Introducción: El estrés oxidativo es crucial para el desarrollo de arteriosclerosis, principal causa de morbimortalidad en población en prediálisis. Nuestro objetivo fue valorar la oxidación de las principales líneas moleculares y discernir si algún biomarcador tenía mejor comportamiento valorando este estrés. Pacientes y método: Estudio observacional en 32 pacientes con MDRD 22,1 ± 1,08 ml/min. Medimos en linfocitos periféricos: malondialdehído, glutatión oxidado/reducido, 8-oxo-deoxiguanosina nuclear y mitocondrial, superóxido dismutasa, glutatión reductasa, glutatión peroxidasa y catalasa, y en plasma F2 isoprostanos y proteínas carboniladas. Correlacionamos los resultados con función renal y factores comórbidos. Resultados: Todos los biomarcadores tuvieron amplias diferencias significativas cuando se compararon con el grupo control peroxidación lipídica: F2 isoprostanos: 821,89 ± 300,47 ng/ml vs. 270 (95,66)* ng/ml (p <0,000); MDA 0,11 (0,11)* vs. 0,7 ± 0,31 nmol/mg prot (p <0,000). Oxidación proteica: GSSG/GSH: 6,89 ± 1,91 vs. 1,39 ± 0,75 (p <0,000); proteínas carboniladas: 7,41 ± 0,84 vs. 3,63 (1,12)*. Daño material genético: 8-oxo-deoxiguanosina nuclear: 7,88 (2,32)* vs. 2,96 (1,78)* y 8-oxo-dG mitocondrial: 15,73 ± 2,28 vs. 13,85 ± 1.44 (p <0,05). Los valores de las enzimas antioxidantes también obtuvieron amplias diferencias significativas. La molécula 8-oxodeoxiguanosina en DNA nuclear fue la que tuvo una relación significativa con el resto de biomarcadores, con homocisteína (r = 0,305; p <0,05), lipoproteína (a) (r = 0,375; p <0,01), 8-oxo-deoxiguanosina mitocondrial (r = 0,411; p <0,05), GSSG/GSH (r = 0,595; p <0,001) y proteínas carboniladas (r = 0,489; p <0,05), y de forma inversa con las proteínas totales (r = -0,247; p <0,01), GSH (r = -0,648; p <0,000), GRS (r = -0,563; p <0,001) y SOD (-0,497; p <0,000). Ninguno de los parámetros tuvo correlación con la función renal. Tampoco se obtuvieron diferencias significativas con la presencia o no de diabetes o la toma de estatinas. * Mediana (amplitud intercuartil). Conclusión: Existe un elevado estrés oxidativo en los pacientes con enfermedad renal avanzada que probablemente se establezca desde fases tempranas de la enfermedad. Entre todos los parámetros estudiados, la molécula de 8-oxo-dG se comportó como el marcador más idóneo.
INTRODUCTION
The prevalence of chronic kidney disease (CKD), in Spain and in the rest of the world, has continued to increase over the last few years. This trend is probably due to the ageing population, the increasing prevalence of conditions such as diabetes and arterial hypertension, and possibly to the existence of better public health programmes that detect and monitor kidney disease in its early stages.1-3
It has been shown that the main causes of morbidity and mortality in CKD patients are cardiovascular4,5 and that oxidative stress and an inflammatory subclinical state can be the end factors responsible for the generation and progression of the arteriosclerotic plaque.5
Excessive generation of pro-oxidation substances and the deficit or loss (in dialysis) of antioxidant substances are responsible for generating oxidative stress and producing irreversible molecular damage. In patients who receive kidney replacement therapy, there may be different added factors involved in stress generation, such as the back-filtration of non-ultrapure dialysis fluid and the resulting flow of endotoxins and complement system activation, the loss of antioxidant substances through highly permeable membranes or the infusion of large amounts of intravenous iron, etc.
The existence of this relationship between oxidative stress and cardiovascular pathology in patients on aemodialysis has already been demonstrated by various studies.6 However, the situation in pre-dialysis patients is different; there are fewer studies and most of them assess an isolated molecular group, which makes it difficult to gain a global understanding of this alteration.
There is evidence to show that in early stages of the disease, this stress level is already elevated.7,8 The predialysis population is characterised as having a large number of traditional risk factors such as hypertension, dyslipidaemia and diabetes mellitus. All have been shown to be cardiovascular risk factors, and it is therefore understandable that oxidative stress would be high in this population. Furthermore, the accumulation of uraemic toxins will contribute to the further increase of the unbalance between defence mechanisms and oxidant attack.
Our purpose was to evaluate the oxidation of different molecular lines (proteins, lipids and genetic material) in order to discern which one might behave as the best oxidative marker in a population group with an advanced stage of renal disease, but without the influence of potential factors arising from dialysis techniques.
PATIENTS AND METHODS
This observational study took place in a group of 32 patients with a previous diagnosis of stage 4 CKD and a control group made up of 67 healthy volunteers. The patient group was gathered from the pre-dialysis patients at the Nephrology Department at the Clinical Hospital of Valencia. All participants signed a consent form in order to take part in the study.
We included those patients who had been clinically stable during the previous six months and excluded those with a neoplastic condition, copious bleeding, inflammatory illness or an active infection, and any who had received treatment with intravenous iron in the three preceding months.
We reviewed patients¿ clinical charts and recorded their demographic data, the aetiology of the renal disease, history of arterial hypertension and number of antihypertensives taken to treat it, dyslipidaemia, statin drug therapy, diabetes mellitus, ischaemic heart disease (defined as having a history of myocardial infarction, angina or an imaging study positive for myocardial infarction), stroke and peripheral arterial disease.
Blood samples were taken following 12 hours of fasting. Haemogram and biochemical screening samples were taken to measure the following: urea, creatinine, lipid metabolism, total proteins, albumin, homocysteine, lipoprotein A, fibrinogen and hs-CRP (high-sensitivity C-reactive protein).
To determine oxidative stress parameters, 14ml of blood was extracted using a vacutainer tube with EDTA as anticoagulant. The samples were placed in the centrifuge immediately to separate plasma.
The characteristics of the groups under study are shown in table 1.
The pre-dialysis group contained 26 males and 6 females, with a mean age of 65.29 ± 15.60 years. The cause of the kidney disease was vascular in 21 cases (65.7%), diabetic in eight cases (25%), tubulo-interstitial nephritis in two (6.2%), and renal polycystic disease in one case (3.1%). The estimated glomerular filtration rate (MDRD formula) at the time of the study was 22.09 ± 6.02ml/min on average.
Laboratory procedures
Mononuclear cells were isolated using Ficoll-Hypaque9 centrifugation, followed by three rinses with saline solution. Mononuclear cells that were resuspended in RPMI 1460 medium (Sigma) and those lysed using RNA/DNA Stabilization Reagent for Blood/Bone marrow (Roche) for DNA extraction were stored at -80∫ C until they were used.
Isolation of nuclear DNA (nDNA) and mitochondrial DNA (mtDNA)
The nDNA was isolated using the Gupta method with the modification described by Muñiz et al.10 by which chloroform-isoamyl alcohol (24:1) is used instead of phenol for protein elimination. Isolation of mtDNA was done using the method developed by Espinosa et al.11
Oxidative stress study
Determining oxidation parameters
Two parameters were established for the study of lipid peroxidation: MDA by high-resolution liquid chromatography in isolated mononuclear cells, measuring the protein concentration in each sample using the Lowry method12 in order to reference these parameters. The esterified F2 isoprostanes were quantified in plasma using the ELISA technique (Cayman Chemicals).
F2 isoprostanes are products deriving from the nonenzymatic oxidation of arachidonic acid present in cell membrane lipids.
Protein stress was studied using the GSSH/GSH relationship with high-resolution liquid chromatography in mononuclear cells, and carbonyl proteins in plasma using the ELISA technique and the method developed by Buss et al.13
Lastly, the analysis of oxidative damage on a genetic material level was carried out by a study of the modified base 8-hydroxy-dG in previously isolated nuclear and mitochondrial DNA by means of high-resolution liquid chromatography.
Antioxidant defence products
Antioxidant defence was evaluated by studying the activity of various enzymes that have been shown to have this capability.
The total activity of the SOD enzyme was determined by the McCord and Fridowich14 method, and catalase, GPX and GSR enzyme activity was determined by the Claiborne and Gunzler15 method; all of the above used spectrophotometric assays.
Statistical analysis
The different numeric variables are expressed as a mean ± standard deviation and as a median and interquartile range for variables without a normal distribution (Kolgomorov- Smirnov test). For the latter, logarithmic transformation was used. When comparing independent samples, we used the Student T-test with the Levene¿s test to assess the equality of variances. For comparing qualitative variables, we used the Chi-square test with Yates¿ correction, or Fisher¿s exact test where there were fewer than five observations. A bivariate correlation with Pearson¿s P-test was used to study different correlations between variables. Values of p ≤= 0.05 were considered significant. We used statistical software SPSS version 15.0.
DISCUSSION
The principal finding of the study is the significant increase of oxidative markers in a pre-dialysis population along all of the studied molecular groups, as well as the deficit in antioxidant defences. The 8-oxo-dG molecule, a nuclear damage marker, had the best correlation compared with the rest of the studied parameters, and we therefore relieve it to be the most reliable marker.
In general, the study of oxidative stress in a CKD population has focussed on the population on dialysis with the fundamental intention of demonstrating the technique¿s effect in this area. However, the pre-dialysis population is characterised by the convergence of multiple cardiovascular risk factors, every one of which is capable of provoking such stress by itself.
Over the last two decades, interest in discovering the causes of the high cardiovascular morbidity and mortality in the CKD population has increased. As a result, oxidative stress has been the subject of many studies, since tests point to it as the physiopathological centre for atheromatous plaque formation. However, in the pre-dialysis population, most of the studies have focussed on scarce oxidative biomarkers, often in populations with heterogeneous characteristics. Our purpose was to evaluate the oxidation of different molecular groups (proteins, lipids and genetic material) in order to discern if one would behave as a better oxidative marker in a grouped population with an advanced stage of renal disease, but without the influence of potential factors arising from dialysis techniques.
In our case, patients in the population have a 100% hypertension prevalence rate with varying degrees of severity, and a diabetes rate above 40%.
Although 65% of our patients have been diagnosed with dyslipidaemia, 59.4% received treatment with statin drugs.
As a result, the mean lipoproteins are within the normal range in the pre-dialysis group.
Lipid peroxidation was studied using two parameters, plasma MDA and F2 isoprostanes. MDA, the end product of lipid peroxidation,16 is one of the most closely studied parameters. Various authors have found high MDA levels in CKD patients on HD.17We also found significantly elevated levels in the CKD group. However, despite being an optimal marker of oxidative stress, MDA is a low molecular weight, hydrosoluble molecule, which means that it can be cleared by the kidneys or dialysed. Recently, de Vecchi et al. suggested that the high MDA levels that we find in these patients are due in part to the low glomerular filtration rate. For this reason, it seems more fitting to quantify MDA molecules bound to macromolecules that prevent this clearance process.18 Meanwhile, measuring plasma F2 isoprostanes has gained aspecial interest in recent years for various reasons. These molecules are more stable than other oxidised lipids (oxidised LDL, for example), and since they are measured in their esterified form in plasma, they do not suffer kidney clearance or filtration by dialysis.19 High F2 isoprostane levels have been shown both in smokers20 and in diabetic patients.21 Handelman et al. found elevated F2 isoprostane levels in patients on haemodialysis, and a correlation with CRP levels in their patients.19
Recently, Cottone et al. measured F2 isoprostanes in an extensive sample of 626 hypertensive patients in different stages of CKD and found a negative correlation with the degree of renal function.22 Donousi et al.23 also found this correlation in a sample of 87 patients with an ample range of stages from 1 to 4, and so have other authors. However, our study was not capable of showing this relationship, perhaps because the patient sample is smaller or possibly because all patients are in a very advanced stage of renal disease. In fact, one of the noteworthy points of Donousi¿s study is that the F2 isoprostane values in stage 3 patients are higher than in stage 4 patients.
Likewise, we consulted the study by Oberg et al.24 containing 60 patients whose mean glomerular filtration rate was 27.11ml/min, which was also unable to demonstrate a relationship with F2 isoprostane levels.
All of these results suggest the hypothesis that lipid peroxidation takes place during early phases of CKD and remains at high levels while the disease progresses.
DNA is a molecular structure that is especially vulnerable to the attack of reactive types of oxygen, and even more so if we consider the mitochondrial DNA molecule. There are few studies on oxidative damage to genetic material in patients with kidney disease, and most are done with patients on haemodialysis.25,26 One of the modifications that DNA can suffer due to the effects of reactive types of oxygen is the molecule 8-hydroxy-2¿-deoxyguanosine (8-OHdG), which is one of the most abundant. It is known that the hydroxyl radical reacts rapidly with the nucleoside guanosine to produce the molecule 8-hydroxy-dG, which has been suggested as a good marker for estimating the production of that free radical. Other reactive forms of oxygen are likely to intervene in this process, above all in patients in whom there is a marked deficit in catalase enzyme activity, which leads to an increase in H2O availability. Oxidative damage to DNA is capable of inducing premature cellular ageing, as well as an increase in the incidence rate of cancer.27
Tarng demonstrated that the 8-hydroxy-dG content in cell DNA provides a reliable measure of oxidative damage to genetic material in peripheral leukocytes in patients on chronic haemodialysis.23 Curiously, patients on haemodialysis have a higher cancer incidence rate than the general population, although it is true that these patients require special attention, as the technique itself can influence the oxidative balance.28 Watanabe et al. measured 8-hydroxy-dG in patients with advanced renal failure (average GFR 6.4ml/min) and found the highest levels in the group with the lowest values for histidine, an amino acid that could act as a marker for the individual¿s
nutritional state. In a group of hypertensive patients, Redón et al. found elevated levels of both nuclear and mitochondrial 8-hydroxy-dG in peripheral mononuclear cells. This group was unable to establish a correlation between the degree of hypertension and the increase of oxidative markers. The authors think this may be due to the fact that other factors may be present in the hypertensive patient, such as increased angiotensin II or a hyperinsulinaemic state, which could have an effect on stress.29 In our population, we find a general increase in both nuclear and mitochondrial 8-hydroxy-dG, with a variety of significant differences when compared with the control group. As with the study of lipid peroxidation, we did not find any correlation with the deterioration of renal function. We think that this may be due to the convergence of multiple comorbidity factors in these patients. In fact, all of the patients were hypertensive, not to mention that more than 40% were diabetic and that our study involved patients in a very advanced stage of kidney disease.
Lastly, we studied oxidative damage to proteins. Witko-Sarsat et al. identified what they called advanced oxidation protein products (AOPPs) by means of an analogy with the advanced glycation end products (AGE) in patients with CKD in different treatment stages: pre-dialysis, haemodialysis and peritoneal dialysis. Patients in predialysis had the lowest levels out of the three groups, although they were significantly higher than those of a control group of healthy individuals. In our study, we assessed protein oxidation by measuring carbonylated proteins. These molecules are an important protein oxidation marker and reflect the formation of aldehyde groups. Heinecke et al. showed that by means of an oxidative reaction mediated by the myeloperoxidase enzyme, common amino acids could be converted to highly reactive aldehydes,30 which have been shown to play an important role in the creation of atheromatous plaque.31 This author underscores the importance that myeloperoxidasemediated reactions have in contributing to the development of arteriosclerotic plaque. Products deriving from myeloperoxidase-mediated oxidation of the amino acid tyrosine, such as dityrosine or 3-chlorotyrosine have been found as part of oxidised LDL molecules and in arteriosclerosis samples in humans.32 In fact, leukocyte myeloperoxidase levels have been related with an increased risk of suffering coronary disease.33 Our patients present significantly higher carbonylated protein levels than the control group, and as with the rest of the oxidation parameters, there was no correlation shown with MDRD, which is probably due to such reasons as having a small number of patients in the study and the convergence of different pro-oxidation factors. However, other authors have shown a correlation between the products derived from protein oxidation and the decrease in renal function, always with a large number of patients and when considering all renal failure stages.34
The oxidative stress study ended with the analysis of the activity of the main antioxidant enzymes and glutathione. Under normal conditions, the concentration of antioxidants is noticeably higher than the concentration of oxidised products. In this way, the continual production of free radicals, deriving from cell metabolism, is regulated and neutralised by the antioxidants. Effective antioxidant protection requires synchronised action from the three studied enzymes: superoxide dismutase, glutathione peroxidase and catalase. We find the activity of all three enzymes to be significantly reduced in the pre-dialysis group, which reflects the lack of balance between oxidative and antioxidant factors. In a recent study of a sample comparing patients undergoing HD with a control group, Moradi et al. found a decrease of up to 50% in GPx values, along with other antioxidant enzymes and molecules. In addition, they found that an HD session did not normalise those levels.35
RESULTS
Cardiovascular risk history
All of the patients received at least one antihypertensive drug, 65% took two drugs and 12.5% took at least three drugs. Diabetes mellitus was present in 13 patients (40.6%), dyslipidaemia in 21 (65.6%) and nine patients (28%) had a history of ischaemic heart disease. On the other hand, 19 patients (59.4%) took statin drugs as a hypolipaemic treatment, which was considered to be a protective factor.
General biochemistry
The biochemistry values obtained from both groups and the statistical differences between the control group and the predialysis group are shown in table 2.
Inflammatory parameters
CRP and fibrinogen values were significantly elevated in comparison with the control group. CRP: 9.76 ± 2.54mg/dl in the pre-dialysis group vs. 1.57 ± 1.67mg/dl in the control group (p < 0.01); fibrinogen: 4.84 ± 0.29mg/dl in the predialysis group vs. 3.55 ± 0.63mg/dl in the control group (p < 0.01) with a significant correlation between both groups (r: 0.574; p < 0.05) and the CRP with the uric acid value (r: 0.398; p < 0.05).
Oxidative stress
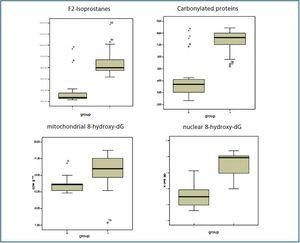
The values for the oxidative stress biomarkers and the differences between both groups are shown in detail in table 3 and figure 1.
Lipid peroxidation
We obtained significant differences between the control and pre-dialysis groups. There was a significant correlation between MDA and HDL-chol (r = 0.406; p < 0.05), but there were no correlations with other oxidative stress parameters.
Protein oxidation
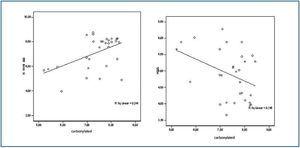
Carbonylated protein values presented a positive correlation with the GSSG/GSH relationship (r = 0.505; p < 0.05) and with nuclear 8-hydroxy-dG (r = 0.489; p < 0.05) (figure 2) and negative with antioxidant enzymes SOD (r = -0.381; p < 0.05) (figure 2) and GSR (r = -0.405; p < 0.05).
Oxidative damage to genetic material
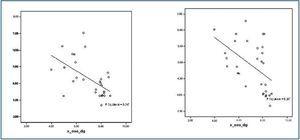
There were significant differences between the study group and the control group for both the nuclear and mitochondrial 8-hydroxy-dG values. 8-hydroxy-dG values correlated significantly with homocysteine (r = 0.305; p < 0.05); lipoprotein (a) (r = 0.375; p < 0.01); mitochondrial 8-hydroxy-dG (r = 0.411; p < 0.05); GSSG/GSH (r = 0.595; p < 0.001) and with carbonylated proteins, as stated above. Additionally, 8-hydroxy-dG values had inverse correlations with the following: total proteins (r = -0.247; p < 0.01); GSH (r = -0.648; p < 0.000) and SOD (-0.497; p < 0.000). (figure 3)
Antioxidant defences
The values for antioxidant enzyme activity for the control and pre-dialysis groups are shown in table 4.
The GSR enzyme showed a significant correlation with GSH (r = 0.552, p < 0.05), SOD (r = 0.396, p < 0.05) and an inverse correlation with nuclear 8-hydroxy-dG (r = -0.563; p < 0.001), GSSG/GSH (r =-0.437, p < 0.05) and carbonylated proteins (r = -0.405; p < 0.05).
The glutathione molecule had a significant inverse correlation with nuclear 8-hydroxy-dG (r = -0.648, p < 0.001), GSSG (r = -0.612, p < 0.001); and carbonylated proteins (r = -0,585, p < 0,001).
GPx had the following correlations: MDA (r = -0.871, p < 0.001) and SOD (r = 0.498, p < 0.005)
SOD was correlated with Nuclear 8-hydroxy-dG (r=-0.497, p < 0.05), MDA (r = -0.459, p < 0.01), GSR (r = 0.396, p < 0.03) and carbonylated proteins.
Relationship with kidney function, diabetes mellitus or statin use
None of the oxidative stress biomarkers showed a significant correlation with MDRD, the presence of diabetes or the use of statin drugs.
CONCLUSION
Despite the limitations of the study, which is an observational transversal study with a limited number of participants, we have observed that there is an important level of oxidative stress in advanced stage CKD patients without dialysis. These findings suggest that oxidative accumulation begins in previous stages of renal failure. Out of all of the studied parameters, the nuclear 8-hydroxy-dG molecule acted as the most reliable marker.
Table 1. Characteristics of the groups under study
Table 2. Biochemical parameters
Table 3. Oxidative parameters
Figure 1.
Figure 2.
Figure 3.
Table 4. Antioxidant molecules