Renal failure is one of the major health issues faced by many people worldwide. 10% of the global population is affected by chronic kidney disease, and the number of dialysis recipients is growing by an estimated 6% annually.1 Consequently, with the increase in the number of patients, the demand for medical membranes and dialyzers is growing.2 To treat approximately 3.2 million end-stage renal disease patients with extracorporeal blood purification, approximately 320 million dialyzers are required worldwide.2 In 2020, the dialyzer market was valued at $3.19 billion and is estimated to reach $4.53 billion by 2025, registering a CAGR of 6.2%.3
The membranes used in hemodialysis are made up of polymer. For the preparation of membrane, dissolution of polymer is done in an organic solvent and the membrane is formed by a de-mixing process with water.4 In the end of the procedure, solvents are removed by conducting the freshly spun fibers through a sequence of water baths.4 Thus, residual solvent and extractable and leachable substances are washed out of the membranes.4
Solvents used in the manufacture of membranes provided their residual levels in the finished product comply with the acceptable limits set by regulatory agencies based on safety data. At worldwide level, the acceptable limits of residual solvents are defined in the “Guideline Q3C (R6) on impurities: guideline for residual solvents” issued by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH).5
Various solvents can be used for preparing the membrane, such as N-methyl-2-pyrrolidone (NMP), N-ethyl-2-pyrrolidone (NEP), N-octyl-2-pyrrolildone (NOP), dimethyl acetamide (DMAC), dimethyl formamide (DMF), dimethyl sulfoxide (DMSO) or gamma-butyrolactone (GBL) and mixtures thereof.6 The solvent will be present in an amount representing the balance to 100wt.% of the polymer solution. The content of the solvent in the polymer solution preferably is from 60 to 80wt.%, more preferably from 67 to 76.4wt.%.6 N-methyl-2-pyrrolidone (NMP) is especially preferred because of its great stability compared to other solvents.6 Most of the dialysis membranes produced and sold in the world, are based on NMP in the production of the polymers polyethersulfones (PES) and polysulfones (PSU) and or of the membrane spinning solutions.7
NMP is not required to be present in the end product for the membrane properties.7,8 Consequently, NMP is removed by washing at the end of the manufacturing process.8 However, even if the solvent is removed from the membrane at the end of manufacturing process, the possibility of remaining residual traces in the finished product still possible.7 Experience residual NMP content in the membranes is well below 0.1% which is the general notifying threshold value for substances of very high concern of the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) requirements of the European Union.7
Health risk for solvents exposureRisk for hemodialysis patientsData regarding the amount of residual NMP in dialyzers after production still a sensitive subject to the industry.7 According to the ICH guidelines, it is considered that amounts of residual NMP of 5.3mg/day or less (corresponding to 530ppm) in pharmaceuticals or medical devices would be acceptable without justification.5 In experimental model, chronic exposition of rabbits to 530ppm (equivalent to 2000mg/m3: 1ppm=4.12mg/m39) of NMP can results in increased γ-GT in blood, with maternal and fetal toxicity.10 NMP has been shown to have neurotoxic, hepatotoxic, carcinogenic, genotoxic and mutagenic effects.11 In rats, NMP produced an increase in hepatocellular adenomas and carcinomas.9
The main route of excretion of NMP and its metabolites is by the kidneys through urine.12 Elimination of NMP is a saturable process, and unchanged NMP is intensively reabsorbed by the glomeruli.12 In reviewing the literature, we found no data of elimination kinetic of NMP in case of impaired kidney or dialysis. However, the physiologic perturbations associated with renal disease can have a pronounced effect on the kinetics of elimination of NMP and its metabolites from the body in the case of ESRD and dialysis.
Risk for membrane manufacturing workersThe NMP volume used for the production of membranes is about 2000 to 4000tons/year.7 Risks to workers are typically linked to acute and chronic exposures. Workers exposed to concentrations of NMP in air ranging from 3 to 6mg/m3, for even short period (30min), reported severe eye irritation, headache and dermatitis.13 Also a broad set of studies across multiple species show that NMP causes developmental toxicity (fetal death or decreased infant birth rates and reduced body weights) and other health effects.13 The United State Environmental Protection Agency (EPA) estimates that 62,000 workers and 2 million consumers – including many women of childbearing age at risk of developmental effects – are exposed to NMP.14 EPA's analysis shows that these exposures present risks of cancer and non-cancer effects significantly above levels that EPA and other risk managers have traditionally considered unacceptable.14
Impact of solvents on environmental pollutionIn recent years, societal awareness about protecting our environment and health has grown, prompting the membrane industry, among others, to fully consider the impact of its manufacturing processes. Due to the waste-intensive nature of membrane fabrication, billions of liters of contaminated wastewater are produced each year.15 The amount of wastewater generated from membrane manufacturing plants can be estimated to be approximately 100–500L of wastewater per square meter of membranes produced,16 and the solvent contamination in the wastewater always exceeds the allowable levels.11
NMP may enter the environment as emissions to the atmosphere, as the substance is volatile and widely used as a solvent, or it may be released to water as a component of municipal and industrial wastewaters.11 The substance is mobile in soil, and leaching from landfills is thus a possible route of contamination of ground water.11 In air, NMP is expected to be removed by wet deposition or by photochemical reactions with hydroxyl radicals. As the substance is completely miscible in water, it is not expected to adsorb to soil, sediments, or suspended organic matter or to bioconcentrate.11 NMP is not degraded by chemical hydrolysis, and its impact on the aquatic environment is crucial; the NMP is recognized as the most organic solvent to Daphnia magna.17
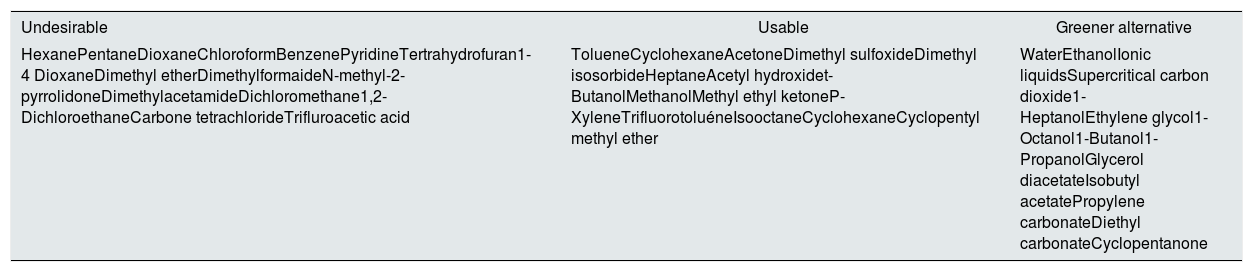
Sustainability for employed solventsTo maintain the sustainable development of membrane technology, it is imperative to replace these toxic materials with environmental-friendly alternatives. In fact, this has been an active membranes research topic around the globe for this very reason.18 In membrane preparation, solvents play a crucial role, and the properties of a solvent and its interaction with the polymer affects the membrane morphology and, thus, performance.19 Hence, identifying a green solvent that can dissolve the polymer of interest is only a prerequisite, as the resulting membrane must exhibit a competitive performance to be adopted by the membrane industry. Furthermore, environmental regulations now restrict the use of toxic solvents for membrane production. Notably, REACH has classified DMF, DMAc, and NMP as substances with very high concerns, and the use of these solvents will be restricted after May 2020.18,20 Recent reports on green solvent alternatives for membrane fabrication are summarized in Table 1.
Recommendations for selecting solvents for membrane fabrication.18
| Undesirable | Usable | Greener alternative |
|---|---|---|
| HexanePentaneDioxaneChloroformBenzenePyridineTertrahydrofuran1-4 DioxaneDimethyl etherDimethylformaideN-methyl-2-pyrrolidoneDimethylacetamideDichloromethane1,2-DichloroethaneCarbone tetrachlorideTrifluroacetic acid | TolueneCyclohexaneAcetoneDimethyl sulfoxideDimethyl isosorbideHeptaneAcetyl hydroxidet-ButanolMethanolMethyl ethyl ketoneP-XyleneTrifluorotoluéneIsooctaneCyclohexaneCyclopentyl methyl ether | WaterEthanolIonic liquidsSupercritical carbon dioxide1-HeptanolEthylene glycol1-Octanol1-Butanol1-PropanolGlycerol diacetateIsobutyl acetatePropylene carbonateDiethyl carbonateCyclopentanone |