Introduction: The Notch signalling pathway is activated in a wide variety of human renal diseases. We have recently demonstrated that the activation of this pathway is not involved in experimental renal fibrosis induced by angiotensin II or hypertension. Objectives: To assess whether the Notch pathway is activated in renal fibrosis related to hypertensive nephrosclerosis. To test the hypothesis, various glomerular diseases characterised by tubulointerstitial fibrosis were analysed. Method: Renal biopsies were performed on patients with hypertensive nephrosclerosis, in comparison with diabetic nephropathy and membranous nephropathy at various stages. Gene and protein expression were evaluated by in-situ hybridisation and immunohistochemistry respectively. Results: In hypertensive nephrosclerosis low renal expression of notch-related proteins was observed. There was no link between tubulointerstitial fibrosis and the levels of these proteins. By contrast, in the glomerular diseases studied we observed high expression of the transcripts Jagged-1, HES-1 and TGF-β and the proteins Jagged-1 y Notch-1, localised primarily in tubuloepithelial cells. The levels of expression of the components of the Notch pathway correlate to the degree of tubulointerstitial fibrosis, which confirms the activation of this pathway in progressive nephropathies. Conclusions: Our data demonstrate that the Notch pathway is not activated in the kidneys of patients with hypertensive nephropathy, which extends the results of experimental models of kidney damage related to hypertension to the realm of human pathology. Our studies provide new information on the complex regulation of the Notch pathway in the kidney.
Introducción: La ruta de señalización de Notch está activada en una gran variedad de patologías renales humanas. Recientemente hemos demostrado que la activación de esta ruta no estaría implicada en la fibrosis renal experimental inducida por angiotensina II o hipertensión. Objetivos: Evaluar si la vía Notch está activada en la fibrosis renal asociada a nefroesclerosis hipertensiva. Para validar la hipótesis se estudiaron varias patologías glomerulares caracterizadas por fibrosis túbulo-intersticial. Métodos: Se utilizaron biopsias renales de pacientes con nefroesclerosis hipertensiva, en comparación con nefropatía diabética y nefropatía membranosa en diferentes etapas de progresión. La expresión génica y proteica se evaluó por hibridación in situ e inmunohistoquímica, respectivamente. Resultados: En nefroesclerosis hipertensiva se observó baja expresión renal de proteínas de la vía Notch, no existiendo asociación entre la fibrosis túbulo-intersticial y los niveles de estas proteínas. Por el contrario, en las patologías glomerulares estudiadas se observó una elevada expresión de los transcritos Jagged-1, HES-1 y TGF-β, y de las proteínas Jagged-1 y Notch-1, localizados principalmente en células túbulo-epiteliales. Los niveles de expresión de los componentes de la vía Notch se relacionaron con el grado de fibrosis túbulo-intersticial, lo que confirma la activación de esta vía en nefropatías progresivas. Conclusiones: Nuestros datos muestran que la vía Notch no está activada en el riñón de pacientes con nefropatía hipertensiva, ampliando los resultados de los modelos experimentales de daño renal asociado a hipertensión a la patología humana. Nuestros estudios aportan nueva información sobre la compleja regulación del sistema Notch en el riñón.
INTRODUCTION
The Notch signalling pathway is involved in cellular proliferation, differentiation and apoptosis processes1,2. The members of this pathway include Notch receptors 1/2/3/4, which have two subunits not covalently linked, and the Jagged 1,2 and delta-like 1,3,4 ligands3. The Notch pathway is activated following the interaction of the Notch receptor and its binder, which causes the proteolytic cut of the transmembrane section of the Notch receptor, via the γ-secretase enzyme. This release the intracellular section of the receptor, called the Notch intracellular domain (NICD). This active domain migrates to the nucleus, binds to the RBP-Jκ transcription factor (recombination signal-binding protein 1 for J-kappa) and activates the target genes, including HES (hairy/enhancer of split) and HERP (HES-related transcription factor-1), which are transcriptional repressors that act as negative effectors of the pathway1.
The Notch pathway is used by multicellular organisms to specify cell fate during the formation of complex structures, including the kidney4, and participates in physiological and pathological processes, such as cancer5, the function and regeneration of the vasculature1, endothelial cell6 differentiation and angiogenesis7. In the kidney, Notch pathway activation in podocytes and renal progenitor cells associated with pathological processes in glomerulonephritis has been described8. In addition, experimental model studies show that the over-expression of Notch in podocytes is associated with albuminuria and glomerusclerosis9,10. In renal biopsies of patients with diabetic nephropathy (ND) we have described an increase in Jagged-1 and HES-1 gene expression11. In an extensive study by Suztak et al., an increase in various components of the Notch pathway was observed in progressive nephropathies12. However, the beneficial effect of modulation of the Notch pathway in kidney disease progression remains controversial9,13,14.
We have recently demonstrated that the Notch pathway activation is not involved in experimental renal fibrosis induced by angiotensin II (Ang II) or hypertension15. Our objective has been to try to transfer the results obtained in experimental models to the realm of human pathology, evaluating whether the Notch pathway is activated in patients with hypertensive nephropathy (HN), comparing them with progressive nephropathies exhibiting different degrees of tubulointerstitial fibrosis.
MATERIAL AND METHOD
Patient samples
The study was based on the retrospective analysis of renal biopsies studied in the Nephrology Department of the Instituto de Medicina de la Universidad Austral de Chile, Valdivia (Chile). The renal biopsies obtained by percutaneous puncture were carried out as a diagnostic and/or prognosis procedure, and with the patient’s informed consent. There were no significant differences in the most relevant clinical data between the HN group and the ND and membranous nephropathy (MN) groups. The concrete patient data from whom the clinical information was taken is as follows: HN: 4 males/6 females; average age: 49.2 years; estimated glomerular filtration rate (eGFR): < 60ml/min/1.73m2 = 4/8; tubulointerstitial fibrosis score 1. Patient data DN + MN = 15 males/9 females; average age: 53.5 years; eGFR <60ml/min/1.73m2 = 11/19, tubulointerstitial fibrosis score 1.69. With respect to pharmacological treatment, the majority of patients in all the groups studied were receiving angiotensin-converting enzyme inhibitors (ACE inhibitors).
The renal biopsies are from patients with histopathological diagnosis of HN (n = 10), with DN (n = 8) and with MN. These cases were divided into patients with non-progressive MN (np-MN; n = 8, absent or slight tubulointerstitial fibrosis) and with progressive MN (p-MN; n = 8, moderate or severe tubulointerstitial fibrosis). As control tissues, biopsies from patients with minimal changes disease (MGL; n = 5) were used, pathology which does not present tubulointerstitial fibrosis. Interstitial fibrosis, defined by Masson’s trichrome technique (which detects the presence of interstitial collagen) and the tubulointerstitial cellular infiltration were classified into four groups, according to the extension and presence of atrophy and tubular degeneration: (0) normal, (1) affecting up to 25 % of the cortex, (2) affecting 25 % to 50 % of the cortex, and (3) extensive damage to more than 50 % of the cortex, as described previously in other studies16. All the biopsies were evaluated by two pathologists, whose readings coincided, without prior knowledge of other studies carried out on these biopsies.
The renal tissue obtained was fixed in formalin 4 % in phosphate buffer and/or Bouin solution. It was then dehydrated and embedded in paraffin following the conventional histological technique. For the development of the techniques described, a series of cuts of 5 μm thickness were made, which were mounted on microscope slides previously treated with 2 % aminopropiltrietoxisilane.
In-situ hybridisation
In order to evaluate the levels of gene expression, in-situ hybridisation, as previously described, was used17. The hybridisation reaction was carried out overnight at 37 ºC using biotin-stained probes (200ng/ml). The detection was made with avadin-alkaline phosphatase conjugate, using NBT/BCIP (nitro bluetetrazolium/5-bromo-4-chloro-3-indolyl phosphate) as the enzyme substrate. The reaction specificity was confirmed by RNase treatment (100mg/ml) or by absence of the probe.
Probes used for Jagged-1 detection (Sigma):5’-CCTGACAGTATTATTGAAAAGGCT-3’, 5’-GTACGGCTGGCAAGGCTTGTACTG-3’, 5’-CACGCCTGCCTCTCTGATCCCTGT-3’.
Probes used for HES-1 detection (Sigma): 5’-CTTCTCTCCTTGGTCCTGGAACAG-3’, 5’-AGCTCGCGGCATTCCAAGCTGGAG-3’, 5’-CTGCGCTGAGCACAGACCCAAGTG-3’.
Probe used for TGF-β detection (MaxinBiotechInc): Biotinylated probe of 163pb, made by PCR using a human cDNA and biotinylated dNTPs.
Immunohistochemistry
Immunohistochemistry was used to evaluate the levels of protein expression, using the following primary antibodies: anti-Jagged-1 (1:50, Santa Cruz), active anti-Notch-1 (1:300, Abcam) and anti-α-SMA (1:50, Dako). For this, endogenous peroxidase was blocked in renal sections using 3 % H2O2 for 20 minutes. The tissue was treated in microwaves with a citrate buffer solution 0.1mM 6.0 pH at 94 ºC for 10 minutes. Secondary biotinylated antibodies were used, followed by HRP-conjugate and revealed with diaminobenzidine. The reaction specificity was determined by primary antibody omission or the use of a non-immune serum.
Statistical analysis
Statistical analysis was carried out using GraphPadInstant software (GraphPadSoftware, San Diego, CA). The evaluation of the intensity and distribution of the staining for immunohistochemistry and in-situ hybridisation was determined using computerised analysis of images, with no knowledge of the group that the sample belonged to, and expressed as mean ± SEM. The Mann-Whitney test was used to determine significant differences between the analysed groups. A P value of <.05 was considered to be statistically significant. The Spearman correlation was used to relate Jagged-1, HES-1 and TGF-β expression and tubulointerstitial fibrosis. A P value of <.01 was considered to be statistically significant.
RESULTS
The Notch pathway in hypertensive nephrosclerosis
The presence of the Notch ligand, Jagged-1, in patients with HN was evaluated using immunohistochemistry. Low levels of Jagged-1 expression were observed in those patients (Figure 1A). For the validation of the results and the technique used, we studied, in parallel, patient samples with DN. np-MN and p-MN, where renal activation of the Notch pathway was previously described13. In these samples, a significant increase in Jagged-1 expression at the cytoplasmic level of tubular cells was observed (Figure 1A). It is worth noting that in patients with MCD, positive staining of Jagged-1 was barely observed, presenting values similar to those of HN (Figure 1B).
NICD was released following the binding of the ligand (Jagged-1) to the Notch receptor, which translocated to the nucleus and acted as a transcription factor.
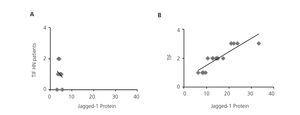
Previous studies have demonstrated that Notch pathway activation is associated with the progression of kidney disease in a large number of disorders, including DN11,12, but there is no clear data on HN. Therefore, we studied the possible correlation between renal fibrosis and Notch pathway activation, analysing serial biopsies from the patient group. In the HN samples, no correlation was found between the degree of tubulointerstitial fibrosis and the intensity of Jagged-1 protein expression (Figure 3A), presenting Jagged-1 values similar to MCD, nephropathy that was not associated with tubulointerstitial fibrosis. By contrast, there was a significant positive correlation on the evaluation of the rest of the study samples (Figure 3B). These results suggest that the activation of the Notch Pathway in HN is not related with the progression of the disease.
The Notch pathway is activated in progressive membranous nephropathy associated with fibrosis
In order to determine the relationship between the Notch pathway and fibrosis in chronic kidney disease and building on previous studies11,12, we evaluated the gene expression of Jagged-1 and HES-1 in patients with p-MN and np-MN, and compared their expression with the control tissue. In the np-MN and p-MN samples there was a marked induction of mRNA Jagged-1 and mRNA HES-1 at the tubular level. In contrast, in patients with MCD, used as controls as they did not present fibrosis, their expression was low or absent (Figure 4A).
Experimental studies have shown that TGF-β is a key growth factor in renal fibrosis. In patients with progressive kidney disease we have previously described that the TGF-β transcript expression is increased18. In Figure 4A, the co-localisation of mRNA of Jagged-1, HES-1 and TGF-β is observed in all the analysed MN cases. Progressive cases show a greater increase in mRNA expression of Jagged-1, HES-1 and TGF-β, compared with non-progressive cases (Figure 4B). In addition, a significant positive correlation between the degree of tubulointerstitial fibrosis and each transcript analysed was found (Figure 4C). We also evaluated the expression of the mesenchymal marker α-smooth muscle actin (α-SMA) and the presence of interstitial collagen in the tissue samples. In the α-SMA control tissue, it was only present in arterioles and there was no evidence of collagen. In np-MN, an increase in α-SMA expression and collagen in the tubulointerstitial area was observed, and with greater extension in p-MN (Figure 4A). Our findings from renal biopsies of patients with p-MN and np-MN show a relation between Jagged-1 induction and tubulointerstitial damage. This suggests that Jagged-1 could participate in fibrosis regulation in the kidney.
DISCUSSION
From biopsies of HN patients, with different degrees of tubulointerstitial fibrosis, we have shown that the Notch pathway is not activated in the kidney. This builds on and confirms our previous in vitro studies and studies in animal models of hypertension and damage mediated by Ang II15, transferring to human pathology the hypothesis that Ang II does not regulate the Notch pathway in the adult kidney.
Ang II actively participates in the progression of kidney damage, as it is able to regulate various pathological processes, such as inflammation and fibrosis. Drugs that inhibit Ang II, ACE inhibitors and Ang II receptor antagonists, are one of the best therapeutic strategies for the treatment of progressive kidney diseases. This is due to their actions beyond the control of blood pressure, providing end-organs protective effects18. Recently, we have described how the Notch pathway is not activated in response to Ang II in renal cell cultures, including podocytes, epithelial tubular cells and fibroblasts. This demonstrates a key difference between the mechanisms activated by TGF-β and Ang II in the kidney15. Previous studies suggest functional interactions between TGF-β and the Notch signalling pathway19. Both signalling pathways are important for cellular differentiation control during development and Jagged-1 expression has been described as dependent on TGF-β in epithelial cells20,21. Various studies have demonstrated that the Notch pathway is essential for epithelial function and also contributes in epithelial-mesenchymal transition (EMT) in embryogenesis and cancer22,23. Studies on tubuloepithelial cell cultures have shown that the pharmacological blocking of this pathway, inhibiting the γ-secretase enzyme, restored the changes in EMT markers induced by TGF-β20,24. However, it does not modulate the ETM caused by Ang II15. The contribution of ETM in renal fibrogenesis is intensely debated24. Consequently, the renal over-expression of Notch in kidney damage models did not modulate ETM markers, although it caused tubulointerstitial fibrosis13. In this study, we have demonstrated that the Notch pathway is not activated in the kidney of HN patients, extending the result observed in experimental models of hypertension and AngII-induced renal damage to the human hypertensive disease.
Various experimental studies have observed that the use of pharmacological inhibitors or soluble Notch ligands mitigates kidney failure9,25. In addition, studies on transgenic mice with Notch deletion at the podocyte level have demonstrated the involvement of this pathway in tubulointerstitial fibrosis13. Experiments carried out on patient samples with p-MN show that Jagged-1 and HES-1 activation are linked with the degree of tubulointerstitial fibrosis and with the increase of TFG-β. This confirms the earlier extensive study that showed Jagged-1, Jagged-2 and Notch-1 activation in progressive human nephropathies12, as well as the previous results of our group in DN11.
Overall, our results show the complex regulation of the Notch pathway in the kidney, confirming the association between Notch pathway activation and renal fibrosis in progressive human pathologies. Currently there are clinical studies using γ-secretase inhibitors in a range of diseases, such as Alzheimer’s disease and leukaemia. As a result, it could also be a new therapeutic target for chronic kidney disease25. However, the fact that Ang II does not regulate this pathway and that it is not activated in patients with hypertension demonstrates the complexity of the Notch system regulation in the adult kidney.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Acknowledgements
This study was financed by the Instituto de Salud Carlos III (ISCIII-RETIC REDINREN RD06/0016, RD12/0021, PI081564, PI11/01854 and PI10/00072) Comunidad de Madrid (S2010/BMD-2321), Sociedad Española de Nefrologia. Agencia Española de Cooperación Internacional (PCI Iberoamerica; A/9571/07), FONDECYT Chile 1080083 and 1120480 and Fundación Lilly. C.L is fellow of ISCIII. Programme to Encourage Research (Programa Intensificación Actividad Investigadora), (ISCIII/Agencia Laín-Entralgo/CM) to AO.
Figure 1. Immunodetection of Jagged-1 in biopsies of patients with minimal glomerular lesion (n = 5), hypertensive nephrosclerosis (n = 10), diabetic nephropathy (n = 8), and non-progressive (n = 8) and progressive (n = 8) membranous nephropathy.
Figure 2. Immunodetection of activated Notch-1 in biopsies of patients with minimal glomerular lesion, hypertensive nephrosclerosis and diabetic nephropathy.
Figure 3. Activation of the Notch pathway not related to renal fibrosis in hypertensive nephrosclerosis.
Figure 4. Activation of the Notch pathway is related to renal fibrosis in membranous nephropathy.