El mieloma múltiple (MM) consiste en la proliferación incontrolada de células plasmáticas con producción de cantidades variables de inmunoglobulinas o sus cadenas. La insuficiencia renal aguda puede ser un síntoma del MM, y a veces su forma de presentación. Las cadenas ligeras libres circulantes (CLL) pueden dar lugar al fallo renal por la precipitación intratubular de ellas, causando una nefropatía por cilindros. El tratamiento del mieloma, una adecuada hidratación y la eliminación de CLL mediante técnicas de aféresis son los tratamientos admitidos actualmente para esta entidad. Se han intentado diversas técnicas de aféresis para intentar eliminar las CLL, siendo la hemodiálisis de larga duración con filtros para eliminar dichas cadenas ligeras (alto cut-off) la que se postula como el tratamiento más eficaz para la nefropatía del mieloma. Métodos: Presentamos cinco casos de nefropatía de mieloma: tres con nefropatía por cilindros (NC) diagnosticada por biopsia renal y dos con alta probabilidad de NC (niveles de CLL > 500 mg/l) tratados con hemodiálisis larga con membrana de alto cut-off. Todos presentaban insuficiencia renal aguda, en cuatro de ellos con necesidad de terapia sustitutiva y uno en situación de insuficiencia renal avanzada. En todos ellos los niveles de CLL fueron muy elevados. Recibieron tratamiento específico para el mieloma más hemodiálisis de alto cut-off hasta alcanzar niveles de CLL < 500 mg/l. Resultados: Cuatro de los cinco pacientes recuperaron función renal, quedando independientes de diálisis. El tiempo de evolución del mieloma desde el inicio de la clínica fue variable (1-6 m). El número de sesiones varió entre 8-16. El paciente de más tiempo de evolución precisó más sesiones y no recuperó función renal. Conclusiones: La hemodiálisis larga con filtros de alto cut-off más tratamiento con quimioterapia del mieloma parece ser un tratamiento eficaz en la insuficiencia renal aguda debida a nefropatía del mieloma. La precocidad en el inicio del tratamiento puede ser un factor determinante de la respuesta.
Multiple myeloma (MM) is the uncontrolled proliferation of plasma cells with variable amounts of production of immunoglobulins or their chains. Acute renal failure can be a symptom of MM, and it is sometimes its form of presentation. Circulating free light chains (FLC) could lead to renal failure due to their intratubular precipitation, causing a cast nephropathy. The treatment of myeloma, adequate hydration and the removal of FLC by apheresis techniques are currently the treatments that are accepted for this disease. Several apheresis techniques have been attempted for the removal of FLC, with long haemodialysis sessions with filters for the removal of these light chains (high cut-off filters) being proposed as the most effective treatment for myeloma nephropathy. Methods: We report 5 cases of myeloma nephropathy: three had cast nephropathy (CN) diagnosed by renal biopsy and the other two had a high probability of CN (FLC levels >500mg/l). They were treated with long haemodialysis sessions with a high cut-off membrane. All patients had suffered acute renal failure; four required renal replacement therapy and one patient had advanced renal failure. In all patients, FLC levels were very high. They received specific treatment for myeloma in addition to high cut-off haemodialysis until they achieved FLC levels of <500mg/l. Results: Four of the five patients recovered renal function, and became independent of dialysis. The progression time for myeloma from the time the first symptoms appeared varied (1-6 months). The number of treatment sessions ranged from 8-16. The patient with the longest progression time required more sessions and did not recover renal function. Conclusions: Long haemodialysis sessions with high cut-off filters in addition to specific myeloma chemotherapy seems to be an effective treatment for acute renal failure due to myeloma nephropathy. The early initiation of treatment could be a determining factor for the response.
Contents
Multiple myeloma (MM) is a neoplastic disease consisting of clonal proliferation of the bone marrow plasma cells which produces uncontrolled amounts of immunoglobulins or their chains (heavy or light) that circulate in the blood as free light chains (FLC) and can appear in the urine (Bence-Jones [BJ] proteinuria). At an intratubular level, these proteins may lead to kidney failure due to their precipitation (cast nephropathy [CN]). Light chains are medium-sized molecules with two isotypes: kappa chains that are primarily monomeric in form (22.5kDa) and lambda chains that commonly occur as dimers (45 kDa).
Myeloma represents 1% of neoplasias in the Western world, with an annual incidence of 5-6/100,000 inhabitants/year. Over one-third of patients are under 65 years of age.1 Acute renal failure occurs in up to 50% of cases of MM and is often reversible with normal treatment based on fluid and electrolyte management,2 but up to 10% of patients will eventually require renal replacement therapy.
Plasma cell leukaemia is a rare variant of MM,3 and constitutes 2%-3% of all myelomas. It is a highly aggressive form of MM with poor short-term survival. Plasma cell leukaemia can be classified into two subtypes: 1. Primary plasma cell leukaemia: when there is already leukaemia development at the time of diagnosis. 2. Secondary plasma cell leukaemia: when it appears as a result of the transformation of a previous MM. Renal failure is a common result.
The causes of renal dysfunction in patients with myeloma include impaired proximal and distal tubular function due to cell damage by filtered light chains, myeloma CN, amyloidosis, light or heavy chain deposit disease, cryoglobulinaemia, interstitial infiltration by plasma cells and rarely proliferative glomerulonephritis or interstitial nephritis. CN occur in 40-60% of cases of myeloma associated with renal dysfunction and can cause acute renal failure.4
Thus, acute renal failure is not an uncommon form of presentation for this disease and its presence will overshadow prognosis and reduce the life expectancy of the patient. In recent years, the introduction of new treatment strategies such as autologous bone marrow transplant and the availability of new therapeutic agents such as thalidomide, lenalidomide and bortezomib have changed the management of myeloma and improved survival. Other therapeutic advances that have changed the prognosis of myeloma are the introduction of apheresis techniques for the removal of light chains, which reverses renal tubular damage.
The removal of substances through the dialysis membranes depends on their molecular weight and the membrane’s pore size. New generations of dialysis membranes with a cut-off close to the native kidney (65 kDa) can be applied in the treatment of various diseases with renal involvement, including rhabdomyolysis and myeloma kidney.2
Acute kidney damage is common in patients with MM, and is often caused by CN, resulting from intratubular precipitation of light chains. This acute kidney damage may become irreversible and usually requires treatment with dialysis. The reduction of plasma FLC is associated with an improvement and sometimes recovery of renal function. Plasmapheresis has been used to remove light chains, with some authors finding an improvement in renal function,4,5 but for others its efficacy is not clear.6 Recent work has shown that the removal of FLC, along with chemotherapy, improves survival in patients with myeloma.7 Early removal is decisive in the recovery of renal function.8 The method of chain removal also appears to affect recovery. Thus, the removal of FLC by plasmapheresis is less effective9 than when the removal is performed by long haemodialysis sessions with high cut-off filters (HCO-HD). This difference appears to be due primarily to the shorter duration of the plasmapheresis technique, which does not allow the chains to be removed, and they increase as a result of their intercompartmental redistribution.10 The current high cut-off dialysers, with a membrane pore size greater than 60kDa, allow the removal of both the kappa and lambda chains.
It has been demonstrated that the recovery of renal function has a positive influence on the survival of patients. Similarly, the type of light chain produced will have an influence on prognosis, and as such, the presence of kappa chains of lower molecular weight gives a better prognosis than the cases in which the lambda chain is produced. The difference in the rate of removal of kappa and lambda chains seems to be most evident when using plasmapheresis, whereas with long haemodialysis sessions, the removal is not very different. Recently, haemodiafiltration techniques with ultrafiltrate reinfusion and passage through resin cartridges have begun to be used, which seems to remove light chains, mainly kappa, effectively,11 at a lower financial cost. However, there has not been sufficient experience with this technique in medical literature to endorse its use, and as such, we will have to wait for the results.
With the current knowledge, we can say that the longer the duration of the technique and the greater the area of the filter, the better the FLC clearance results will be. It also seems clear, for most authors,12 that the removal of the aforementioned FLC, either through the more effective HCO-HD technique or by plasmapheresis, should be carried out at an early stage13 and be combined with a specific chemotherapy treatment to slow down the production of these anomalous chains. In patients with normal renal function, the use of a suitable form of chemotherapy will reduce the levels of circulating FLC to low figures. Nevertheless, in patients with impaired renal function, FLC clearance decreases, with plasma levels remaining high for a long period of time, and as such toxicity is maintained at a tubular level and damage is caused that may eventually be irreversible, even when the form of chemotherapy treatment is suitable. Currently, treatment with dexamethasone and bortezomib-based chemotherapy regimens seem to be among the most effective for treating myeloma.14-16 Furthermore, by using high cut-off dialysers, we can accelerate the removal of FLC, limiting its tubular damage in myeloma.17,18
METHOD
Patients: we present five patients, two males and three females, with an age range of between 51 and 68 years, diagnosed with monoclonal gammopathy with very high levels of serum FLC and in all cases with acute renal failure. In three of the cases, the presence of light chain CN (myeloma nephropathy) was displayed by renal biopsy and in the two patients in whom renal biopsy could not be performed (due to severe thrombocytopenia in one case and systemic antiplatelet therapy in the other), the levels of light chains in urine and serum FLC were very high, which along with the symptoms, meant that their profile was highly suggestive of CN. Four of the patients were diagnosed with MM (two cases of IgG and two of IgA), while in one case the diagnosis was acute plasma cell leukaemia. Four patients clinically presented acute renal failure and were admitted directly to Nephrology. All the cases were newly diagnosed gammopathies and all received treatment with chemotherapy simultaneously with dialysis. The form of chemotherapy received is displayed in Table 1.
In all cases, treatment with hydration and correction of electrolyte imbalance was initiated prior to the diagnosis of suspected myeloma kidney, without any significant improvement being obtained in any patient. In four cases, it was necessary to conduct acute haemodialysis due to the degree of renal failure, while one patient had moderate renal failure and the technique was indicated to remove light chains. The time between developing symptoms and admission varied between two and six months (Table 1).
Consent: once informed, all patients signed their specific consent for vascular access and the high cut-off filter dialysis technique.
Treatment protocol: HCO-HD of 6-8 hours: Theralite® (Gambro) of 1.1m2 (first patient) and 2m2, until achieving serum FLC figures of <500mg/l or recovering renal function, becoming independent of dialysis, for a maximum of 16 sessions. During the sessions, albumin supplements were administered (200ml of 20% albumin solution ml/session), phosphorus (1 vial of Fosfoevac® in dialysate/session), magnesium (1500mg/session, 15% magnesium sulphate, one vial during the last hour of dialysis) and potassium (20-40mEq/session), with subsequent adjustments in accordance with the analysis.
Dialysate: Dialsol 313-A (3mEq/l Ca++ and 1.5mEq/l K+).
Introduction of heparin into the circuit: initial heparin sodium of 0.5mg/kg plus 10mg/hour.
Vascular access: initially, a central venous catheter was temporarily placed in patients for haemodialysis. Subsequently, in patient 1, due to progression, an autologous AVF was carried out and a tunnelled catheter was placed in patient 4.
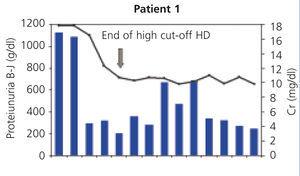
Monitoring: we monitored pre and post-dialysis levels of renal function, calcium, phosphorus, magnesium, potassium and albumin, and we determined pre and post-dialysis FLC levels for each session. In the first of the patients, FLC was not able to be determined in our centre, and as such, it was monitored through B-J proteinuria levels in urine.
Response to treatment: the renal response was classified in accordance with criteria of the European Group for Blood and Marrow Transplantation: 1) complete response, if the patient has a glomerular filtration rate (GFR) of ≥60ml/min; 2) partial response, when the GFR increases >100% or changes from GFR <15ml/min to GFR between 30-60ml/min); or 3) minor response if the GFR increases >50% or changes from a rate of <15 ml/min to 15-30ml/min or from a rate of 15-30 ml/min to 30-60ml/min.
RESULTS
All patients received specific treatment for the haematological disease, in accordance with the haematology protocol (Table 1). Patient 2, who was diagnosed with acute primary plasma cell leukaemia, received treatment with mega-CHOP and once renal function normalised and after the marrow responded to chemotherapy, they underwent bone marrow transplantation with satisfactory results.
In patients 1, 3 and 5, a percutaneous renal biopsy was performed, showing CN in all three, while in patients 2 and 4 no biopsy was carried out due to the increased risk of bleeding (Table 1).
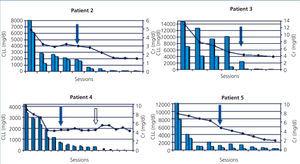
The five patients were treated with HCO-HD for 6 hours. The mean number of sessions was 12±3.1 (8-16). The first received 7 daily sessions and 9 on alternate days with 1.1m2 Theralite® filters (Gambro), while the other four received 5 daily sessions, followed by a variable number (7, 3, 9 and 5) of sessions on alternate days with 2m2 Theralite® filters (Gambro), until FLC blood figures <500mg/l were achieved, except in patient 4, in whom the technique was discontinued after 14 sessions after observing stability in pre and post-dialysis FLC figures, even though the desired 500mg/l had not been achieved. In this case, given the lower chain removal efficiency, we increased the duration of dialysis to 8 hours, in spite of which the efficacy did not improve and given the limited effectiveness of the technique, it was decided to discontinue the clearance treatment. At this time, renal function was stable (serum creatinine of 4mg/dl) and levels of light chains were at 3000mg/l (Figure 1). Once HCO-HD was discontinued, FLC continued to decrease very slowly until levels below 500mg/l were achieved, one month later, as a result of the response to myeloma treatment.
In all patients, serum FLC levels decreased during HCO-HD treatment, most strikingly in the first five (daily) sessions, at the end of which in 2 of the 4 patients, FLC levels were below 1000mg/l, in another patient they were at 1600mg/l and in patient 4, the decrease was slower (Figure 1). In patient 1, who was monitored by B-J proteinuria levels, in the absence of serum FLC, levels of the latter also decreased (Figure 2). In some patients, we observed a clear interdialysis increase in FLC figures, most strikingly in patient number 3, while in the rest, this phenomenon was more latent.
Plasma creatinine improved in parallel with decreasing levels of FLC, stabilising when levels dropped from 500 mg/l in patients 2, 3, 4 and 5, whereas in patient 1, although plasma creatinine decreased as result of dialysis, it remained at figures high enough to require renal replacement therapy.
The progression of kidney function is shown in Table 2 and individually in Figure 1 and Figure 2. In four of the patients (patients 2, 3, 4 and 5), renal function recovered satisfactorily, allowing replacement therapy to be discontinued after completion of the treatment with HCO-HD. In one case, it was considered that the response was complete, in another, partial, and in the other two, minor. This recovery was maintained until death in one patient, and to date in the remainder (26, 18 and 12 months).
We analysed the five patients individually:
Patient 1 presented with a wide array of symptoms: severe anaemia, 20% plasma cells in bone marrow aspiration, severe renal failure (serum creatinine at diagnosis of 18mg/dl), high monoclonal component (3.6g/dl), and when they were referred to our centre, the process had progressed for at least six months. Conventional haemodialysis was begun before the diagnosis was confirmed. At that time, we did not have FLC blood determination, but on finding a very high removal of urinary light chains in urine, we considered performing a renal biopsy, which confirmed the diagnosis of suspected CN and the presence of a damaged tubular epithelium with mixed inflammatory infiltrate and severe interstitial fibrosis. With the diagnosis of CN, treatment with HCO-HD was begun with a 1.1m2 filter (one week after admission) without the recovery of renal function being achieved. Although BJ proteinuria levels decreased with HCO-HD in addition to the specific myeloma treatment (vincristine, doxorubicin and dexamethasone [VAD] plus bortezomib), renal function was not recover and the patient remained in need of replacement therapy (Figure 1); we do not have the FLC levels of this patient due to infrastructure problems in the centre. Subsequently, this patient had myeloma progression and is currently receiving a new line of treatment (bortezomib and adriamycin) for which we are awaiting the response.
Patient 2 was diagnosed with acute leukaemia of primary plasma cells with monoclonal lambda component and a high production and renal removal of these lambda light chains (B-J proteinuria >37,000mg/l), as well as high levels of serum FLC (8030mg/l). Since the patient was undergoing systemic antiplatelet therapy, with a major risk of bleeding if a renal biopsy was performed at that time, the latter was rejected in favour of establishing the histological diagnosis of CN and it was decided to start treatment with HCO-HD, considering the presence in blood of high levels of lambda type FLC. The response to treatment was complete, with normal renal function being achieved. Subsequently, an autologous bone marrow transplant was performed, after which normal renal function was maintained (serum creatinine 0.8mg/dl), with the absence of proteinuria.
In patient 3, the time from the beginning of symptoms until we were consulted was two months. Treatment was initiated with hydration measures and there was improvement in renal function, but later it worsened again, showing very high FLC figures (14,800mg/l) and renal biopsy was proposed. The patient displayed CN and as such, it was decided to start treatment with high cut-off filter. Response to treatment was favourable. In this case, we observed a significant recovery in serum FLC figures during the first sessions (Figure 1). Renal function improved; after completion of treatment with HCO-HD, creatinine remained stable (creatinine 3mg/dl), and the patient became independent of dialysis, although the glomerular filtration rate was around 20ml/min. Subsequently, due to myeloma relapse, it was necessary to intensify chemotherapy and as a result of this the patient developed an infectious complication that resulted in exitus.
Patient 4 had a progression of at least three months of symptoms at the time of admission. They had advanced renal failure and anaemia within transfusion range. The bone marrow aspirate revealed the presence of 56% plasma cells and the determination of serum FLC showed the presence of very high lambda FLC levels (greater than 41,000mg/l). Treatment was initiated without histological study due to haemorrhagic risk. Response to treatment with HCO-HD was good, with a significant decrease in the levels of light chain following the first sessions, as is shown in Figure 1, and levels subsequently stabilised around 3000mg/l. Following the discontinuation of dialysis, the patient displayed a progressive reduction in serum FLC levels to values below 500mg/l after one month of follow-up. Renal function has remained stable until the present day and the patient is independent of dialysis. From the haematological point of view, despite the initial aggressiveness of symptoms, the patient is currently in remission.
Patient 5 presented renal failure symptoms, with a progression of at least two months of anaemia. Given the presence of high FLC levels and renal biopsy with the presence of CN, we began HCO-HD. Clinical response from the haematological point of view with VAD and bortezomib was good. FLC reduction was parallel to the recovery of renal function, which allowed the technique to be discontinued after 10 sessions with a glomerular filtration rate above 20ml/min. Currently, almost 1.5 years after diagnosis, the patient is in haematological remission with creatinine levels stable at 1.8mg/dl.
All patients had an excellent clinical tolerance to the technique. All sessions passed with good haemodynamic stability. According to the protocol, they were supplemented with albumin, phosphorus, magnesium and potassium, adjusted in accordance with the analytical controls carried out. We did not observe any technical complications in the extracorporeal technique or related to vascular access.
DISCUSSION
We present five cases of monoclonal gammopathy with renal failure due to light chain nephropathy. Four of them were diagnosed with MM and one with acute primary plasma cell leukaemia.
In the case of patient 1, we believe that the fact that treatment was not carried out early, with six months' progression of symptoms prior to renal biopsy, may had an influence on progression, although some authors have recently19 found improved renal function in patients with longer progression. Moreover, in our case we believe that the presence of tubular damage and renal interstitial fibrosis on biopsy may be the fundamental reason for the absence of renal function recovery. On the other hand, the filter used had a smaller surface (1.1m2), a fact that could have had an influence on response, although due to the absence of serum FLC determination at that time, we could not assess the degree of efficacy in the removal of FLC. According to authors such as Hutchinson, the type of light chain may influence prognosis, and as such, the kappa chain-producing myelomas respond better to treatment when they are monomeric forms of lower molecular weight that could be removed more easily by employing different techniques.20 In patient 1, the chain produced was a kappa chain and we demonstrated that its excretion in urine decreased very significantly, but it is possible that kidney damage was already irreversible (as expected from renal biopsy findings). The patient currently remains on haemodialysis with progression of the disease and has begun a new line of chemotherapy.
Patient 2 was diagnosed with primary plasma cell leukaemia with highly aggressive symptoms and very high FLC levels, and as such he was started on treatment to remove these chains, without histological confirmation. Primary plasma cell3 leukaemia is characterised by its appearance in younger patients, as in our case in which it appears in the youngest of the five patients. The prognosis seems better than for secondary leukaemia and in our case, it had the best response. Bone lesions and hypercalcaemia are less common in primary plasma cell leukaemias, which more commonly present organomegalies and lymphadenopathies. In our patient, there was hepatosplenomegaly and cutaneous infiltration. There is usually a lower quantity of serum M component than in typical forms of MM, which is also the case for our patient, as well as the presence of renal failure, which is also common.
In this case, the response to treatment was very good. We did not find in literature any case of plasma cell leukaemia in which patients underwent extracorporeal clearance techniques for the removal of FLC. Our patient progressed favourably, recovering renal function ad integrum, which allowed a bone marrow transplantation to be performed.
Patient 3, when we were consulted due to the presence of renal failure in a patient with a diagnosis of myeloma that was being treated, already had a severe thrombocytopenia secondary to the chemotherapy treatment that they were receiving, and therefore renal biopsy was not performed at that time, due to haemorrhagic risk. The patient initially improved on receiving medical treatment, but after further deterioration of renal function, and after recovery from thrombocytopenia, renal biopsy was performed without complications. The response to treatment with high cut-off dialysis was good, with a better recovery in serum FLC figures being observed during the first four sessions, which we believe to be indicative of a significant generation of FLC at the plasma cell level, which still maintained high activity despite chemotherapy. We observed very high serum FLC figures at the start, which are also indicative of a high activity of the plasma cell clone. Renal function improved, and dialysis was discontinued, but myeloma progressed unfavourably and exitus occurred due to infectious complications.
Despite the long progression of symptoms at the time of diagnosis, patient 4 responded well to treatment with HCO-HD, with a significant initial decrease in background levels of light chains. Subsequently, we observed a stabilisation of FLC levels at around 3000mg/l and found that the technique was not effective in removing the latter despite an increase in the number of hours of each session (8 hours). At this point and given that renal function had improved, remaining at figures that did not require renal replacement therapy, we decided to discontinue haemodialysis. After analysing the situation, we thought that the low removal efficacy could be due to the formation of lambda chain polymers with higher molecular weight and thus they were not removed by HCO-HD. This phenomenon is described in literature,21 although we could not demonstrate this point. In this case, as with patient 1, there was some delay in the haematological diagnosis which we believe could have influenced the progression and non-complete recovery of renal function, in light of the data we have today, which tells us that the early removal of FLC is urgent in order for an improvement in the prognosis of the disease.22 In this case, a renal biopsy was not able to be carried out at the time of diagnosis, since the patient was receiving antiplatelet treatment with aspirin. The clearance technique was initiated in view of serum FLC figures and antiplatelet therapy was discontinued in order to be able to carry out biopsy after 10 days. In the end, it was not possible due to thrombocytopenia secondary to chemotherapy administration (VAD plus bortezomib), and as such, given the good response to treatment, it was rejected. After almost two years of follow-up, serum creatinine of 3.6mg/dl was maintained and from a haematological point of view, the patient is in complete remission.
Patient 5 had a history of at least two months of anaemia, and we understand this to be at least the time of progression of the process. The response to treatment was good, with FLC reduction parallel to recovery of renal function as is reflected in the literature.23 Once treatment was initiated on alternate days, we observed some degree of recovery in interdialysis levels of FLC, which eventually became negligible. We believe that the shortest progression, in this case, could favour a good response to treatment.
In our experience, in lambda light chain-producing myeloma, three of the cases generally had a good response to treatment, with adequate recovery of renal function, except in the case of patient 4, in whom recovery was not complete, which was probably due more to the duration of the disease until the beginning of HCO-HD than the anomalous chain type produced. Some authors have recently have found equal efficacy in the removal of the two types of chains.24 As for the other two patients, in one of those with kappa myelomas, there was no response to treatment (long progression period and associated interstitial renal damage) and in the other we cannot establish progression, given their early death after the technique was ended.
Generally, at the beginning of treatment with high cut-off filters, the different authors8 were more selective in their indication and specified that evidence of CN by renal biopsy was required in order to begin the technique, while authors who carried out plasmapheresis sometimes began it without histological evidence.25 Over time, there has been greater permissibility in this regard. Some of these authors, such as Hutchison,17 in their latest publications performed a biopsy in less than 60% of patients, since, as in our case, sometimes it is not technically possible to do so in our clinical context. On one hand, this allows for earlier treatment, without the requirement to wait for the histological results, but on the other hand, data from a biopsy can predict the prognosis26 and as such, if there is only data for CN, the prospect of a good progression is higher than if there is more chronic damage, which would allow us to not unnecessarily prolong treatment at a high financial cost. Our view is that, where it is technically possible, a renal biopsy should be performed, with a diagnostic and prognosis purpose. Given that we believe early treatment is a priority, we think that, once high FLC blood levels have been determined, there should not be any delay in initiating the technique while waiting for the possibility of performing a renal biopsy or obtaining its result, although, whenever possible, a renal biopsy should technically be performed.
In some articles published recently,27 the authors find complications in up to 28% of the sessions, many of them related to coagulation; we did not have to deal with any major complications and in only one case was there a circuit coagulation, forcing a filter change. Neither did we have any haemorrhagic or infectious complications related to the technique or vascular access. The technique was very well tolerated in all instances.
In short, we believe that the life expectancy of patients with myeloma depends on the haematological disease itself, although renal involvement will modify it substantially. Renal prognosis is, in our experience, clearly related to the progression of time until HCO-HD is started. If treatment is performed early, removing circulating FLC with this type of haemodialysis, while decreasing the production of the latter by the plasma-cell clone, the recovery of renal function, albeit partial, can be achieved, long-term prognosis seems very positive and stability in renal function can be maintained at least in the medium term.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 2. Clinical and progression data
Figure 1. Progression of renal function and free light chains
Figure 2. Progression of renal function and Bence-Jones proteinuria
Table 1. Initial epidemiological and clinical data