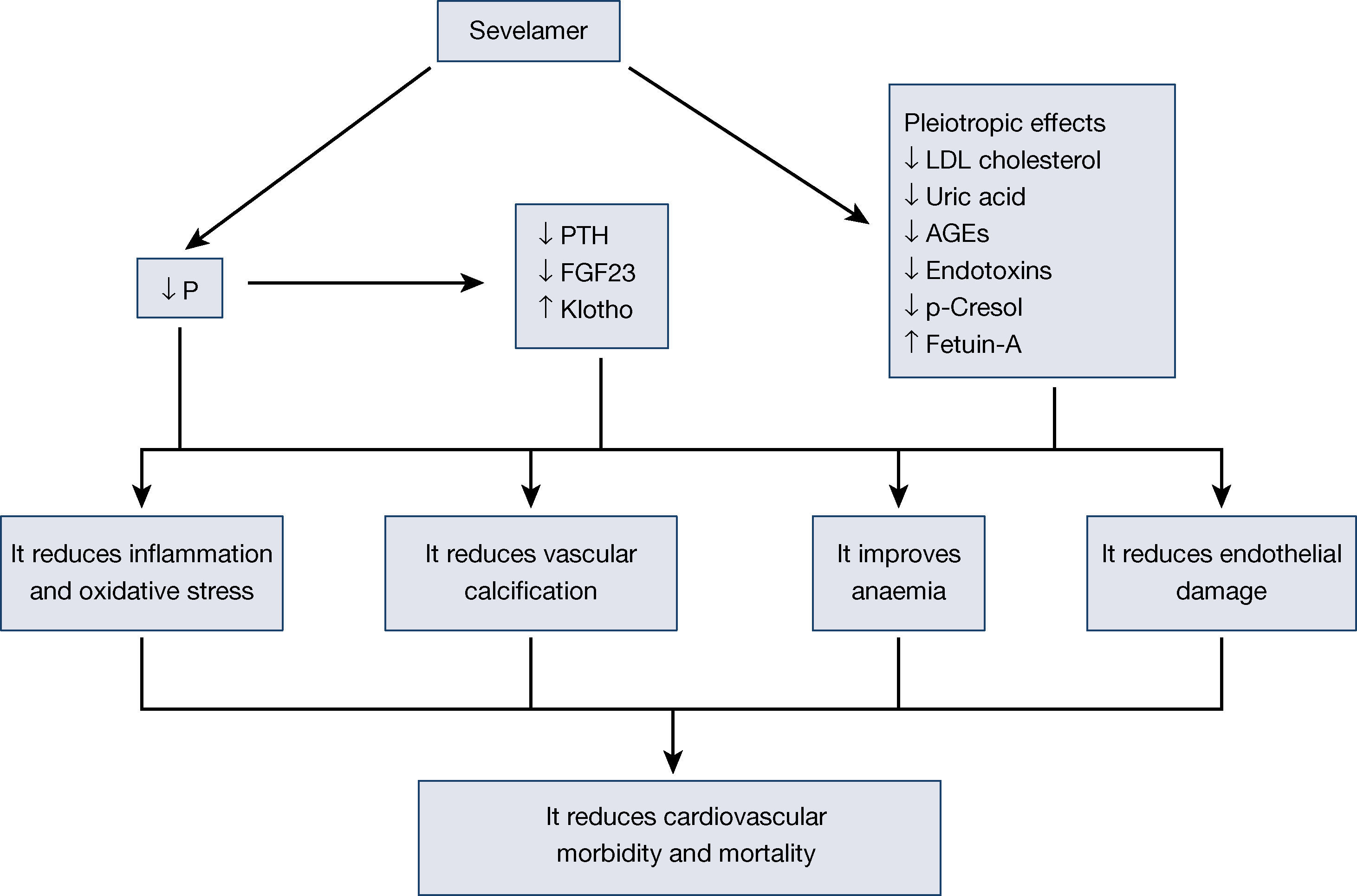
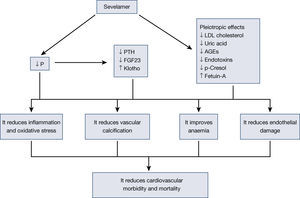
Sevelamer is a non-calcium phosphate binder used in advanced chronic kidney disease (CKD) and in dialysis for hyperphosphataemia control. Several experimental, observational studies and clinical trials have shown that sevelamer has pleiotropic effects, beyond hyperphosphataemia control, including actions on inflammation, oxidative stress, lipid profile and atherogenesis, vascular calcification, endothelial dysfunction and the reduction of several uremic toxins. This is the biological basis for its global effect on cardiovascular morbidity and mortality in patients with chronic kidney disease. This review focuses on these pleiotropic actions of sevelamer and their impact on cardiovascular health, with the experience published after more than ten years of clinical expertise.
El sevelamer es un captor no cálcico de fósforo que se utiliza en la ERC avanzada y en diálisis para el control de la hiperfosforemia. Varios estudios experimentales, observacionales y ensayos clínicos han mostrado que el sevelamer tiene efectos pleiotrópicos, más allá del control de la hiperfosforemia, incluyendo acciones sobre la inflamación, el estrés oxidativo, el perfil lipídico y la aterogénesis, la calcificación vascular, la disfunción endotelial y la disminución de diversas toxinas urémicas, todo lo cual sería la base biológica de su efecto global sobre la morbilidad y la mortalidad cardiovascular en pacientes con enfermedad renal crónica. En esta revisión, se hace énfasis en estas acciones pleiotrópicas del sevelamer y su impacto en la salud cardiovascular, con la experiencia publicada después de más de 10 años de experiencia clínica.
Patients with chronic kidney disease (CKD) show an important increase in cardiovascular morbidity and mortality compared to the general population.1. Between 40% and 75% of patients who start dialysis have a cardiovascular disease (CVD), which accounts for 44% of the deaths of these patients and constitutes the most important cause of total cardiovascular morbidity and mortality, adjusted for age and gender2,3. One of the unusual cardiovascular risk factors in these patients is altered mineral metabolism4.
At present, phosphorus is considered an important element which causes vascular damage in people with normal renal function, and particularly in patients with renal failure5. Control of its absorption to prevent overload is a usual practice in patients with kidney disease, but doubts have been raised about its usefulness in other patients. Diet and the use of phosphate binders are ways in which this control is achieved6.
There are different types of phosphate binders, but each shows distinguishing features, which may include advantages other than the already proven reduction in phosphorus absorption. Recently, the lower mortality of non-calcium versus calcium binders has been reported as non-calcium binders shown better survival results7. These studies have awoken interest in these binders once more, mainly in sevelamer and lanthanum. In this review, we focus on the analysis of sevelamer. This molecule has been used in clinical practice for more than ten years, and many studies have shown its effects on patients with kidney disease. In both forms (carbonate and hydrochloride), sevelamer is a metaland calcium-free non-absorbable phosphate binder or chelating agent that is used for the control of hyperphosphataemia in chronic kidney disease (CKD)8. Its concomitant administration with food reduces the absorption of this ion. Regarding its composition, it contains multiple amines, separated by one carbon in the polymer skeleton, which are partially ionised in the intestine and interact with phosphorus molecules by means of hydrogen and ionic bonds. Therefore, dietary phosphorus is bound in the gastrointestinal tract, captured and eliminated through the intestine, thus reducing serum concentrations9.
Besides reducing phosphorus absorption due to its capacity to capture it in the intestine without added calcium, sevelamer is capable of modifying adaptive mechanisms, such as reducing fibroblast growth factor 23 (FGF23) or parathyroid hormone (PTH). Moreover, since it was first marketed, other effects of this molecule have been confirmed, which are known as pleiotropic effects. Reductions have been described in lipids10, changes in bone structure11, inflammation12, oxidative stress13, anaemia14, as well as fetuin-A15, among others. All these effects of sevelamer have been associated with a reduction in vascular calcification, an improvement of cardiovascular lesions and, therefore, a reduction in mortality16.
The pleiotropic effects of sevelamer, as it is not absorbed, are due to the intestinal effect of this molecule. Its binding to bile salts and its capacity to capture other molecules in the intestine seem to have other beneficial effects. This effect simply reinforces the importance the intestine has in uremic patients and opens the possibility of new treatments where the intestine is the site of action in patients with CKD and related complications17. In this review, we analyse the different effects published with sevelamer, avoiding a detailed analysis of its phosphate binding effect on the intestine (Figure 1).
1Sevelamer, bone and mineral metabolism, and FGF23a) FGF23
Fibroblast growth factor 23 (FGF23) is a member of the fibroblast growth factor superfamily that displays phosphaturic action, inhibition of 1α-hydroxylase in the proximal tubules, and 24-hydroxylase activation18,19, which is why vitamin D activity is reduced. FGF23 plays an important role on vitamin D and phosphorus metabolism20–22. In uremia, both metabolisms are altered.
Both in healthy subjects and patients with CKD, FGF23 is secreted by osteocytes and osteoblasts as a response to oral phosphate overload19,23,24. In addition, it is known that serum FGF23 levels increase at the early stages of chronic kidney disease in an initial attempt to avoid phosphorus retention, which increases phosphaturia. With the progression of CKD, in very advanced stages, hyperphosphataemia appears despite increased levels of FGF23 and PTH. Although this aspect is still being researched, it seems that the FGF23 receptor and its Klotho coreceptor are expressed in many cell types, including cardiomyocytes, vascular wall, kidneys, and parathyroid glands25,26. Moreover, elevated FGF23 is associated with the development of endothelial dysfunction and cardiac hypertrophy in patients with CKD27.
Several recent studies have shown the capacity of sevelamer to decrease FGF23 thanks to its capacity to reduce phosphorus absorption in the intestine. Koiwa et al. proved that the use of sevelamer hydrochloride reduces serum FGF23 levels in dialysis patients, presumably through the inhibition of phosphate loading in the intestine. Serum FGF23 levels significantly decreased after four weeks of treatment with sevelamer hydrochloride + CaCO3 from the pretreatment levels (p <0.05), while no changes were found in patients treated with CaCO328 alone.
Oliveira et al. confirmed this effect of sevelamer on FGF23 in patients with CKD. In a 6-week randomised study, the effect of two phosphorus chelating agents (calcium acetate and sevelamer) on PTH and FGF23 was studied in patients with stage 3 and 4 CKD. During treatment with both chelating agents, there was a progressive decrease in serum PTH and urinary phosphorus, but there were no changes in serum calcium or serum phosphorus. Significant changes for FGF23 were only observed in patients treated with sevelamer29.
The reduction in FGF23 increases calcitriol by reducing 24-hydroxylase activity and increasing 1α-hydroxylase activity. However, there have also been reports of a slight decrease in intestinal absorption of lipids and liposoluble vitamins, including vitamin D, derived from binding to bile acids30.
The reduction in serum FGF23 levels with sevelamer has a beneficial effect on vascular endothelial function in patients with kidney disease as it improves vascular flow-mediated vasodilation, which is an endothelial damage marker. In addition, FGF23 correlates with asymmetric dimethylarginine (ADMA) and it is an endogenous inhibitor of the nitric oxide synthase enzyme, a pathway which could cause vascular dysfunction in patients with kidney disease31.
b) Klotho
The reduction in FGF23 is accompanied by an increase in serum Klotho. These changes are already observed 48hours following treatment. Serum FGF23 and phosphorus decrease while sevelamer is used and the increase in Klotho is proportional to the decrease in phosphorus32.
c) Effect of sevelamer on the WNT-beta-catenin pathway
Olivera et al. examined the effects of phosphorus chelating agents, sevelamer hydrochloride and calcium acetate, on energy-regulating hormones and WNT-beta-catenin pathway in stage 3 to 4 CKD. The study showed that there is an important alteration of the WNT pathway in CKD, which is reflected by the increased sclerostin and the deregulation of energy-regulating hormones. Many of these alterations may be mitigated by treatment with phosphorus chelating agents, although more so with sevelamer hydrochloride than with calcium acetate because it significantly decreases serum FGF23, sclerostin and leptin and also significantly increases alkaline phosphatase levels33.
2Sevelamer and the absorption of molecules of intestinal origina) Sevelamer and its effect on endotoxins
Haemodialysis patients are characterised by having an increased chronic inflammation, which causes a high comorbidity that is also increased by a poor nutritional status. Endotoxin (ET) is a glycolipid component of the cell wall of Gram-negative bacteria that is a powerful stimulus for the activation and release of proinflammatory cytokines (for example, IL-1, IL-6, and TNF), which adversely affect protein metabolism and nutritional status34. These proinflammatory cytokines are increased in haemodialysis patients and have been associated with a higher risk of cardiovascular disease, hospitalization and death35–37. In addition, low levels of albumin are associated with increased inflammatory markers such as IL6, CRP, TNFα.38–40.
Cross-sectional observational studies conducted in haemodialysis patients have found elevated serum ET levels. These are associated with lower serum albumin, an increase in proinflammatory cytokines and C-reactive protein, which is related to important cardiovascular damage, and, in extreme cases, they may even cause septic shock41. Albumin has also proven to have anti-inflammatory properties by binding to the endotoxin and reducing the expression of proinflammatory markers42,43.
Possible sources of ET include bacterial translocation from the gastrointestinal tract34. In stage 5 CKD, the gastrointestinal barrier function is compromised by oxidative stress, circulatory compromise, hypoxia of the intestinal wall, reduced motility and bacterial overgrowth44. Hypoperfusion and intestinal oedema induce changes in permeability, facilitating bacterial translocation through the gastrointestinal lumen towards the blood current45. The administration of iron supplements, which is a common treatment for anaemia in patients with end-stage CKD, may also promote intestinal bacterial growth35,46. In addition, iron is an essential requirement for most microorganisms, and it has been shown that iron overload may improve bacterial growth and virulence47,48.
Some observational studies have shown that haemodialysis patients who are treated with sevelamer show lower ET levels, with the consequent decrease in proinflammatory cytokines and CRP, and a simultaneous increase in serum albumin43.
Sevelamer may bind non-specifically to negatively charged biomolecules, such as portion A of the negatively charged lipid of the ET, thus reducing in vitro and in vivo serum concentrations. In vitro experiments show that sevelamer binding to ET is dose-dependent49. Sevelamer is known to bind to negatively charged bile acids, thus acting as a bile acid sequestrant, which may reduce low-density lipoprotein concentrations9.
Several small, short-term studies have shown an association between treatment with sevelamer and reductions in ET, soluble CD14 and proinflammatory markers, such as CRP and IL-634. A recent 8-week randomised crossover study in patients with stage 2 to 4 CKD proved that treatment with sevelamer carbonate reduces advanced glycation end products (AGE), haemoglobin A1C, and inflammatory biomarkers50. These preliminary data suggest potential benefits of sevelamer in reducing proinflammatory cytokines.
b) Sevelamer and cholesterol
Sevelamer binds to negatively charged bile acids, acting as a bile acid sequestrant which may reduce low-density lipoprotein concentrations9. The beneficial effects attributed to sevelamer on cardiovascular mortality are partly due to the hypolipaemic action of the molecule51. The capacity to reduce intestinal cholesterol absorption with sevelamer is well-known thanks to several studies which report this effect10,52. This hypolipaemic effect has been widely described in our field. In peritoneal dialysis, sevelamer has been shown to reduce phosphorus and cholesterol in a multicentre crossover trial conducted in Spain53. In our field, this effect has also led to reduce the need for statins in patients with chronic kidney failure54.
c) Sevelamer and p-Cresol
Among the uremic toxins are those molecules produced by the altered intestinal flora in patients with kidney disease. The most widely studied toxins are derived from the metabolism of aromatic amino acids by this abnormal intestinal f lora and which generate molecules such as phenols and indoles. These are absorbed into the blood current and constitute uremic toxins with high clinical implications. One of these phenols is p-Cresol, a molecule that has proven to have an important role in renal function impairment, such as in vascular damage in patients with kidney disease55. The binding power of sevelamer in the intestine does not seem to be exclusive of dietary phosphorus. Sevelamer has been described as a potential absorbent of these molecules of intestinal origin, such as p-Cresol56. This binding power has been quantified in 10-15% of indoles and 40-50% of p-Cresol depending on intestinal pH57. However, in other studies, sevelamer has not proven to reduce the absorption of these molecules in CKD rat models58.
Recently, a study of 57 peritoneal dialysis patients reported that patients who were being treated with sevelamer showed lower p-Cresol levels. Although it is a cross-sectional observational study where such an effect cannot be attributed to this drug, it would undoubtedly support the evidence presented by other authors59.
d) Sevelamer and glycation products
Advanced glycation end products (AGE) are highly inflammatory, oxidative and atherogenic molecules formed as a result of the oxidation of carbohydrates, lipids and amino acids60. These molecules build up to a greater extent in patients with kidney disease, as renal function gets impaired. It is known that intestinal absorption of AGE determines AGE plasma concentrations61. Sevelamer reduces intracellular and serum levels of AGE in patients with stage 3 to 4 CKD with diabetic nephropathy50 and in diabetic patients on dialysis62. One of these molecules, pentosidine, is reduced with sevelamer, but increases with calcium binders63. A recent 8-week randomised crossover study in patients with stage 2 to 4 CKD proved that treatment with sevelamer carbonate reduces AGE, haemoglobin A1C, and several inflammatory biomarkers50. These preliminary data suggest the potential benefits of sevelamer in reducing proinflammatory cytokines50.
e) Sevelamer and uric acid
Uric acid is a molecule which induces oxidative stress and endothelial damage in patients with CKD64. There is controversy about whether or not sevelamer is capable of reducing serum uric acid levels. Several studies have observed a reduction in serum uric acid in haemodialysis65 and peritoneal dialysis66 patients. Other authors, however, have not found this effect67.
f) Sevelamer and fetuin-A.
Fetuin-A is a glycoprotein that is decreased in situation of systemic inflammation68. Serum fetuin-A levels are lower in patients with CKD than in healthy controls, possibly due to the presence of inflammation, as suggested by the negative correlation between fetuin-A and C-reactive protein69.
Caglar et al. found that sevelamer has short-term effects on fetuin-A levels and consequently on endothelial dysfunction. In an 8-week randomised prospective study in 50 patients with stage 4 CKD, the sevelamer effect was compared to calcium acetate effect on fetuin-A levels and endothelial dysfunction. Fetuin-A levels and flow-mediated dilation were determined both at baseline and after treatment. Patients with CKD showed significantly lower fetuin-A levels. The use of sevelamer led to a significant increase in fetuin-A concentration with dilation improvement, while no significant difference was observed in the calcium acetate group. In a multiple regression analysis, flow-mediated dilation was independently related to fetuin-A70.
3Sevelamer, endothelium and inflammationThere is evidence that sevelamer reduces the inflammatory status in patients with kidney disease. Chennasamudram et al. compared the effects of sevelamer carbonate and calcium carbonate on endothelial function (EF) and inflammation in peritoneal dialysis (PD) patients with type 2 diabetes mellitus (T2DM). EF biomarkers, proinflammatory cytokines, albumin, calcium, phosphate and lipids were measured at baseline and at the end of each treatment. It was observed that treatment with sevelamer carbonate has beneficial effects compared to calcium carbonate as far as inflammation decrease is concerned, since serum endothelin-1, plasminogen activator inhibitor-1, C-reactive protein and interleukin-6 levels were reduced. It also significantly improved the lipid profile compared to calcium carbonate71. Navarro et al. have equally shown how sevelamer reduces C-reactive protein, IL-6, endotoxin levels and CD14 concentrations in haemodialysis patients, while calcium binders are not capable of doing so72.
All the foregoing effects would be either directly or indirectly involved in the reduction of the inflammatory state. Phosphorus has been shown to be a potent inflammatory molecule that causes vascular damage73, so its mere reduction would entail a decrease. FGF23 and PTH are also proinflammatory molecules which have shown their implication in vascular damage74.
Vascular and endothelial inflammation is responsible for cardiovascular damage in uremic patients75. However, as has already been described, each molecule involved, whose blockage is related to the pleiotropic effects of sevelamer, individually shows an association with inflammation. Their significance in inflammation, either separately or jointly, has not been determined yet. Oxidised LDL cholesterol, bacterial endotoxins, AGE and bacterial toxins, such as p-Cresol, would increase inf lammatory cell activity and oxidation76.
4.- Sevelamer and anaemiaCurrent information about the chelating effects of phosphorus on anaemia in patients with CKD is quite scarce. Anaemia is another important complication in HD patients, and it is associated with a reduced quality of life, higher cardiovascular morbidity and increased mortality77–79.
Aasebø et al. observed that patients who received a higher dose of sevelamer showed higher haemoglobin levels than those treated with a lower dose, although this association was not significant in the multivariate analysis80.
Ikee et al. studied whether the use of vitamin D, sevelamer and cinacalcet analogues affect the capacity of response to erythropoiesis-stimulating agents (ESA) in haemodialysis patients who are treated with them. Preliminary data showed an independent association between the sevelamer dose and the capacity of response to ESA in haemodialysis patients. The univariate analysis showed a significant association between the ESA resistance index and transferrin saturation (TSAT) rate, vitamin D analogue dose, and sevelamer dose. In the multivariate analysis, the sevelamer dose and the TSAT turned out to be determining factors independent from the ESA resistance index81.
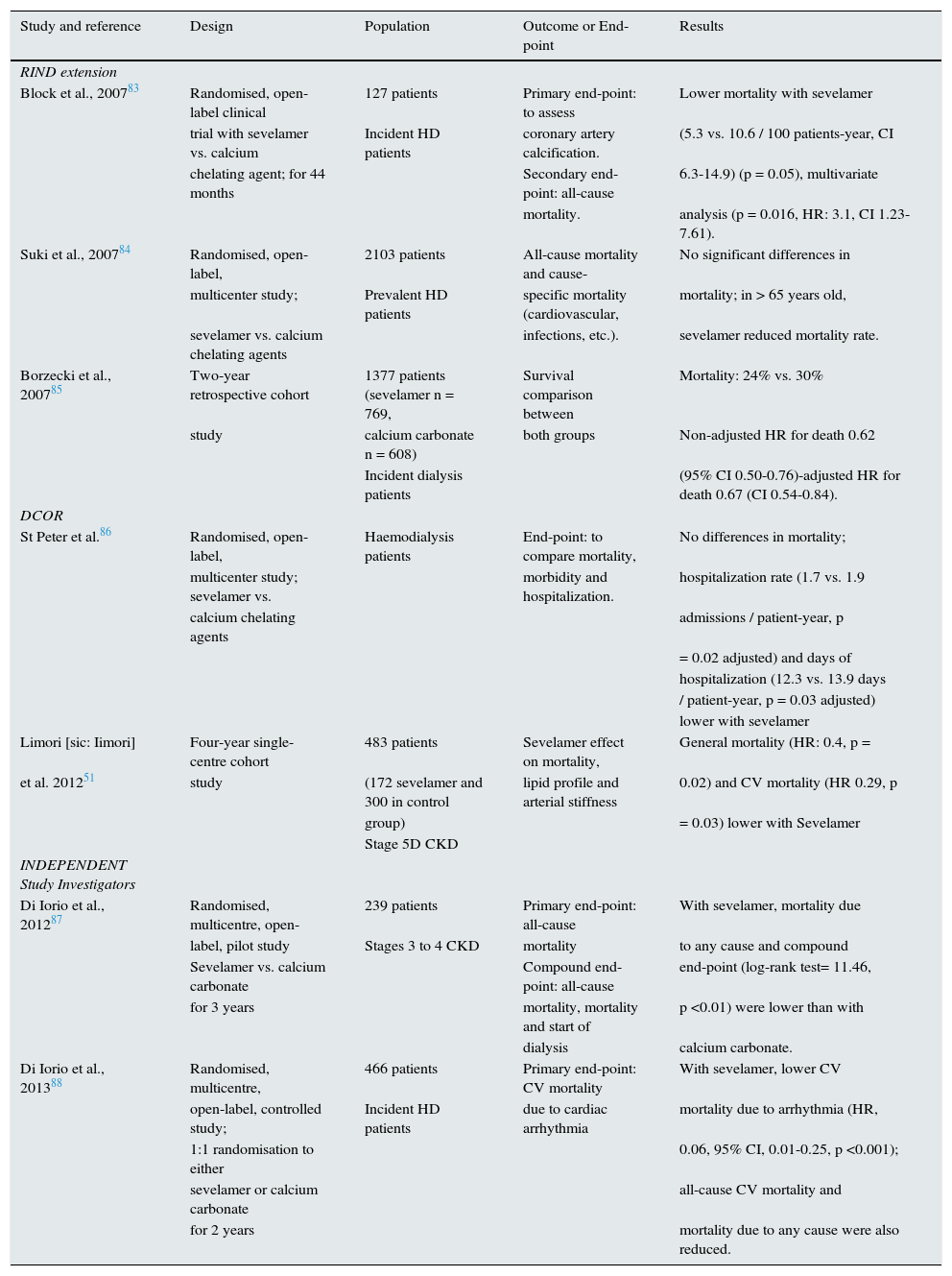
Main studies where Sevelamer has been associated with an improvement in mortality.
| Study and reference | Design | Population | Outcome or End-point | Results |
|---|---|---|---|---|
| RIND extension | ||||
| Block et al., 200783 | Randomised, open-label clinical | 127 patients | Primary end-point: to assess | Lower mortality with sevelamer |
| trial with sevelamer vs. calcium | Incident HD patients | coronary artery calcification. | (5.3 vs. 10.6 / 100 patients-year, CI | |
| chelating agent; for 44 months | Secondary end-point: all-cause | 6.3-14.9) (p = 0.05), multivariate | ||
| mortality. | analysis (p = 0.016, HR: 3.1, CI 1.23-7.61). | |||
| Suki et al., 200784 | Randomised, open-label, | 2103 patients | All-cause mortality and cause- | No significant differences in |
| multicenter study; | Prevalent HD patients | specific mortality (cardiovascular, | mortality; in > 65 years old, | |
| sevelamer vs. calcium chelating agents | infections, etc.). | sevelamer reduced mortality rate. | ||
| Borzecki et al., 200785 | Two-year retrospective cohort | 1377 patients (sevelamer n = 769, | Survival comparison between | Mortality: 24% vs. 30% |
| study | calcium carbonate n = 608) | both groups | Non-adjusted HR for death 0.62 | |
| Incident dialysis patients | (95% CI 0.50-0.76)-adjusted HR for death 0.67 (CI 0.54-0.84). | |||
| DCOR | ||||
| St Peter et al.86 | Randomised, open-label, | Haemodialysis patients | End-point: to compare mortality, | No differences in mortality; |
| multicenter study; sevelamer vs. | morbidity and hospitalization. | hospitalization rate (1.7 vs. 1.9 | ||
| calcium chelating agents | admissions / patient-year, p | |||
| = 0.02 adjusted) and days of | ||||
| hospitalization (12.3 vs. 13.9 days | ||||
| / patient-year, p = 0.03 adjusted) | ||||
| lower with sevelamer | ||||
| Limori [sic: Iimori] | Four-year single-centre cohort | 483 patients | Sevelamer effect on mortality, | General mortality (HR: 0.4, p = |
| et al. 201251 | study | (172 sevelamer and 300 in control | lipid profile and arterial stiffness | 0.02) and CV mortality (HR 0.29, p |
| group) | = 0.03) lower with Sevelamer | |||
| Stage 5D CKD | ||||
| INDEPENDENT Study Investigators | ||||
| Di Iorio et al., 201287 | Randomised, multicentre, open- | 239 patients | Primary end-point: all-cause | With sevelamer, mortality due |
| label, pilot study | Stages 3 to 4 CKD | mortality | to any cause and compound | |
| Sevelamer vs. calcium carbonate | Compound end-point: all-cause | end-point (log-rank test= 11.46, | ||
| for 3 years | mortality, mortality and start of | p <0.01) were lower than with | ||
| dialysis | calcium carbonate. | |||
| Di Iorio et al., 201388 | Randomised, multicentre, | 466 patients | Primary end-point: CV mortality | With sevelamer, lower CV |
| open-label, controlled study; | Incident HD patients | due to cardiac arrhythmia | mortality due to arrhythmia (HR, | |
| 1:1 randomisation to either | 0.06, 95% CI, 0.01-0.25, p <0.001); | |||
| sevelamer or calcium carbonate | all-cause CV mortality and | |||
| for 2 years | mortality due to any cause were also reduced. |
CV: cardiovascular, CKD: chronic kidney disease, HD: haemodialysis, HR: hazard ratio
All the effects described up to now have a direct effect on the patient's clinical situation and are translated into a reduction in cardiovascular mortality.
Cardiovascular disease as an important cause of mortality in patients with CKD, and hyperphosphataemia seems to be one of the most important factors involved. Phosphorus has been associated with the development of inflammation and atherosclerosis in patients with CKD, as well as in the general population with normal renal function, since it favours endothelial dysfunction, increases intima-media thickness, and leads to higher cardiovascular mortality82.
Vascular calcification progression is one of the late markers of vascular damage; for this reason, its slowdown or disappearance contributes to a longer survival. Coronary artery calcification (CAC) is slower in haemodialysis patients treated with sevelamer than in those treated with calcium-based phosphorus chelating agents89,90. This effect is due to several sevelamer actions. Sevelamer reduces low-density lipoprotein cholesterol (LDL-C). In the CARE-2 study, Ounibi et al. tried to verify whether the intensive reduction in LDL-C levels with atorvastatin (reaching serum levels lower than 70mg/ dL in 203 prevalent haemodialysis patients treated with calcium acetate) could result in CAC progression rates similar to those shown by patients treated with sevelamer. The scoring change in CAC was assessed by means of electron beam computed tomography. Haemodialysis patients treated with calcium acetate or sevelamer for a year, with an exhaustive LDL-C control (<70mg/dL), experienced a similar CAC progression91.
Di Iorio et al. examined whether the use of sevelamer instead of a phosphate calcium chelating agent improves cardiovascular (CV) survival in incident haemodialysis patients in a randomised, open-label, controlled, parallel-group study with a 36-month follow-up. Cardiovascular death due to cardiac arrhythmia was considered the primary endpoint. After a mean follow-up of 28 ± 10 months, 128 deaths were recorded (29 and 88 due to cardiac arrhythmia and all causes of CV death). Patients treated with sevelamer experienced lower cardiovascular mortality due to cardiac arrhythmia compared to patients treated with calcium carbonate (HR, 0.06, 95% CI, 0.01-0.25, P <0.001). Similar results were observed for all cardiovascular mortality causes. These results show that sevelamer improves survival in incident haemodialysis patients compared to a calcium-based phosphate binder88.
Furthermore, Maizel et al. studied the therapeutic effects of sevelamer on CKD-induced cardiovascular alterations; for this purpose, a recently developed mouse model was used, with chronic kidney failure, but with no high blood pressure, hypercholesterolemia or aortic calcification82. This mouse model is characterised by the fact that after six weeks of chronic kidney failure, the mice developed cardiovascular abnormalities, including left ventricular hypertrophy (LVH), diastolic dysfunction, aortic stiffness and endothelial dysfunction92. Animals started treatment with sevelamer after 6 weeks of CKD induction and were reassessed 8 and 14 weeks later. Following the first 8 weeks of treatment with sevelamer, mice with chronic kidney failure showed a reduction in serum phosphate levels and an improvement in systolic expansion of the aortic root, pulse wave velocity and diastolic function; LVH remained unchanged. After 6 additional weeks with sevelamer, LVH had not progressed. FGF23 levels were not reduced until 14 weeks after treatment with sevelamer. In the multiple regression analysis, serum phosphate, unlike FGF23, correlated independently with left ventricular diastolic function and mass. Therefore, sevelamer primarily improved aortic stiffness and diastolic dysfunction and secondarily prevented LVH development in mice with CKD82.
Chue et al. raised the hypothesis that the decrease in phosphate gastrointestinal absorption with the use of sevelamer carbonate would reduce serum levels of phosphatonins such as FGF-23, thus reducing LV mass and arterial stiffness, as well as improving LV systolic function and diastolic function in patients with early-stage CKD. For such purpose, a randomised, double-blind, placebo-controlled study was conducted with 120 patients with stage 3 nondiabetic CKD. After 40 weeks, no statistically significant differences were found between sevelamer and placebo with respect to the left ventricular mass, systolic and diastolic function measured by magnetic resonance imaging, or the carotid-femoral pulse wave velocity. This study does not provide any evidence that sevelamer carbonate improves left ventricular mass, LV function, or arterial stiffness in stage 3 nondiabetic CKD93.
Cardiovascular protection offered by sevelamer seems to be partly due to the reduction in phosphorus absorption, with no added calcium, as well as lipid reduction. Calcium-based phosphorus chelating agents are commonly used to treat hyperphosphataemia; however, it has been observed that they increase calcium load, so calcium-free phosphorus chelating agents are recommended94,95. Sevelamer produces a significant decrease in serum phosphorus levels without altering serum calcium levels, thus causing lower vascular calcification rates. In addition, treatment with sevelamer reduces total and low-density lipoprotein cholesterol, apolipoprotein B, α2-microglobulin and C-reactive protein levels, while increasing high-density lipoprotein levels96. In this context, Iimori et al. examined the effects of sevelamer on mortality, the lipid profile and arterial stiffness in patients with stage 5D chronic kidney disease. They concluded that a lower mortality in patients being treated with sevelamer HCl can be partly explained by an improvement in dyslipidaemia and arterial stiffness.97.
The beneficial effect of all binders, except for aluminium, in the survival of haemodialysis patients has been recently verified by the COSMOS study. It was also observed how sevelamer, administered either alone or in combination, improved these patients’ survival98. For years, other authors have purported phosphorus control efficacy, with no added calcium or aluminium, to be one of the advantages of sevelamer in patients with chronic kidney failure99.
ConclusionSevelamer is a phosphate binder that acts in the intestine, preventing absorption without being absorbed. Its use has been associated with lower cardiovascular mortality. To a great extent, this effect is due to its phosphate-binding capacity and a reduction in its adaptive mechanisms, such as FGF23 and PTH, and to the fact that it is a calcium-free molecule. Over more than 10 years of experience with sevelamer, several effects derived from its capacity to bind other molecules in the intestine, which are important for the reduction in cardiovascular risk, have been demonstrated. The reduction in LDL cholesterol, AGE, phenols, uric acid or endotoxins, all of which are of intestinal origin, seems to have an effect on the reduction in the inflammatory status and oxidation of these patients. All these actions have significant effects on concrete clinical aspects, such as anaemia, vascular calcification, atherogenesis and endothelial dysfunction, all of which result in improved survival of patients with CKD.
Conflicto de interesesThe authors declare potential conflicts of interests: Fees for presentations: EGP has received fees for presentations from Sanofi, Shire and Abbvie. JE has received fees for presentations from Sanofi and Abbvie.
Fees as consultant: AO Sanofi consultant.
Instituto de Salud Carlos III [Carlos III Health Institute] (PI10/00072), REDINREN (Red de Investigación Renal [Renal Research Network]) (RD012/0021).