EMVs.28 This discrepancy could be related with the effect of the treatment with statin and PA, but since it was hard to find diabetic patients without treatment (Statin and PA) in our sample, we could not pursue this issue any further.
Our pilot study has some limitations. The control group consists of healthy subjects of unpaired age with those of the study population and without associated comorbidity, but it is essential to include these results given the absence of normal reference values for these factors. Finally, it is important to emphasize the data variability, given that they are unconventional measures. To try to minimize this possible error, we use inter-assay pattern lines that allow to homogenize results. In addition, we determined each sample in triplicate, using the average of the determinations.
ConclusionsThe present study has shown that CKD patients present modifications in the count of circulating EMVs, and in the VEGF and AOPP levels. The use of statins and AP is associated with a correction of this effect, indicating the existence of pleiotropic mechanisms on endothelial function in this group of patients. In the population with CKD, age also seems to be a determinant in the number of EMVs. Further studies are needed to clarify whether EMVs act as subrogated biomarkers or are themselves effectors of endothelial damage.
Conflicts of interestThe authors have no conflicts of interest to declare.
FinancingProyecto FIS, Instituto Carlos III. 2014 Expediente: Q2818018JPI14/00806.
We would like to thank Antonio J. Sánchez López PhD, Scientific Coordinator of the biobank of IIS HUPH-M and Paula López, statician H.U. Puerta de Hierro Majadahonda, Nephrology, for their technical assistance in the development of this work.
Please cite this article as: García Menéndez E, Marques Vidas M, Alique M, Carracedo J, de Sequerac P, Corchete E, et al. El uso de estatinas y antiagregantes se asocia con cambios en los marcadores de disfunción endotelial en la enfermedad renal crónica. Nefrologia. 2019;39:287–293.