Tolvaptan (Jinarc®) is a vasopressin V2 receptor antagonist whose action leads to a decrease in intracellular cAMP levels.1
This drug is indicated for patients aged 18–60 years diagnosed with autosomal dominant polycystic kidney disease (ADPKD)2–4 in cases of rapid progression and CKD stages 1–4.5,6
It first became available on the European market in 2015 following the promising results of the TEMPO 3:4 clinical trial.7 Dosage is divided into two daily intakes, starting at 45 + 15 mg, with progressive increase up to the full dose of 90 + 30 mg,1 maintaining the highest dose tolerated by the patient and monitoring the main side effects: hepatotoxicity and polyuria.8
There are no published cases in the literature identifying toxicoderma as an adverse effect.
We present the case of a 45-year-old woman being followed up for ADPKD since 1992. Her previous personal history included being a former smoker, with no other unhealthy habits, and no known drug allergies. Regarding previous illnesses, she had high blood pressure on dietary treatment and dyslipidaemia being treated with statins.
Her family history included her mother being diagnosed with ADPKD and starting renal replacement therapy (RRT) at the age of 48, and a sister also affected by the disease who had been on peritoneal dialysis since the age of 49.
Magnetic resonance imaging9 was performed in April 2018, fulfilling the criteria for Mayo Clinic class 1E rapid progression5. Therefore, in June 2018, treatment was started with tolvaptan at a dose of 45–15 mg every 12 h, with normal liver function tests prior to starting the drug, with a glomerular filtration rate (GFR) on starting the drug of 53 ml/min/1.73 m2.
In September 2018, the dose was increased to 60–30 mg every 12 h, and in November 2018, the full dose of 90–30 mg was initiated, with no clinical or analytical adverse effects of the drug having been reported so far.
In December 2018, the patient presented with a skin rash accompanied by palpitations, without associated respiratory distress, so it was decided to discontinue the drug.
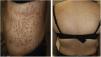
The lesions consisted of pruritic erythematous papules, some with mild hyperkeratosis, on the trunk, upper extremities and face. A skin biopsy was performed as indicated by dermatology and treatment was started with Adventan® emulsion (Fig. 1).
Skin biopsy showed mounds of parakeratosis, mild spongiosis, minimal very focal lymphocytic exocytosis on a dermis with a mild superficial perivascular lymphocytic infiltrate (Fig. 2).
She was assessed by allergology and the condition was attributed to a possible allergy to tolvaptan, with disappearance of the lesions in May 2019. The manufacturing pharmaceutical company was notified of the pharmacological alert.
At the patient's request, with the consensus of a multidisciplinary session and taking into account the benefit of the drug in disease progression, it was decided to reintroduce the drug at low doses (45–15 mg), with close monitoring by the different medical specialties. In August 2019, lesions reappeared on the patient's forehead and cheeks, so the dose was reduced to 30–15 mg.
Low doses of the drug are currently being maintained, with control of skin lesions and stable renal function (creatinine 1.06 mg/dl and urea 37 mg/dl, GFR 61 ml/min).
Skin reaction is an extremely rare adverse effect in patients treated with tolvaptan, and no similar case has been reported in the literature to date.
In our opinion, in addition to the exceptional rarity of the case, two essential points should be highlighted: 1) the importance of a multidisciplinary approach in this type of patient (joint assessment by dermatology, allergology and nephrology, with the participation of the hepatology and gastroenterology departments also being common) to properly assess the risk/benefit ratio of using the drug; and 2) it should also be emphasised that, even in unusual situations such as this, treatment can be administered at lower doses than those established to maintain the beneficial effect of the drug.
We believe that being able to continue with tolvaptan preserves the principle of patient autonomy and will result in a better prognosis of long-term renal function.
In conclusion, the drug has been maintained at a low dose, with good tolerance and sporadic appearance of minimal skin lesions (one or two), with close dermatological follow-up.
FundingNo funding was received.
Conflicts of interestThere are no conflicts of interest.