Hyponatremia caused by inappropriate secretion of antidiuretic hormone (SIADH) is not uncommon in patients with cancer, in relation with the tumor or with chemotherapy. Drugs classically associated with SIADH are cyclophosphamide (intravenous, high dose), vincristine, vinblastine, vinorelbine, cisplatin, melphalan, ifosfamide and methotrexate.1
In multiple myeloma (MM), hyponatremia secondary to SIADH is not frequent2,3 and is usually due to one of the indicated drugs. During the last decade new drugs have been introduced as bortezomib, a proteasome inhibitor, in which hyponatremia has been described as a rare side effect during the clinical development, prior to authorization, tested in various chemotherapy regimens. In the post-authorization stage, SIADH-related cases are extremely rare when used alone or associated with dexamethasone (Bor-D).4,5
Here we describe a case of light chain MM treated with Bor-D that undergoes a complete response with disappearance of light chains in the 24-hour urine collection and develops an early SIADH.
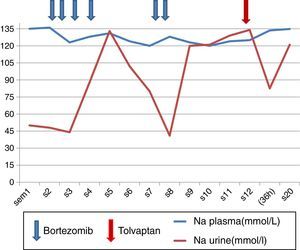
A 81-year-old female with preserved general functions, which begins with a pain in the lumbar region of one month duration that reveals a pathological fracture in L3. As a previous history, hypertension controlled with ACE inhibitors, stable ischemic heart disease, antiaggregated and on beta-blockers. The physical examination shows an adequate nutritional status and euvolemic. Complementary tests reveal a discrepancy between proteinuria quantified by quotient and that obtained in elemental urine, which is confirmed by elimination of light chain kappa type of 3150μg/24h; Preserved renal function, mild anemia and absence of serum paraprotein. Bone marrow aspirate demonstrates infiltration by clonal plasma cells, and lumbar MRI plasmocitomas in D11 and L3. Diagnosed with MM, treatment with Bor-D is initiated, with no changes in its usual medication. Receives a first cycle on days 1, 4, 8 and 11 with good clinical tolerance. From day +8 the patient begins to show disorientation, drowsiness, lethargy and asthenia and a true hyponatremia was documented with elevated urine osmolarity, and normal adrenal and thyroid hormone studies. The evolution of plasma Na (Nap) and urinary Na during the first cycle (4 first doses) and half of the second cycle (5 and 6 doses) are shown in Fig. 1. After the 6th dose, it was decided to discontinue treatment to evaluate a causal relationship with SIADH, in the absence of other drugs, other causes being ruled out and considering the criteria of temporal sequence.
In spite of discontinuation of the treatment, an early and complete response was obtained with elimination of light chains in 24h urine collection practically undetectable at week3 of Bor-D initiation (Table 1). After water restriction she is discharged, without completely normalizatiom the Nap (cations urine/plasma: 0.5–1), with remission of the described symptoms. Readmitted 2 months later, because disorientation and myoclonus. On physical examination the patient remains euvolemic, without hypotension or presence of edema. Hyponatremia persists (120mEq/l) with increased urine osmolarity, and again normal thyroid and adrenal hormonal study. Cranial CT without findings. The water restriction (cations urine/plasma >1) does not correct the Nap value. At that time, treatment with tolvaptan was initiated at a dose of 15mg/day and NaP was corrected within 36h (Fig. 1) without complications, and the symptomatology disappeared. The treatment is maintained intermittently for about 4 weeks and is subsequently discontinued, with no reoccurrence of symptoms, despite the fact that the Nap figures have remained at the lower limit of the normal range.
Biochemical data at baseline and post-bortezomib treatment: Plasma and urinary Na, urinary K (mmol/l,), plasma Cr, plasma urea, uric acid (mg/dl), osmolality in blood and urine (mOsmol/kg) and light chains in urine (g/24h).
| Pre-BOR | Post-BOR 1.° | Post-BOR 2.° | |
|---|---|---|---|
| Plasma Na | 135 | 123 | 120 |
| Plasma osmolarity | – | 268 | 260 |
| Na/K urine Urine osmolarity | – | 88/55/606 | 41/46/298 |
| Plasma Cr | 0.84 | 0.34 | 0.44 |
| Plasma urea | 36 | 8 | 19 |
| Light chains in urine | 3.1 | 0.13 | 0.11 |
| Plasma uric acid | 5.1 | 2.2 | 2 |
The mechanism of development of an anti-tumor drug-related SIADH is not clearly defined, although it is suspected that there is through stimulation of vasopressin release, or by potentiating the antidiuretic effect of vasopressin through a direct tubular effect. Symptomatic hyponatremia is an effect that limits the dose of the treatment. Bortezomib is a protease inhibitor authorized in April 2005 by the EMEA as a second-line drug in the treatment of MM. Limiting side effects were peripheral sensory neuropathy such as paresthesia or pain that can occur up to 30–40% and it is reversible in most cases. Also thrombocytopenia occurring in up to 30% of cases. More common non-serious effects are weakness, fatigue and GI disorders.6 Hyponatremia is regarded as infrequent. Post-authorization we have found only 2 references of SIADH attributed to bortezomib. Paydas presents a case of MM IgG kappa, treated with bortezomib, melphalan and prednisone. The picture unfolds similarly to ours on the 8th post-treatment day. It is solved with the usual handling. Retrospectively this author demonstrates by immunohistochemical positivity to ADH in monoclonal plasma cells, concluding as a developmental mechanism an equivalent to tumor lysis syndrome. The second case is a patient diagnosed with a mantle lymphoma in which hyponatremia (112mEq/l) develops late (+43 days after initiation of Bortezomib), requiring treatment suspension.4,5 In our case, we did not performed the immunohistochemical study and we do not have vasopressin determination that would have helped to clarify the mechanism, although the prolonged duration of hyponatremia that our patient experienced is against the mechanism proposed by Paydas. This case of SIADH is considered a rare complication of treatment with bortezomib, that may force the suspension of the drug and to consider tolvaptan as an effective therapeutic alternative applied on a temporary basis.
Please cite this article as: Carreño A, Hernández B, Mayoralas Á, Calle C. Síndrome de secreción inadecuada de hormona antidiurética (SIADH) secundario a bortezomib en mieloma múltiple de cadenas ligeras. Tratamiento con tolvaptán. Nefrologia. 2017;37:558–559.