Lupus nephritis (LN) is the first manifestation of systemic lupus erythematosus (SLE), and it determines the prognosis of patients. Around 30% of cases do not achieve a complete response, and 10–30% of cases progress to end-stage renal disease within 10 years.1 We present two cases of refractory LN treated with voclosporin, a calcineurin inhibitor (ICI) approved in Spain in 2024 for proliferative or mixed LN, in combination with mycophenolate mofetil (MMF).2,3
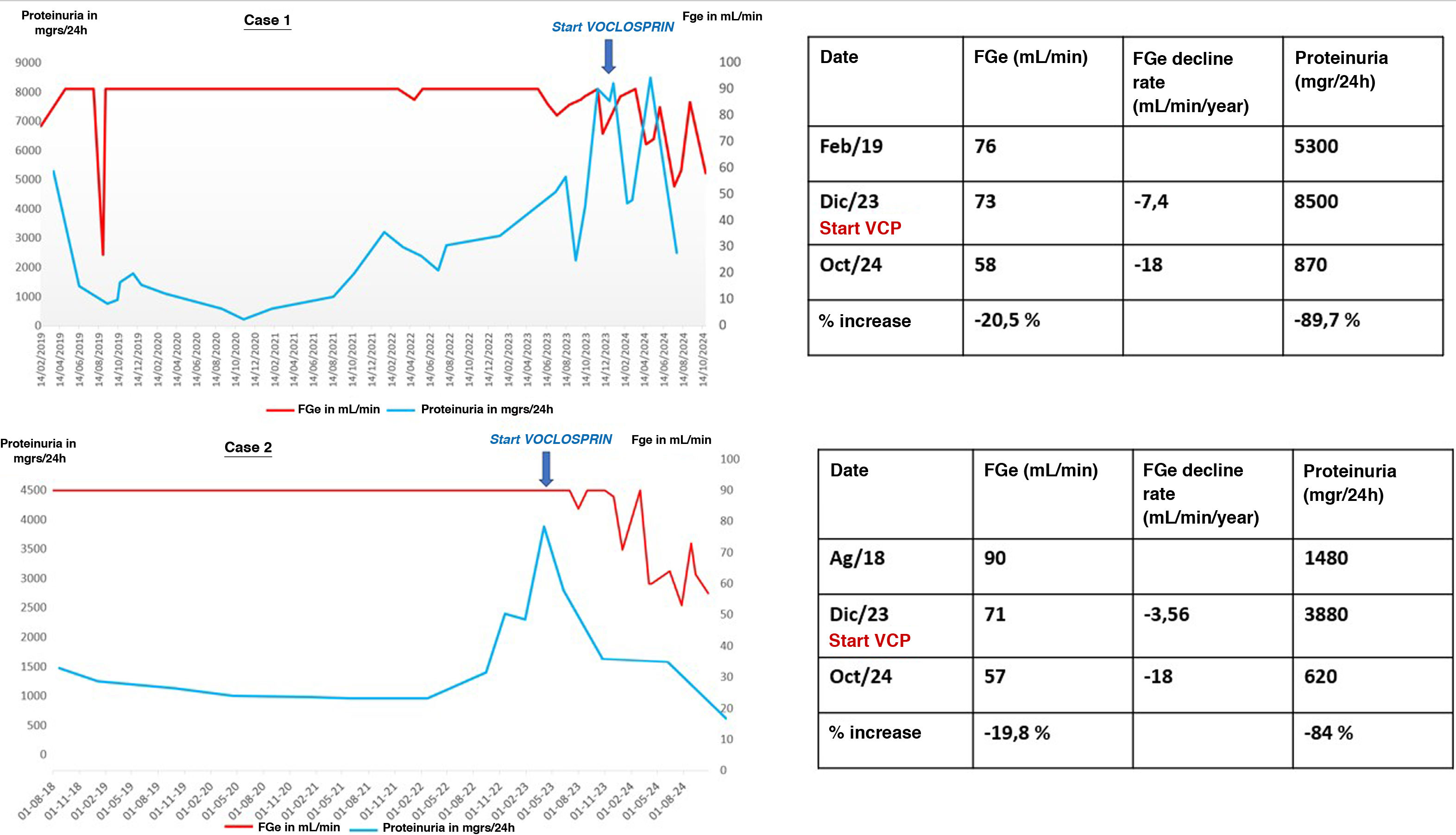
The first case is a 36-year-old Venezuelan woman with a history of hypertension (HTN) that began in 2014 during postpartum with hand edema, Raynaud’s syndrome, dry syndrome, leukopenia, thrombocytopenia, and hypocomplementemia with positive antinuclear autoantibodies (ANA), anti-U1-RNP, SSa, SSb, Sm and antiphospholipid antibodies. She began treatment with corticosteroids, hydroxychloroquine and azathioprine. Since then, she developed various complications, including nephrotic proteinuria with preserved glomerular filtration rate (GFR) in 2016 and pleuropericarditis in 2017. She moved to Spain and began follow-up at our center. A renal biopsy was performed with a diagnosis of class V LN. Between 2019 and 2023, due to various clinical and infectious complications and the absence of renal remission, she underwent various treatments that failed to induce remission (Table 1). A new renal biopsy was performed with a result of LN class III + V with an activity index of 5/24 and chronicity of 5/12. It was decided to start anifrolumab and voclosporin at an initially reduced dose to minimize the risk of infection, increasing to the full dose three months later after confirming tolerance and the absence of infectious complications. During this period, a decrease in disease activity was achieved (anti-DNA negative, lymphopenia resolved and hypocomplementemia to a lesser degree), with proteinuria greater than 89% (Fig. 1), meeting DORIS remission criteria4 and allowing a significant reduction in corticosteroid dose. The only notable complication is a recurrence of pyoderma gangrenosum, which was successfully treated with skin micrografts. She is currently continuing treatment with anifrolumab, MFM, voclosporin and prednisone.
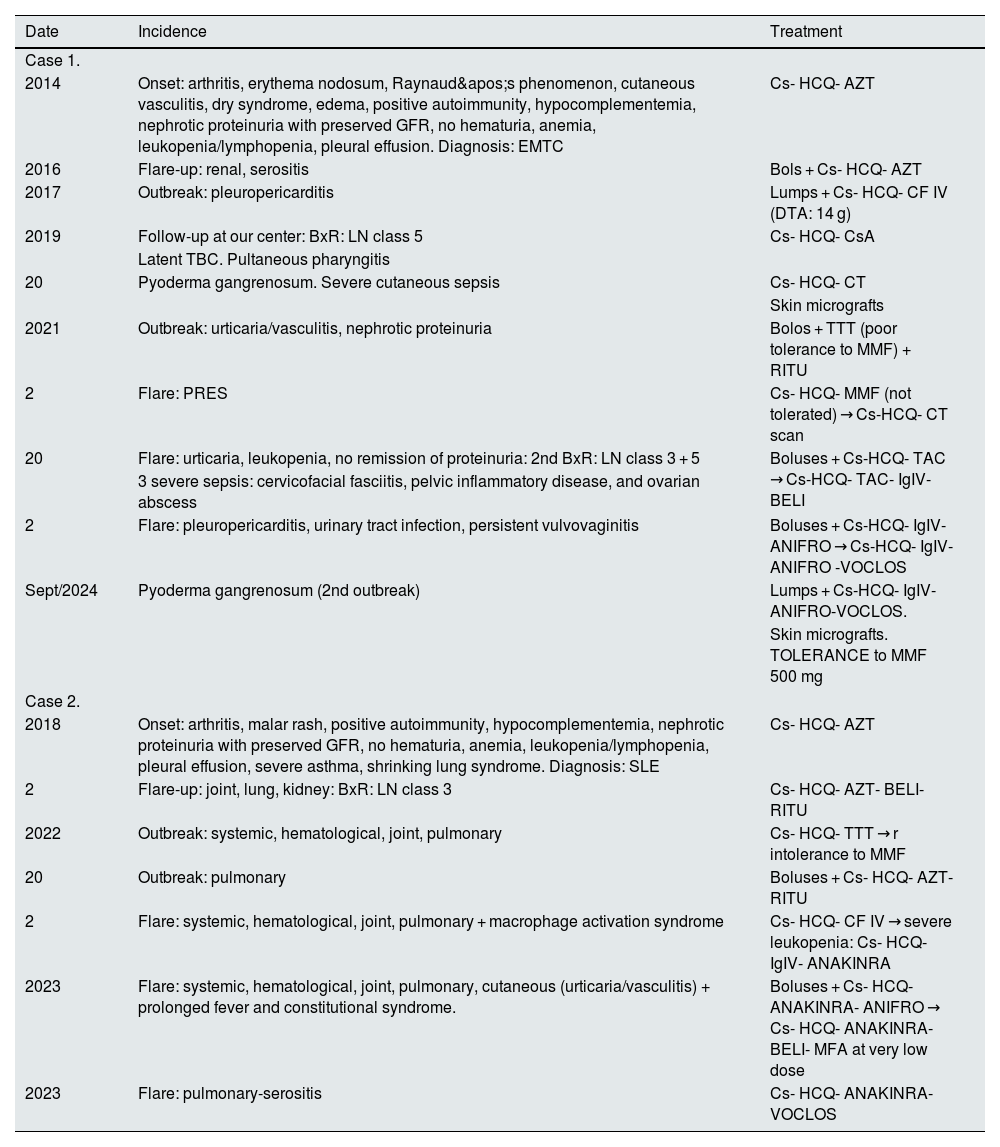
Clinical and treatment evolution of cases 1 and 2.
| Date | Incidence | Treatment |
|---|---|---|
| Case 1. | ||
| 2014 | Onset: arthritis, erythema nodosum, Raynaud's phenomenon, cutaneous vasculitis, dry syndrome, edema, positive autoimmunity, hypocomplementemia, nephrotic proteinuria with preserved GFR, no hematuria, anemia, leukopenia/lymphopenia, pleural effusion. Diagnosis: EMTC | Cs- HCQ- AZT |
| 2016 | Flare-up: renal, serositis | Bols + Cs- HCQ- AZT |
| 2017 | Outbreak: pleuropericarditis | Lumps + Cs- HCQ- CF IV (DTA: 14 g) |
| 2019 | Follow-up at our center: BxR: LN class 5 | Cs- HCQ- CsA |
| Latent TBC. Pultaneous pharyngitis | ||
| 20 | Pyoderma gangrenosum. Severe cutaneous sepsis | Cs- HCQ- CT |
| Skin micrografts | ||
| 2021 | Outbreak: urticaria/vasculitis, nephrotic proteinuria | Bolos + TTT (poor tolerance to MMF) + RITU |
| 2 | Flare: PRES | Cs- HCQ- MMF (not tolerated) → Cs-HCQ- CT scan |
| 20 | Flare: urticaria, leukopenia, no remission of proteinuria: 2nd BxR: LN class 3 + 5 | Boluses + Cs-HCQ- TAC → Cs-HCQ- TAC- IgIV-BELI |
| 3 severe sepsis: cervicofacial fasciitis, pelvic inflammatory disease, and ovarian abscess | ||
| 2 | Flare: pleuropericarditis, urinary tract infection, persistent vulvovaginitis | Boluses + Cs-HCQ- IgIV-ANIFRO → Cs-HCQ- IgIV-ANIFRO -VOCLOS |
| Sept/2024 | Pyoderma gangrenosum (2nd outbreak) | Lumps + Cs-HCQ- IgIV-ANIFRO-VOCLOS. |
| Skin micrografts. TOLERANCE to MMF 500 mg | ||
| Case 2. | ||
| 2018 | Onset: arthritis, malar rash, positive autoimmunity, hypocomplementemia, nephrotic proteinuria with preserved GFR, no hematuria, anemia, leukopenia/lymphopenia, pleural effusion, severe asthma, shrinking lung syndrome. Diagnosis: SLE | Cs- HCQ- AZT |
| 2 | Flare-up: joint, lung, kidney: BxR: LN class 3 | Cs- HCQ- AZT- BELI- RITU |
| 2022 | Outbreak: systemic, hematological, joint, pulmonary | Cs- HCQ- TTT → r intolerance to MMF |
| 20 | Outbreak: pulmonary | Boluses + Cs- HCQ- AZT- RITU |
| 2 | Flare: systemic, hematological, joint, pulmonary + macrophage activation syndrome | Cs- HCQ- CF IV → severe leukopenia: Cs- HCQ-IgIV- ANAKINRA |
| 2023 | Flare: systemic, hematological, joint, pulmonary, cutaneous (urticaria/vasculitis) + prolonged fever and constitutional syndrome. | Boluses + Cs- HCQ- ANAKINRA- ANIFRO → Cs- HCQ- ANAKINRA- BELI- MFA at very low dose |
| 2023 | Flare: pulmonary-serositis | Cs- HCQ- ANAKINRA- VOCLOS |
ANIFRO: anifrolumab; AZT: azathioprine; BELI: belimumab; BOLUS: 3 doses of methylprednisolone 250 mg; BxR: renal biopsy; Cs: corticosteroids (prednisone); CFIV: intravenous cyclophosphamide; CsA: cyclosporine A; DTA: total accumulated dose; EMTC: mixed connective tissue disease; HCQ: hydroxychloroquine; IgIV: intravenous immunoglobulins; MFA: mycophenolic acid; MMF: mycophenolate mofetil; NL: lupus nephropathy; PRES: posterior reversible encephalopathy syndrome; RITU: riruximab; TAC: tacrolimus; Tbc: tuberculosis; TTT: triple therapy; VOCLOS: voclosporin.
The second case is that of a 44-year-old Venezuelan woman with a history of hypothyroidism, asthma and iron deficiency anemia of gynecological origin who in 2016 presented with arthritis, cutaneous lupus, shrinking lung syndrome, nephrotic proteinuria with preserved GFR, hypocomplementemia and positive ANA with anti-DNA. She began treatment with corticosteroids, hydroxychloroquine and azathioprine. In 2020, she began follow-up at our center for arthritis, pleuropericardial serositis and proteinuria. A renal biopsy showed class III LN (chronicity index 2/12). Since then, several severe systemic and renal flare-ups have occurred despite multiple treatments (Table 1). In 2022, she began to experience macrophage activation syndrome. Anakinra was added to the treatment with good systemic response, but renal refractoriness persisted, so in November 2024, treatment with voclosporin and low-dose MFM was initiated due to digestive intolerance. Proteinuria decreased by 84% in the following 3 months (Fig. 1) and complete renal and system l remission was achieved except for biological activity data (lymphopenia, high DNA titers and hypocomplementemia) with decreasing corticosteroid doses.
In both cases, a rapid and sustained decrease in proteinuria, a good tolerance profile, metabolic control and safety were observed, and the dose of corticosteroids could be reduced. At the same time, a decrease in GFR of around 20% was observed due to functional and reversible circumstances.
Voclosporin is a calcineurin inhibitor (CNI) with a structure similar to cyclosporine A, modified in a functional group of the amino acid residue 1, which gives it greater binding power to calcineurin and linear kinetics that do not require monitoring of plasma levels. It acts on the lymphocyte activation process by inhibiting the calcineurin-dependent NF-κB pathway, which leads to a decrease in cytokine synthesis, including interleukin-2, reduced lymphocyte proliferation, and stabilization of the podocyte cytoskeleton and slit diaphragm architecture through its action on synaptopodin.5 In the Aurora 1 and Aurora 2 studies, complete renal remission was achieved in 41% and 50.9% of patients, with a higher risk of developing HTN and a reversible and functional decrease in GFR.6–9
We present two cases of complex LN, with multiple previous treatment regimens that have been ineffective and accompanied by severe systemic infectious and renal complications, in which voclosporin has improved disease control, reducing the need for corticosteroids, significantly improving proteinuria, and reducing systemic complications, despite a decline in GFR of around 20%. We have only found one published case of voclosporin in combination with anifrolumab.10
In short, the addition of voclosporin to the therapeutic arsenal for LN opens up a promising treatment option for patients with significant proteinuria, which should be confirmed in the future.
FundingThis article has no sources of funding.
The authors declare that they have no conflicts of interest.