Se ha descrito que el nivel circulante del receptor soluble de la uroquinasa (suPAR) podría ser útil para diferenciar la glomeruloesclerosis focal y segmentaria idiopática de las formas secundarias, pero los resultados publicados son discordantes. En el presente estudio, se analiza la variabilidad intraindividual, las variables clínicas y anatomopatológicas asociadas con los niveles de suPAR y si los niveles circulantes de suPAR permiten diferenciar las formas de glomeruloesclerosis focal y segmentaria (GFS) idiopáticas de las GFS secundarias, independientemente de la presencia de síndrome nefrótico y de la fase de actividad. Métodos: Se estudiaron 35 pacientes afectos de GFS idiopática y 48 con GFS secundaria (83 en total). Se realizaron mediciones de suPAR circulante en el momento del diagnóstico y/o tras la remisión y se analizaron las correlaciones entre niveles de suPAR y variables demográficas, clínicas y bioquímicas. La capacidad de suPAR para diferenciar entre ambas formas de GFS se analizó mediante curvas ROC y análisis de regresión logística. Resultados: En ambas formas de GFS, los niveles de suPAR fueron independientes de la proteinuria y del subtipo histopatológico de GFS y se asociaron significativamente a la edad y a la función renal. Tras ajustar por ambas variables, los niveles de suPAR fueron significativamente superiores en los enfermos con GFS idiopática, tanto en fase de síndrome nefrótico como en situación de remisión parcial o total. El nivel de suPAR con mayor sensibilidad (80 %) y mayor especificidad (73 %) para discriminar entre formas idiopáticas y secundarias fue de 3443,6 pg/ml (área bajo la curva [ABC] 0,78 ± 0,083, p < 0,001). En el análisis de regresión logística, tras ajustar por edad, función renal y presencia de síndrome nefrótico, los niveles de suPAR se asociaron de forma independiente con el diagnóstico de GFS idiopática, pero el modelo tuvo un mal ajuste para categorías de riesgo bajas, en las que tendió a clasificar las formas primarias como secundarias (χ 2 = 11,2 p = 0,027). Conclusiones: Los niveles de suPAR carecen de sensibilidad para diferenciar entre GFS idiopática y secundaria. Sin embargo, valores de suPAR superiores a 4000 ng/ml son altamente específicos de GFS primaria, por lo que, ante un patrón morfológico de GFS asociado a proteinuria no nefrótica, indicarían una baja probabilidad de GFS secundaria.
Background: It has been reported that the circulating level of the soluble urokinase receptor (suPAR) could be useful for distinguishing idiopathic from secondary focal segmental glomerulosclerosis, but the results published are conflicting. In this study, we analyse the intraindividual variability and clinical and anatomopathological variables associated with the suPAR levels and if circulating suPAR levels allow the different forms of focal segmental glomerulosclerosis (FSGS) to be distinguished, i.e., idiopathic forms from secondary FSGS, regardless of the presence of nephrotic syndrome and the activity phase. Method: We studied 35 patients affected by idiopathic FSGS and 48 with secondary FSGS (83 in total). We carried out measurements of circulating suPAR at the time of diagnosis and/or after remission and we analysed correlations between suPAR levels and demographic, clinical and biochemical variables. The ability of suPAR to distinguish between both forms of FSGS was analysed by ROC curves and logistic regression analysis. Results: In both forms of FSGS, suPAR levels were independent of proteinuria and the histopathological subtype of FSGS and they were significantly associated with age and renal function. After adjusting for both variables, suPAR levels were significantly higher in patients with idiopathic FSGS, both in the nephrotic syndrome phase and in partial or complete remission. The most sensitive suPAR level (80%) and the most specific (73%) for discriminating between idiopathic and secondary forms was 3443.6pg/ml (area below curve [ABC] 0.78±0.083, P<.001). In the logistic regression analysis, after adjusting for age, renal function and presence of nephrotic syndrome, suPAR levels were independently associated with the diagnosis of idiopathic FSGS, but the model was poorly adjusted for low risk categories in which it tended to classify primary forms as secondary forms (χ 2 = 11.2 p=.027). Conclusions: SuPAR levels lack sensitivity for differentiating between idiopathic and secondary FSGS. However, suPAR values greater than 4000ng/ml are highly specific to primary FSGS, and as such, with a morphological FSGS pattern associated with non-nephrotic proteinuria, they would indicate a low probability of secondary FSGS.
INTRODUCTION
Focal segmental glomerulosclerosis (FSGS) is a glomerular disease with many potential aetiologies and pathogeneses.1 It is classified as idiopathic or secondary depending on whether or not an aetiology responsible for it is identified.2 Distinguishing between idiopathic and secondary forms is of therapeutic and prognostic interest, given that in the treatment guidelines, only patients with idiopathic forms who present with nephrotic syndrome are considered candidates for immunosuppressant treatment.3 The differentiation between both is based on the clinical profile and the renal ultrastructural examination by electron microscopy.4,5 This classification is based exclusively on clinical and morphological criteria and has arbitrary connotations, since it does not include any aspect related to the pathogenesis.
Idiopathic FSGS can present in the form of outbreaks, and as such, if at the time of diagnosis there is proteinuria but not clinical nephrotic syndrome, it is difficult to determine whether it is an idiopathic form in partial remission or a secondary form for which no specific aetiology has been identified. This differentiation is of prognostic importance, since in the first case, the patient may suffer further outbreaks of activity sensitive to immunomodulation with the possibility of recurrence after transplantation, while the second is not the expected progression.6,7 In idiopathic forms, there is a hypothesis that podocyte injury is caused by a circulating factor or factors.8-10 In a recent study,11 soluble urokinase-type plasminogen activator receptor (suPAR) was identified as a potential soluble factor responsible for podocyte injury. A pathogenic model related to suPAR was described and it was proposed that a serum level above 3000pg/ml11 could be a sensitive and specific biomarker for distinguishing idiopathic FSGS from other diseases that cause nephrotic syndrome. As such, suPAR levels could be useful for distinguishing between idiopathic and secondary forms of FSGS. In this study, however, high dispersion was observed in suPAR levels and patients with non-nephrotic proteinuria levels were included, along with patients for whom no levels of serum albumin or renal biopsy data were available. For these reasons, there is the possibility that the group of patients with FSGS included both idiopathic forms with nephrotic syndrome and secondary forms with non-nephrotic proteinuria or patients with idiopathic forms in partial remission.
The results of this study are difficult to extrapolate to studies carried out with different classification criteria and, to date, although some data confirm that suPAR levels are higher in patients with idiopathic FSGS than in other nephropathies,12 others do not confirm these results13 and there is no evidence that, in patients with secondary FSGS, suPAR levels are independent of the cause responsible for the latter. Data on the relationship between suPAR levels and the disease activity in FSGS are limited and controversial, and little information exists on suPAR levels in patients with FSGS who are in total or partial remission.14 Furthermore, the data reported in the different studies published to date are based on just one measurement of suPAR levels, and as such, there is no information on the variability of values when they are measured in the same individual over different periods without a change in the clinical characteristics of the disease.
The aims of this study are: 1) To analyse clinical, biochemical and pathological variables associated with circulating suPAR levels in patients with idiopathic and secondary FSGS. 2) To analyse whether, in patients with secondary FSGS, suPAR levels differ depending on its aetiology. 3) To analyse the variability of suPAR levels when they are measured in the same individual, without there being changes in the clinical profile of the disease. 4) To analyse whether circulating suPAR levels allow for a distinction between idiopathic FSGS and secondary forms, independently of the presence of nephrotic syndrome and the activity phase.
MATERIAL AND METHOD
This is an observational cross-sectional study comparing groups of patients diagnosed with primary and secondary FSGS.
Sample
We studied 35 patients diagnosed with idiopathic FSGS and 48 patients with secondary FSGS. The diagnosis was carried out via renal biopsy and using clinical criteria. The patients were classified as idiopathic only if they fulfilled all the criteria listed below: 1) Histological diagnosis of focal segmental glomerulosclerosis with evidence of diffuse podocyte effacement in electron microscope 2) Presence of clinical nephrotic syndrome at the time of diagnosis. 3) Exclusion of secondary aetiologies, including: reduction of renal mass, morbid obesity, nephropathy associated with the human immunodeficiency virus, heroin or cocaine use, use of analgesics, vesicoureteral reflux and obstructive sleep apnoea. 4) Absence of family history of chronic kidney disease or renal replacement therapy. 5) In patients under 30 years of age (n=4), absence of demonstrable NPHS2 gene mutations.
Secondary FSGS was diagnosed when there was evidence of proteinuria without clinical nephrotic syndrome, FSGS lesions in the renal biopsy, non-diffuse podocyte effacement in the electron microscope and evidence of a responsible aetiology.
When blood samples were obtained to measure suPAR levels, the patients’ demographic, clinical and biochemical variables were recorded. Serum creatinine was measured by the IDMS-traceable compensated method (Hitachi Modular P-800 Roche Diagnostics, Germany). The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula.15 Measurements of suPAR levels were carried out on serum samples using a commercial ELISA kit (Human uPAR Quantikine® ELISA kit; R&D Systems, Minneapolis, MN, USA; intra-assay variability: 4.1%-7.5%; inter-assay variability: 5.1%-5.9%).11 To analyse reproducibility of measurements, three or more suPAR measurements were taken during the nephrotic phase before starting treatment in 11 out of 20 patients who were studied at the time of diagnosis.
Pathological analysis of renal biopsies
The biopsies were stained with haematoxylin and eosin, PAS (Periodic acid–Schiff)- methenamine and Masson's trichrome for morphological analysis and immunofluorescence studies were carried out with antibodies against IgA, IgG, IgM, C3, fibrinogen and light chains and were processed for an electron microscope study. FSGS lesions were classified in accordance with the criteria of D’Agati et al.16
Statistical analysis
Quantitative variables were expressed as the mean ± 1 standard deviation and the qualitative variables were expressed as a proportion. Group means were compared using variance analysis for more than two groups or the Student’s t-test for independent data and, proportion means were compared using the χ2 test or Fisher's exact test. The correlation between quantitative variables was analysed using Pearson's correlation coefficient. Intra-individual variability after repeated measurements of the suPAR values was calculated by Pearson's coefficient of variation. To identify the variables independently associated with suPAR serum values, a univariate analysis was carried out, and subsequently, a step-by-step multiple regression analysis, with the circulating suPAR level being considered as the dependent variable. The sensitivity and specificity of suPAR levels for identifying patients with idiopathic FSGS was analysed using ROC curves. Subsequently, a step-by-step logistic regression analysis was carried out to identify the independent predictors of idiopathic FSGS diagnosis and its discriminative capacity was analysed by risk categories, using the Hosmer–Lemeshow test.17 A P value <.05 was considered to be statistically significant. Statistical calculations were carried out using the SPSS 20.0 software.
Definitions
Values >3.5g/day were considered to indicate nephrotic range proteinuria. Nephrotic syndrome was defined as proteinuria >3.5g/day associated with hypoalbuminaemia <3.5g/dl. Complete remission: proteinuria <0.3g/day in two consecutive tests. Partial remission: proteinuria <3.5g/day and >0.3g/day.
The study complied with the regulations set out in the Declaration of Helsinki and was approved by the hospital’s ethics committee.
RESULTS
83 patients with FSGS were included in the study, 42.2% (n=35) had primary FSGS and 57.8% (n=48) had secondary FSGS. In both groups, we observed a higher number of males, which was significant in the case of patients with secondary FSGS. In 20 patients with idiopathic FSGS, suPAR levels were measured during the nephrotic syndrome phase before they received any treatment and in 15 during total (n=2) or partial (n=13) remission after finishing immunosuppressant treatment (these 15 patients displayed nephrotic syndrome at diagnosis and received treatment with steroids n=15, calcineurin inhibitors n=11 and mycophenolate mofetil n=5). The group of 48 patients with secondary FSGS included 6 morbidly obese patients, 12 patients with chronic reflux nephropathy, 11 with FSGS associated with reduction of the renal parenchyma, 4 patients with prolonged use of non-steroidal anti-inflammatory drugs and other analgesics and 15 patients with glomerulosclerosis lesions associated with arteriolo-nephroangiosclerosis.
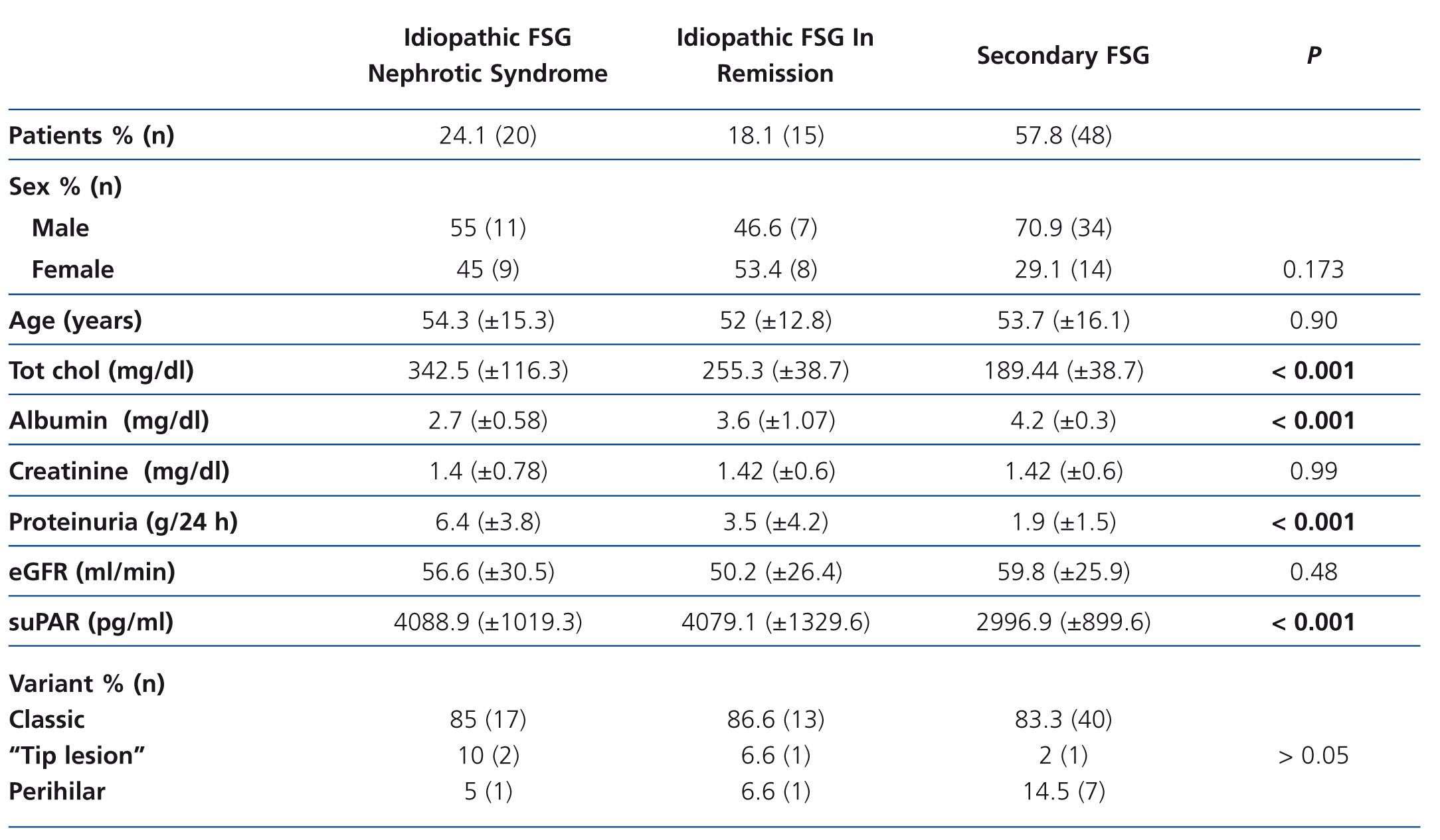
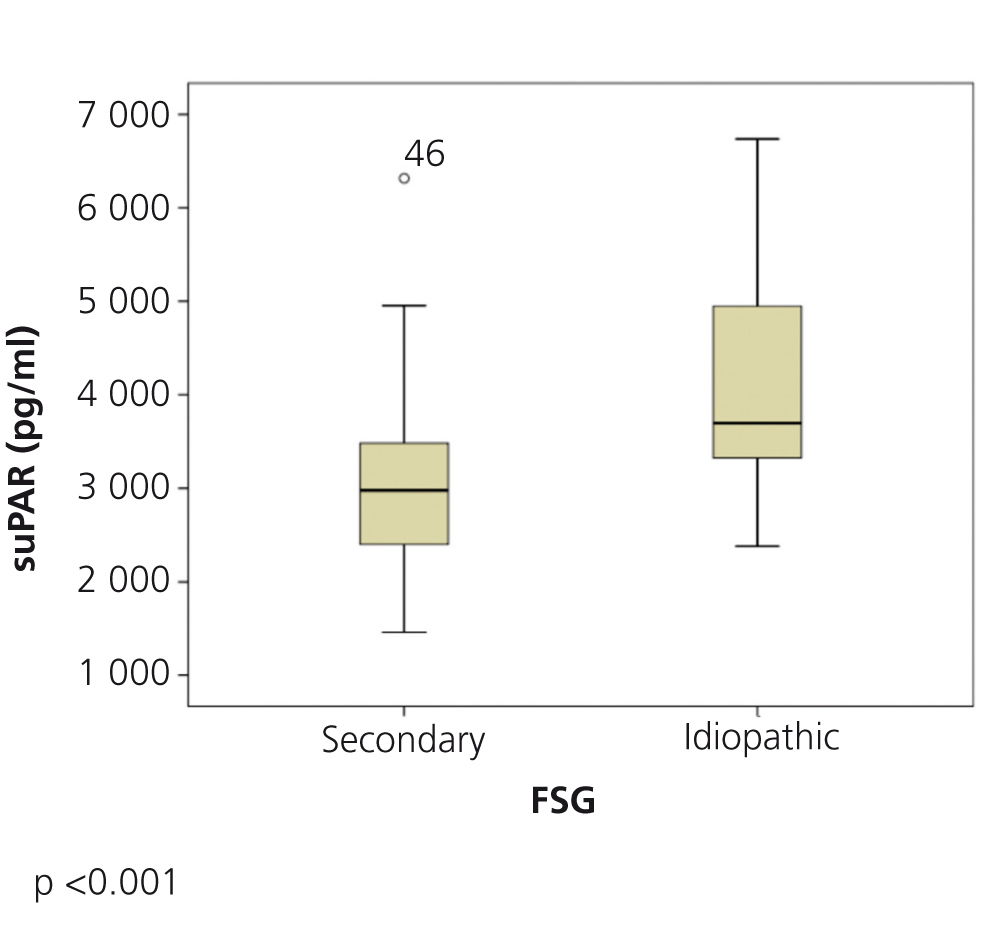
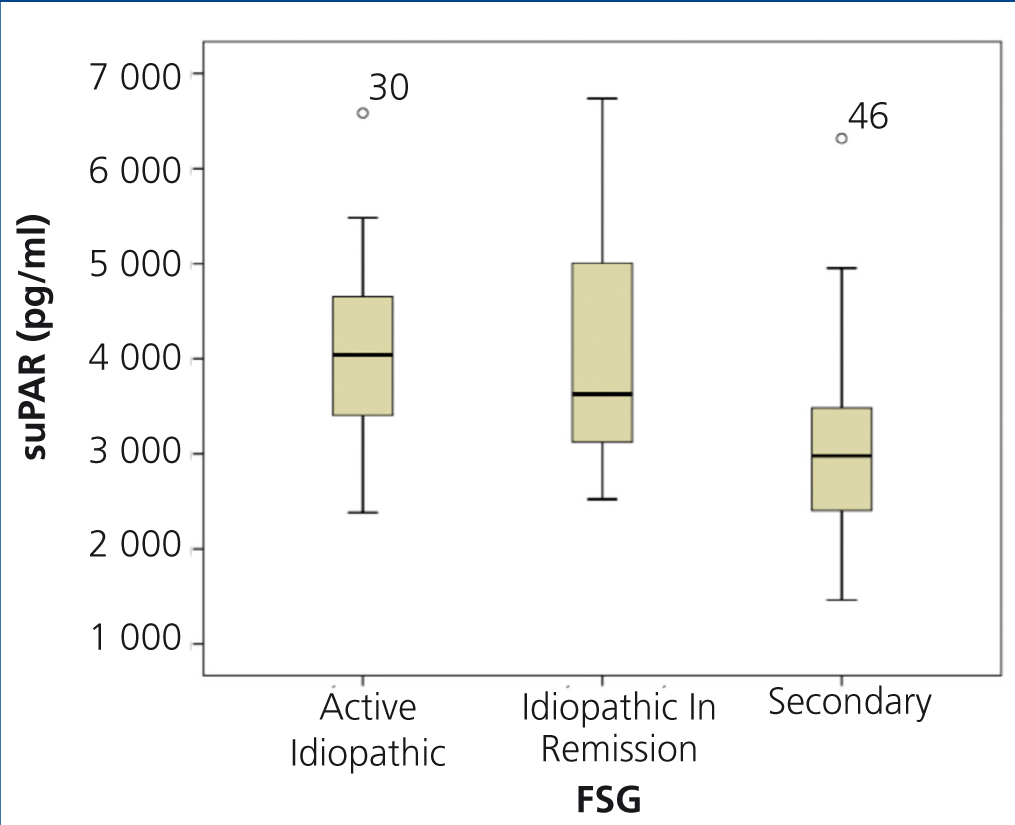
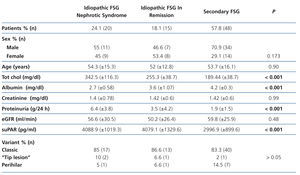
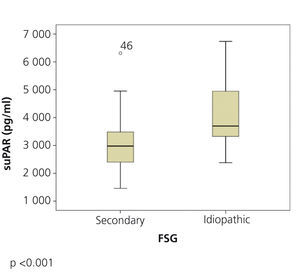
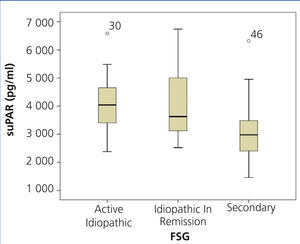
The clinical and demographic characteristics of the patients studied are summarised in Table 1. There were no differences in terms of age, creatinine or glomerular filtration rate between the groups. Patients with FSGS studied during the nephrotic syndrome phase displayed significantly higher total cholesterol and LDL values, lower albumin values and higher proteinuria than the other two patient groups. Circulating suPAR levels were significantly higher in patients with idiopathic FSGS, both in the nephrotic phase and in total or partial remission, than in patients with secondary FSGS (Table 1 and Figure 1). No significant differences were observed between idiopathic FSGS in the nephrotic syndrome phase and in remission (Table 1 and Figure 2). In the group of 15 patients in whom the suPAR level was measured after remission of the nephrotic syndrome, we did not observe differences between patients in total (n=6) or partial (n=9) remission (3745.3±661.3 vs. 3642.1±1056.9pg/ml, P=.48).
The histological variant of FSGS most frequently observed in the three groups was the classic variant and it was not significantly associated with serum suPAR values (Table 1).
On analysing suPAR values from samples taken from one patient at different times, we observed intra-individual variation equal to or less than 11%.
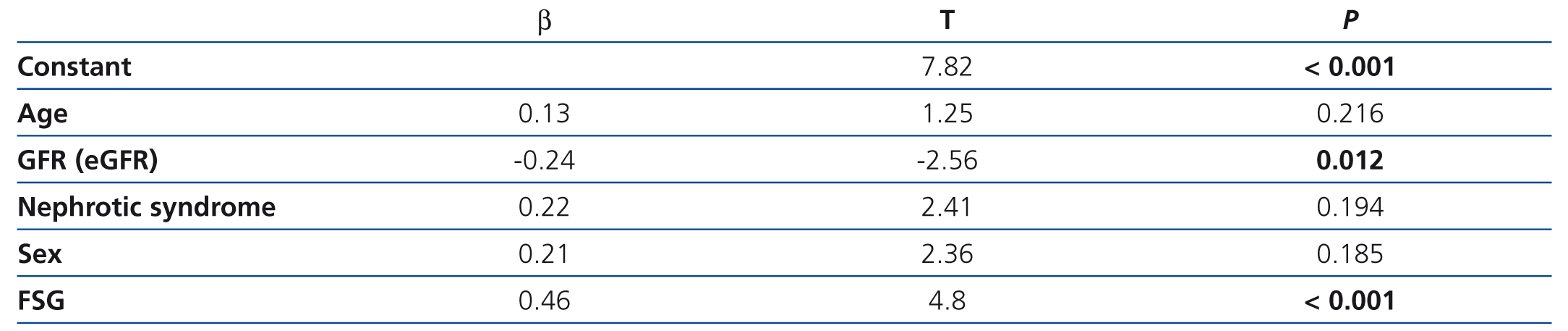
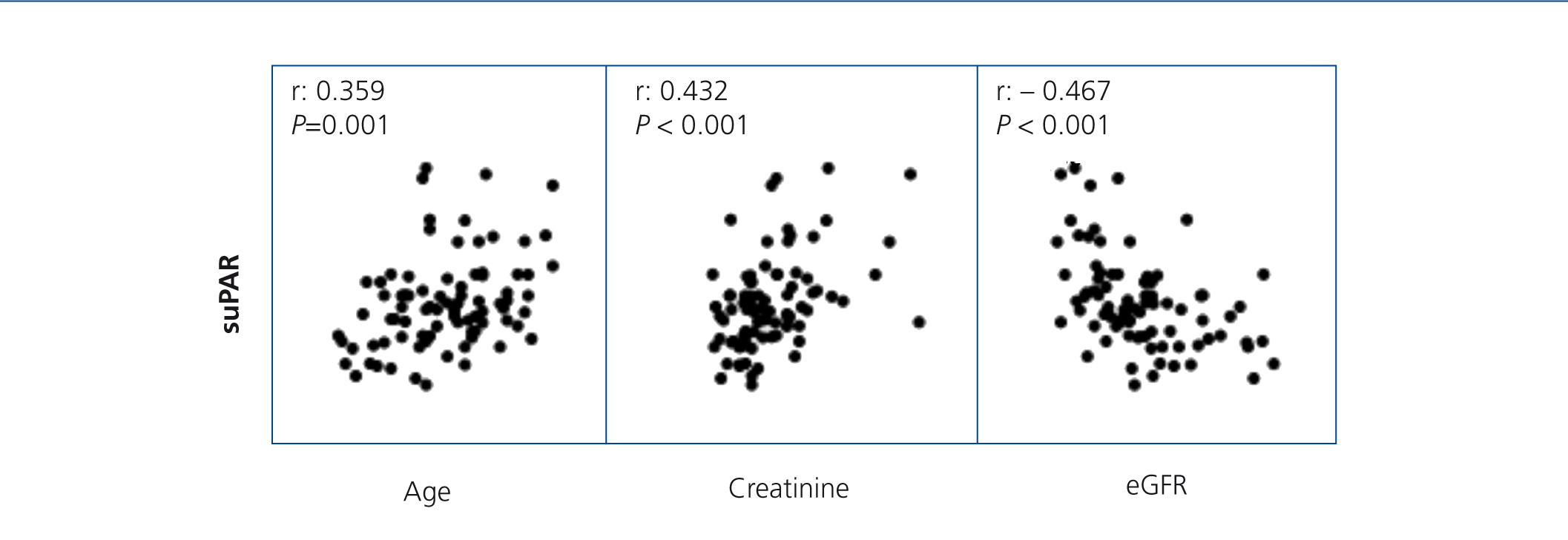
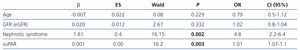
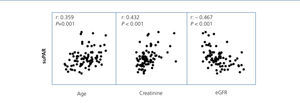
On analysing the variables associated with suPAR levels in the whole sample studied, we observed that, as well as being related to the form of FSGS, suPAR levels were significantly associated with age (r=0.36, P=.02) and renal function (creatinine and eGFR) (r=0.432, and r=–0.467, p ≤.001; respectively) (Figure 3). In the multiple regression analysis, we observed that, after adjusting for age and glomerular filtration, the diagnosis of idiopathic FSGS was an independent predictor of suPAR levels. Overall, the model explained the 39% variability of these levels (Table 2). We did not observe an association between suPAR levels and any of the clinical or biochemical characteristics of the disease at diagnosis (data not displayed). On analysing the variables related to suPAR levels in patients with secondary FSGS, age and the glomerular filtration rate were identified as the only independent predictors, which explains the 32% variability. We did not observe a significant association between suPAR levels and the aetiology of secondary FSGS.
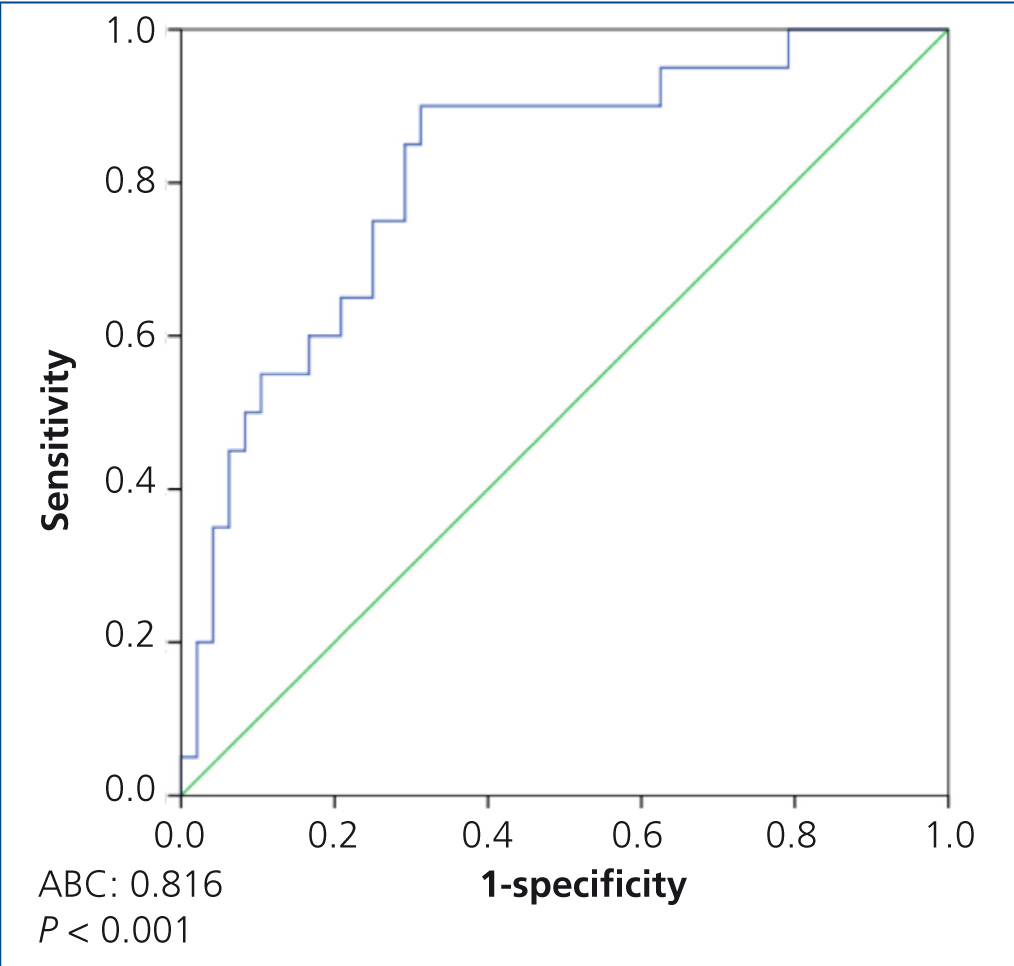
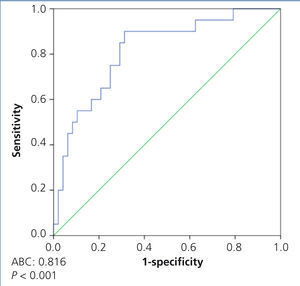
In the ROC curve analysis, which included all patients, we observed that the most sensitive (85%) and the most specific (71%) suPAR value for discriminating between primary and secondary forms was 3336.9pg/ml (area under the curve [AUC] 0.81±0.056, p<.001) (Figure 4).
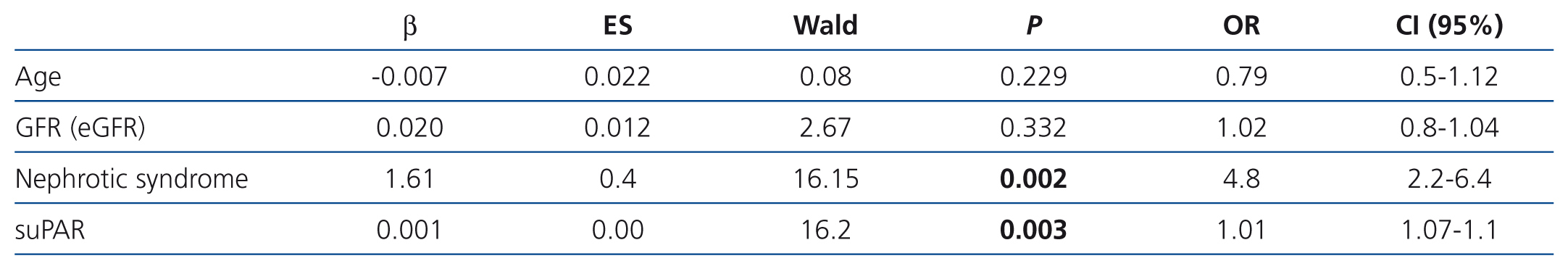
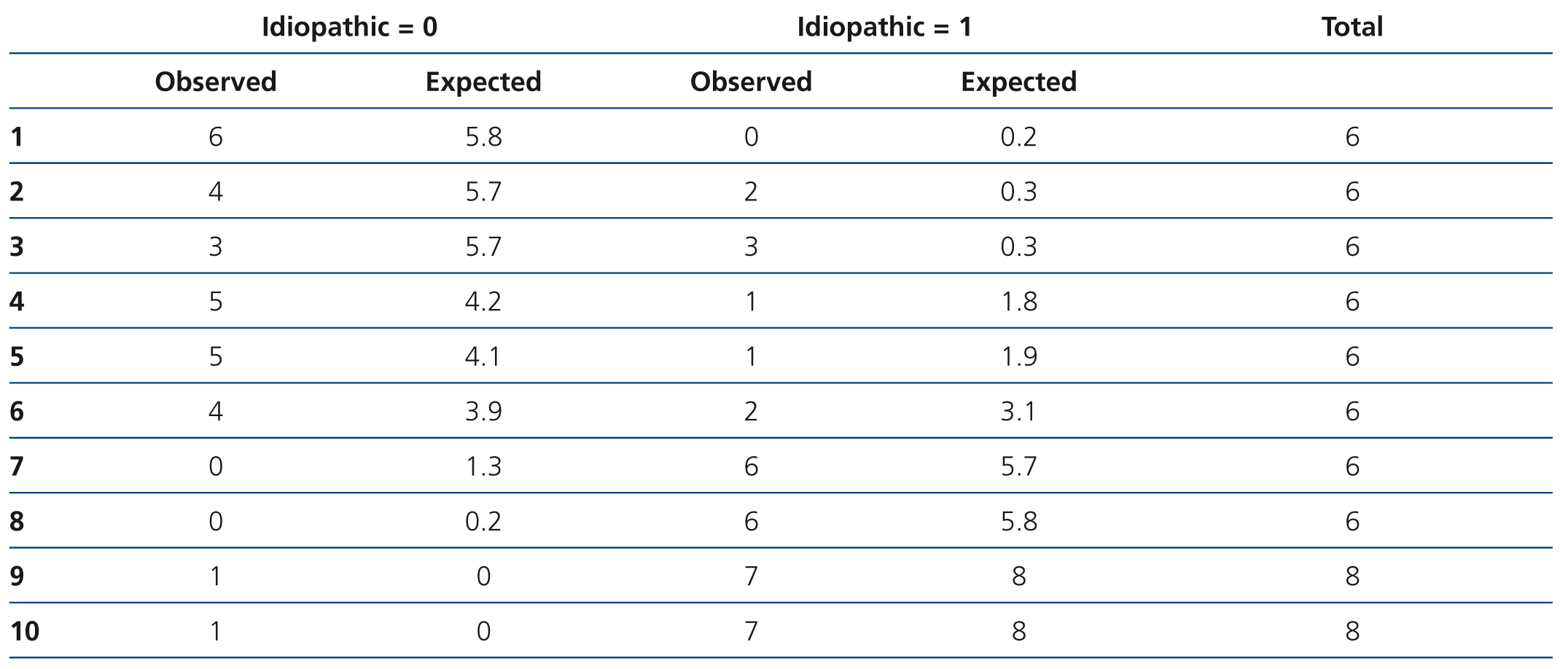
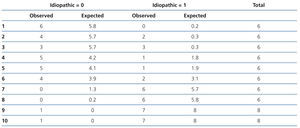
In the logistic model (Table 3), the variables independently associated with the diagnosis of idiopathic FSGS were the presence of clinical nephrotic syndrome and the level of circulating suPAR. Overall, the logistic model adjusted for age and renal function had a higher predictive capacity than when there was no adjustment for the aforementioned variables (AUC: 0.81±0.068 vs. 0.74±0.134 [p=.034]). However, the model was not well-adjusted for all risk categories (χ2: 11.2 p=.027). In high-risk categories, the difference between cases observed and those expected were not statistically significant, while in low-risk categories, the model had a significant tendency to classify idiopathic forms as secondary (Table 4). The capacity of suPAR to distinguish between idiopathic and secondary FSGS forms was similar both when the analysis included 15 patients with FSGS secondary to arteriolo-nefroangioesclerosis and when this subgroup was excluded from the analysis.
DISCUSSION
The results of our study contribute the following data of interest about the clinical usefulness of measuring suPAR in patients with FSGS: firstly, they show that suPAR levels, when they are measured by enzyme immunoanalysis in the same individual on different occasions, without the clinical characteristics of the disease changing, have intra-individual variations that are not higher than 11%, which indicates that the measurements are reliable and reproducible, and that changes higher than these values cannot be attributed to the measurement technique. Secondly, these data contribute to highlighting the existence of notable differences in suPAR levels in different patient groups with FSGS. The suPAR values observed in the group of patients with idiopathic FSGS are similar to those reported by Wei et al.14. However, both the mean values observed in our patients and those reported by Wei14 are higher than those reported by Huang et al.12. These differences in absolute levels between studies could be due to aspects related to the lack of measurement technique standardisation, different classification criteria for idiopathic and secondary forms, or potential ethnic influences in the distribution of suPAR levels, which have been detected in previous studies13 and would need to be analysed in more extensive studies with the inclusion of patients of different ethnicities. Thirdly, our data indicate that, in patients with idiopathic and secondary FSGS, suPAR levels are independent of proteinuria and the pathological form of FSGS, but are significantly associated with age and renal function. As such, both variables must be taken into account when analysing the value of suPAR levels in order to discriminate between idiopathic and secondary forms of FSGS. The relationship between suPAR levels, age and the glomerular filtration rate coincides with that reported to date in all previously published studies.12-14 After adjusting for both variables in the multivariate analysis, our data coincided with those published in previous studies12,14 and indicated that the suPAR level was significantly higher in patients with idiopathic FSGS than in those with secondary forms. The forth aspect of interest in our study is the relationship between suPAR levels and the disease activity in patients with idiopathic FSGS. Data published to date indicate that, in native kidney FSGS, changes relating to suPAR levels are independent predictors of the likelihood of achieving complete remission following treatment, after adjusting for age, sex, ethnicity, glomerular filtration rate and baseline suPAR levels. However, it has been reported that, in patients who go into remission following treatment, the increase in suPAR levels after remission is associated with a higher risk of proteinuria recurrence.14
Since our results lack follow-up of progression, they do not contribute new data to those previously published on the relationship between levels and activity, but they indicate that, independently of the phase in which they are measured, circulating suPAR levels are significantly higher in idiopathic FSGS than in secondary forms. As proof thereof, in the logistic regression analysis, the circulating suPAR level was associated with the diagnosis of idiopathic FSGS, independently of the presence of nephrotic syndrome. Although in the univariate analysis no significant differences were observed in age or renal function between idiopathic and secondary forms, considering that both variables were significantly associated with suPAR levels, they were introduced in the logistic regression analysis as potential confounding variables. Overall, the logistic model adjusted for age and renal function had a higher predictive capacity than when there were no adjustments for these variables, but it was only well-adjusted for high-risk categories. In low-risk categories, represented by the absence of nephrotic syndrome, low suPAR values and a high glomerular filtration rate, the model had a significant tendency to classify idiopathic forms as secondary. These data are explained by the high overlapping of values between groups, fundamentally due to the elevated number of patients with idiopathic FSGS who displayed low suPAR levels and, overall, they coincide with those recently reported by Huang et al.12
Although suPAR sensitivity for distinguishing between idiopathic and secondary forms is low, our data indicate that values higher than 3800ng/ml are uncommon in secondary FSGS and are highly specific to idiopathic FSGS, and as such, with a morphological FSGS pattern associated with non-nephrotic proteinuria, values greater than this figure could indicate a low probability of secondary FSGS.
The main limitation of this study is the size of the sample studied. However, given the high overlapping of values that was observed at low suPAR concentrations between idiopathic and secondary FSGS, it seems indisputable that, even with an increase in the number of patients studied, a low suPAR value would not permit the diagnosis of idiopathic FSGS to be ruled out. Another limitation to bear in mind is the potential classification error between idiopathic and secondary FSGS. The selection criteria used in this study minimised but did not exclude the possibility of idiopathic FSGS being classified as a secondary form, since in any case, the presence of currently known responsible aetiologies was systematically investigated and in all patients studied in the total or partial remission phase, there was evidence of previous nephrotic syndrome and a favourable response to treatment. However, within the patient group initially classified as secondary FSGS, we could not rule out the possibility of some displaying idiopathic FSGS in partial remission. Bearing this limitation in mind, we consider it unlikely that this would have led to a systematic error that would have had a significant influence on the results.
In summary, our data indicate that measuring circulating suPAR levels in patients with FSGS provides reliable and reproducible results. Both in idiopathic FSGS and in secondary forms, suPAR levels are independent of proteinuria and the histopathological subtype of FSGS and are significantly associated with age and renal function. After adjusting for both variables, patients with idiopathic FSGS displayed significantly higher suPAR levels than patients with secondary FSGS, both in the nephrotic syndrome phase and in partial or total remission. The high overlapping of primary and secondary values causes suPAR values not to provide useful information in clinical practice for distinguishing between both entities. Nevertheless, with a morphological FSGS pattern associated with non-nephrotic proteinuria, suPAR values greater than 3800ng/ml could indicate a low probability of secondary FSGS.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Clinical-demographic variables according to patient groups
Table 2. Independent predictors of variability in circulating suPAR levels in the multiple regression analysis adjusted for age, nephrotic syndrome and sex
Table 3. Logistic regression model for predicting the diagnosis of idiopathic focal segmental glomerulosclerosis
Table 4. Analysis of the logistic models discriminative capacity according to risk categories by the Hosmer-Lemeshow test
Figure 1. Soluble urokinase-type plasminogen activator receptor values in idiopathic and secondary focal segmental glomerulosclerosis
Figure 2. Soluble urokinase-type plasminogen activator receptor values in active idiopathic focal segmental glomerulosclerosis, FSGS in remission and secondary FSGS
Figure 3. Soluble urokinase-type plasminogen activator receptor correlation with age, serum creatinine and estimated glomerular filtration rate
Figure 4. ROC curve to distinguish between idiopathic and secondary focal segmental glomerulosclerosis