Diabetes is a major cause of chronic kidney disease worldwide. Managing CKD in diabetic patients is complex due to accumulation of comorbid conditions such as hypertension, cerebrovascular and peripheral artery disease, as well as increased risk of infection and malnutrition. Reaching end-stage kidney disease, many diabetic patients will choose peritoneal dialysis. This review explores the epidemiology, outcomes, and specific management challenges of diabetic patients undergoing peritoneal dialysis. Literature from PubMed and MEDLINE from 2000 to 2023 was methodically reviewed. In a patient population with increased cardiovascular risk and unique metabolic challenges, the need for individualized treatment strategies in order to improve clinical outcomes is underscored.
La diabetes es una de las principales causas de enfermedad renal crónica en todo el mundo. El manejo de la enfermedad renal cronica en pacientes diabéticos es complejo debido a la acumulación de condiciones comórbidas como hipertensión, enfermedad cerebrovascular y arterial periférica, así como un mayor riesgo de infección y desnutrición. Al llegar a la etapa terminal de la enfermedad renal, muchos pacientes diabéticos optarán por la diálisis peritoneal. Esta revisión explora los resultados y los desafíos específicos del manejo de los pacientes diabéticos en diálisis peritoneal. Se revisó metódicamente la literatura de PubMed y MEDLINE desde 2000 hasta 2023. En una población de pacientes con mayor riesgo cardiovascular y desafíos metabólicos únicos, se subraya la necesidad de estrategias de tratamiento individualizadas para mejorar los resultados clínicos.
Diabetes mellitus (DM) is one of the main causes of chronic kidney disease worldwide and is a significant cumulative cardiovascular (CV) risk factor.1
The management of diabetic chronic kidney disease (CKD) patients is challenging in every stage, as it is often associated with other comorbid states such as hypertension, cerebrovascular and peripheral artery disease, increased risk of infection and malnutrition.
Reaching end stage kidney disease, an individualized “life plan” for kidney care should be discussed with patients and in the absence of technique-associated contraindications, many of these will choose peritoneal dialysis (PD) as a form of kidney replacement therapy (KRT).
Contrary to hemodialysis modality, which is the main KRT worldwide,2 few studies have addressed particularities regarding the management of diabetes mellitus patients undergoing peritoneal dialysis. Clinical studies involving PD cohorts are often small and variability in local prescription protocols lead to difficulty in interpretation of results.
In this review we intend to address epidemiology, outcomes, particularities in prescription and diabetes mellitus management in this subgroup of patients. Incident diabetes mellitus during the course of PD treatment modality is beyond the scope of this review.
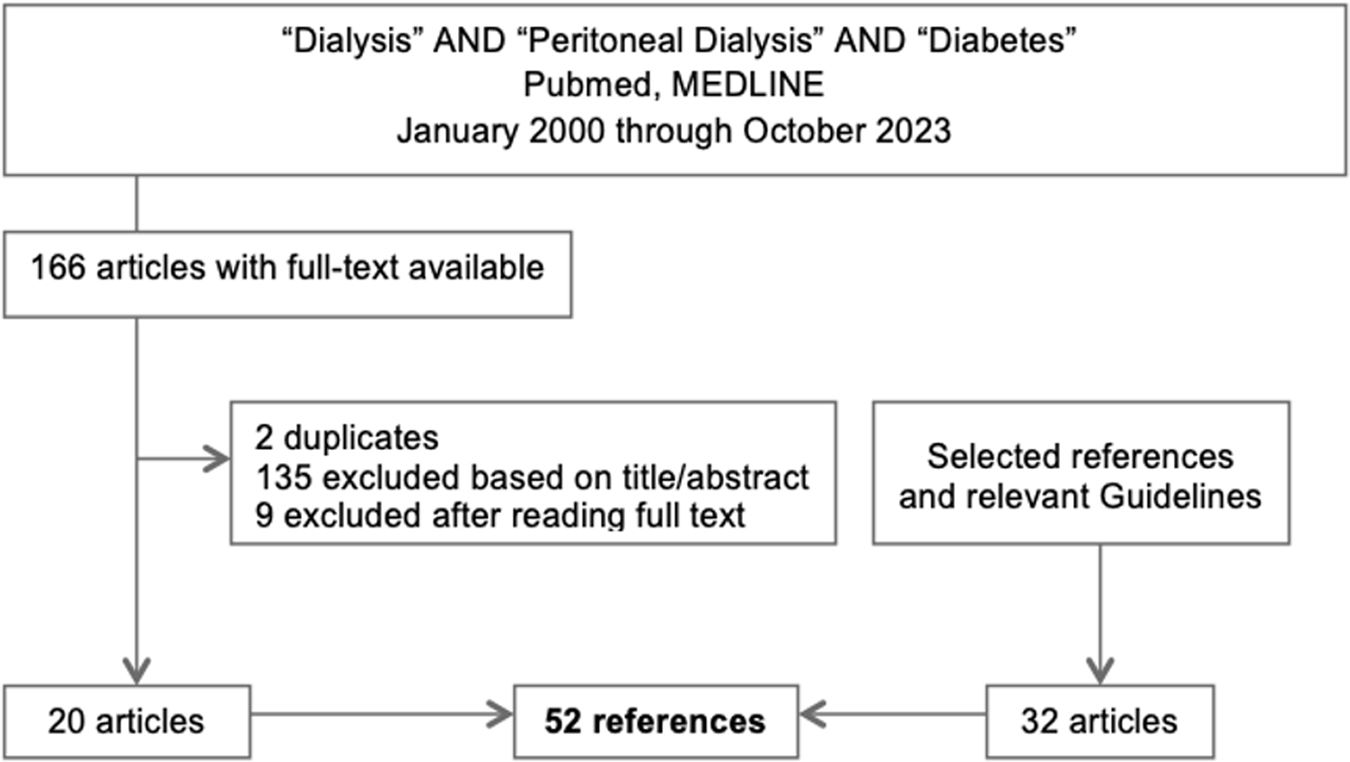
Main textSearch methodologyWe reviewed literature using PubMed, MEDLINE from 2000 through October 2023. A search query with the terms “dialysis”, “peritoneal dialysis” and “diabetes” retrieved 166 articles of which full text was available. These included narrative reviews, systematic reviews and meta-analysis, observational prospective and cross sectional studies, randomized control trials and case reports. After excluding duplicates, 135 were excluded based on title and abstract, 9 excluded after reading the full text. Selected references deemed relevant were also reviewed as well as guidelines in the field where also included in this review (Fig. 1.)
Effect of dialysis modality on cardiovascular and survival outcomes in diabetic patientsMortality between patients treated with PD varies between and within countries however has been reported to be similar to that of HD.3,4 In recent years patient survival on PD has been reported to improve in many countries.5
The role of PD as an option KRT for patients with diabetic kidney disease is well established and used world-wide.6 Survival advantages on dialysis modality selection in diabetic end stage kidney disease population are controversial throughout literature, with mainly low quality evidence and meta analysis reaching opposite conclusions.7
A meta-analysis of observational cohort studies (17 studies, including n=504304 dialysis patients) revealed increased mortality rate of diabetic patients in Asian countries on PD when compared to HD, with a HR of 1.46 (95% CI 1.23–1.75) vs. HR 1.11 (95% CI 1.01–1.21) in non-Asian countries. Authors noted marked heterogeneity of analyzed data and included older cohorts meaning that advances in PD including availability of icodextrin, and low-GDP neutral pH fluids are not reflected in all studies.8 This has also been supported by another Asian review and meta-analysis, outlining diabetes, among others, as a risk factor for mortality in PD patients.9
Increased CV mortality in diabetic PD patients with metabolic syndrome when compared to HD has been suggested by a recent meta-analysis (8 observational studies, n=790 patients on KRT). Survival advantage associated with large body size (commonly referred to as the obesity paradox in CKD) was less evident in PD than HD patients. Authors speculate that exposure to increased dextrose concentrations (and therefore calories) in the PD dialysate may be responsible for this difference.10
On the other hand, lower risk of CV events was observed in PD patients by a Chinese meta analysis on the efficacy and safety of PD and HD in diabetic kidney failure. The study included 6 small cohorts, with n=635 diabetic patients on PD and n=719 on HD. The PD group showed less CV events with an OR 0.42 (95% CI: 0.28, 0.62). However, as is frequently observed in frequent studies with PD patients, a high risk performance and detection bias was reported by authors.11
Generally, PD and HD modalities are considered adequate options for diabetic patients. Some studies however seem to demonstrate differential temporal advantage in favor of PD in patients with elevated CV risk, including diabetic population. The apparent survival benefit in the first years seems to be lost over time12 although these differences may be causal, possibly due to differences in comorbidities at the start of treatment and better preservation of residual kidney function in PD patients.13,14
All in all, choice of dialysis modality (HD vs. PD) and within dialysis modality (chronic ambulatory peritoneal dialysis vs. automated peritoneal dialysis) in diabetic patients seems to depend on the general criteria that also apply to the non-diabetic ESKD population.
Glycemic control particularities and targets in peritoneal dialysisSelf-monitoring blood glucose and continuous glucose monitoringSelf-monitoring of blood glucose (SMBG) and continuous glucose monitoring (CGM) are used in the general diabetic population for assessing short term glycemic variability.
SMBG is the most common form of monitoring glycemic control including in diabetic patients that initiate PD. False elevations of blood glucose measures occur with glucose dehydrogenase pyrroloquinoline quinone (GDH-PQQ) based tests in patients on icodextrin. Patient education is essential as misinterpretation of readings may lead to inappropriate insulin injection.15
On the other hand, CGM measures interstitial glucose levels by a subcutaneous sensor. These are reliable indicators of real-time blood glucose concentrations, validated in small samples of diabetic HD and PD patients.16,17 Currently, 2 systems have been approved specifically for use in the PD population.15
KDIGO Diabetes Guidelines outline as a practice point that a measure of average blood glucose expressed in the units of HbA1c (%) can be derived from CGM data, known as the Glucose Management Indicator (GMI).18 This indicator can be useful to infer glycemic control in individuals where HbA1c is suspected to be discordant with real measured blood glucose levels and diabetic control. Use of GMI in patients on dialysis is outlined in these guidelines, taking into account that the indicator should be regularly re-assessed and appropriate individualized targets should be defined.
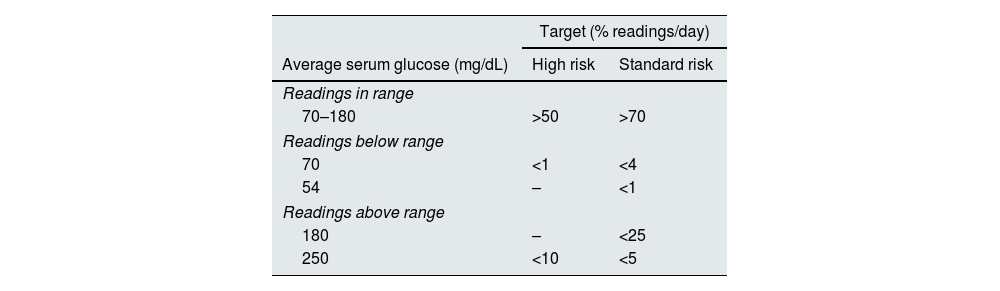
Metabolic control targets of average serum glucose for patients in high-risk groups (including patients with kidney disease) have been issued by an International consensus report.19 In the high risk group, these recommendations are generally less strict allowing for less time in the targeted range, however are more strict in the low range in order to avoid hypoglycemia which can be particularly deleterious (Table 1.).
Metabolic control targets of average serum glucose for DM type 1/type 2 according to Risk Groups.
| Target (% readings/day) | ||
|---|---|---|
| Average serum glucose (mg/dL) | High risk | Standard risk |
| Readings in range | ||
| 70–180 | >50 | >70 |
| Readings below range | ||
| 70 | <1 | <4 |
| 54 | – | <1 |
| Readings above range | ||
| 180 | – | <25 |
| 250 | <10 | <5 |
High risk defined as individuals with a higher risk of complications, comorbid conditions (e.g., cognitive deficits, kidney disease, joint disease, osteoporosis, fracture, and/or cardiovascular disease), and those requiring assisted care, which can complicate treatment prescriptions.
The question if the use of CGM systems can improve outcomes in dialysis patients remains unanswered by the lack of robust evidence. Availability and costs are a key factor in the possibility of generalized implementation of these systems.
Hemoglobin A1cHemoglobin A1c (HbA1c) is a generally acceptable measurement of long-term glycemic control in the general population. Concerns on the accuracy and precision of HbA1c measures in patients with diabetes and advanced kidney disease, particularly on dialysis have been reported in numerous papers and guidelines, including KDIGO and ADA consensus report 2022.17 Limitations of HbA1c in the setting of ESRD include the presence of anemia due to shortened erythrocyte half-life as well as metabolic acidosis, leading to its likely underestimation.16 In part, this may explain controversial results between HbA1c level measurement rates and mortality rates in dialysis patients.
Association between HbA1c levels and all-cause mortality has not been shown in some cohorts in the first 2 years on PD.20 On the other hand, other cohorts of diabetic patients on PD have demonstrated poor glycemic control measured by HbA1c to be associated with higher all-cause mortality, mainly infection-related deaths.21
Higher variation of HbA1c, reflecting both episodes of hypo and hyperglycemia, was seen to be associated with increased mortality in an observational study of 325 patients from the Swedish Renal Registry.22 Other studies have also shown U-shaped relationship between HbA1c and mortality in PD patients showing increased mortality com HbA1C<6 and >8.23
Although the most adequate range of HbA1c in the dialysis population is unknown, current recommendations are extrapolated from the general diabetic population and guidelines suggest monitoring of twice per year, up to 4 times per year if erratic results or recent changes in anti-diabetic therapy.18
Other serum markersBased on the limitations of HbA1c, other glycemic control markers have been proposed as being more suitable for this patient population.
Serum fructosamine, also known as total glycated serum protein (GSP), is a measure of all glycated proteins (mainly albumin). Measures of glycated albumin (GA), on the other hand, reflect the albumin that is covalently bonded to glucose. Both values should be corrected for albumin levels, which are very frequently reduced in PD population. Reflection of glycemia in a shorter timeframe (2–4 weeks) when compared to HbA1c is obtained using these biomarkers. Contrarily to HbA1c, GA is not affected by anemia and red blood cell turnover, meaning it may be more appropriate in CKD patients.24,25
Correlation between HbA1c with glucose levels as well as other markers including GA and total GSP were observed in glycemic indices in the dialysis evaluation (GIDE) Study. This included n=282 (16%) diabetic patients on peritoneal dialysis. Even so, these correlations are progressively weaker as CKD advances.16,26
Total GSP (including GA as ∼90% of these proteins) seems to be an inferior biomarker in PD population when compared to GA.17 GA seems to correlate better with blood glucose when compared to HbA1c in the HD population,27 however, this has not been clearly demonstrated in the PD population.
In the cohort of PD patients from the national registry of PD patients in Japan (n=1282), authors reported that measurements of GA above 20% reference values were associated with decreased survival in this patient population, hypothesizing that it would be a more robust indicator for outcomes in PD patients.20
Although all methods have potential disadvantages, experience and general availability of HbA1c assays seem to stand out as the main advantage of this long-term glycemic control evaluation method. If decisions are based on constant re-evaluation and adequate interpretation in context of each individual patient as opposed to looking at the simple numerical value, it may still be an adequate tool for glycemic management.
Anti-diabetic therapy and peritoneal dialysisInsulinDespite association of insulin resistance and uremia, as CKD progresses, GFR decline requires dose adjustments and a reduction of 50% is recommended in patients with end-stage kidney disease.28
Although there is evidence to support that PD therapy improves uremia-associated insulin resistance,28 large insulin losses in the peritoneal dialysate and severe insulin resistance at baseline have been reported in most CAPD patients.1 The rationale based on this finding would be the need for additional insulin in PD patients in particular. There are however no specific recommendations for these adjustments, currently mainly based on expert opinions.29
A series of clinical considerations and expert opinions on the insulin regimen employed are provided by the authors of a comprehensive revision in which a multiple-daily-injection regime associated with long-acting and short-acting insulin on meal times is preferred both to avoid glycemic variability related to dialysate dextrose and hypoglycemia episodes. Of course, this would require important patient compliance and would be easier if the patient were already on this scheme before PD initiation. Basal insulin morning administration is encouraged to reduce hypoglycemia during the night. On the other hand, patients on automated peritoneal dialysis, night basal insulin may be preferred. Another important point is the need for adjustment, and probable necessary dose reduction in the setting of PD-free rest days.24
Particular attention in insulin adjustment is required in patients transitioning from PD to HD. In a systematic review on the subject, authors found scarce recommendations on prior adjustment of insulin in this scenario, warranting further research on the area.29
Intraperitoneal insulin administration has been compared to usual subcutaneous administration in a meta-analysis, showing apparent superiority in terms of glycemic control. However this route of administration was also associated adverse lipid profile and increased cardiovascular risk.30 There is currently no evidence to support this route of administration in the diabetic PD patients.31
Overall, to avoid adverse effects of both excessive and poor glycemic control, insulin prescription should be tailored to the degree of patient comprehension and glycemic control target, taking into account the PD prescription in order to avoid high glycemic variability. A constant re-evaluation is key and support of a multidisciplinary diabetes team is ideal.
SGLT2 inhibitorsThe most recently approved class of oral anti-diabetic medications are the sodium-glucose co-transporter 2 (SGLT2) inhibitors. Their glucose lowering effect depends upon GFR, meaning that glycemic benefit is low once GFR is <45ml/min/1.732. However, since proven to improve cardiovascular outcomes as well as kidney outcome in CKD, SGLT2 inhibitors have extensively exceeded indications as anti-diabetic agents alone.32,33
Currently, drug withdrawal upon initiation of KRT is supported by the lack of evidence of safety of these medications in dialysis settings.18
Physiological rationale for the use of iSGLT2 in PD patients in terms of RRF preservation, peritoneal membrane protection and overall CV mortality benefits has been proposed base on experimental studies.34
SGLT2 receptors are expressed on peritoneal mesothelial cells human cells and their expression seems to increase with PD duration and also in the setting of peritoneal sclerosis. It has been therefore hypothesized that this receptor plays a role in pathological changes on the peritoneal membrane and may be a therapeutic target.
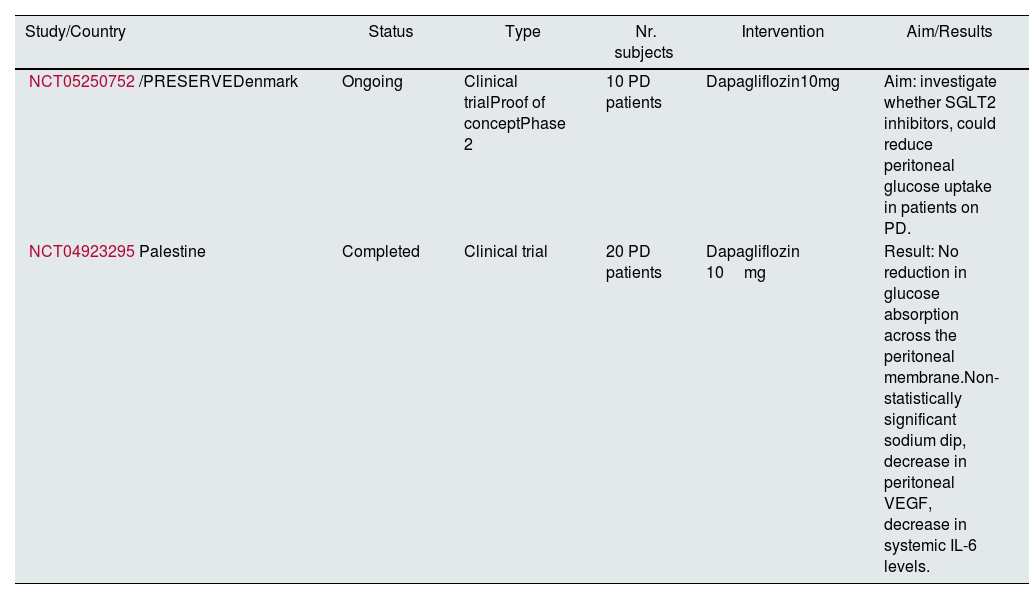
Recent results from the DARE-ESKD Phase 1 trial (NCT05343078), on Pharmacokinetics and Dialyzability of Dapagliflozin in Dialysis Patients including a total of 7 patients (2 on peritoneal dialysis) have shown that dapagliflozin was well tolerated, slightly dialyzable, and had non-accumulating pharmacokinetic properties35 (Table 2).
Trials on SGLT inhibitors in peritoneal dialysis.
| Study/Country | Status | Type | Nr. subjects | Intervention | Aim/Results |
|---|---|---|---|---|---|
| NCT05250752/PRESERVEDenmark | Ongoing | Clinical trialProof of conceptPhase 2 | 10 PD patients | Dapagliflozin10mg | Aim: investigate whether SGLT2 inhibitors, could reduce peritoneal glucose uptake in patients on PD. |
| NCT04923295Palestine | Completed | Clinical trial | 20 PD patients | Dapagliflozin 10mg | Result: No reduction in glucose absorption across the peritoneal membrane.Non-statistically significant sodium dip, decrease in peritoneal VEGF, decrease in systemic IL-6 levels. |
Clinical trials using of SGLT2 inhibitors in PD patients to determine glucose absorption from PD fluid and effect on ultrafiltration are also underway.36,37
Other anti-diabetic drugsIt is important to understand the metabolism of anti-diabetic drugs commonly used in diabetic patients may be affected by PD.38
Glucagon-like peptide 1 (GLP-1) receptor agonists dulaglutide and exenatide are contraindicated when glomerular filtration rate drops below 15 and 30ml/min/1.73m^2, respectively. Although one might argue that the aid in weight loss will help these patients achieve optimal weight for transplant enrollment, we must be aware that limited data exist for the use of the remaining GLP-1 receptor agonists among dialysis patients and therefore, these agents should be used with caution.31
Dipeptidyl peptidase-4 (DPP4) inhibitors can be used in dialysis patients. All except linagliptin require dose adjustment throughout CKD progression.16 Besides their role as anti-diabetic drugs, cell level and animal model studies, in line with observational clinical data have suggested that DPP4 plays a role e in peritoneal fibrosis and functional impairment. Therefore, one might argue that its inhibition may contribute to peritoneal fibrosis prevention and preservation of the peritoneal membrane.39
Peroxisome proliferator-activated receptor-α (PPAR-α) agonists, thiazolidinediones (TZD) are oral anti-diabetic agents thought to act through increased GLUT1 and GLUT 4 cell surface expression, leading to increased glucose uptake and reduced serum glucose levels. Beneficial effects on lipid metabolism have been attributed to this class of drugs. In a small randomized crossover trial, daily 15mg of pioglitazone in CAPD patients (both with and without DM) showed improved insulin resistance, adipokine balance, with reduced markers of inflammation.40 In the diabetic PD population, pioglitazone may be continued without any dose adjustments on PD-free days without increases risk of hypoglycemia.24 The main drawback to its use is related to its adverse effect profile on fluid retention, meaning that it is usually an alternative to other agents with a better safety profile.
Metformin, a classic oral anti-diabetic agent, is contraindicated in severe CKD due to risk of life threatening lactic acidosis as it is eliminated through the kidneys. Other benefits such as cardio-protective properties have been attributed to this drug and these are particularly relevant in the dialysis population. A review on the safety of metformin in maintenance dialysis patients reported three prospective observational studies that included PD patients (total n=119), none of which presented lactic acidosis, although episodes of such events have been seen in case reports. These findings are however of low evidence quality and randomized control trials are inexistent in this population.41
Implications of peritoneal dialysis solutions on diabetic patientsPD dialysate composition includes physiological concentrations of chloride, calcium, sodium, magnesium and either lactate or bicarbonate as a buffer, associated with an osmotic agent to allow for water flow (UF). Glucose is the standard osmotic agent, although icodextrin and amino acids are also available options.42
Reducing glucose exposure in PD solutions is beneficial for various reasons, including the attempt to overcome some degree of insulin resistance in the diabetic patient.1 Biocompatibility of a PD solution is defined as its capacity to leave the anatomical and functional properties of the peritoneal membrane unmodified over time. Therefore, low-GDP neutral pH solutions aim to reduce glucose exposure and hence glucose associated toxicity.35 However, beneficial effects of these solutions on peritoneal membrane function have not been consistent in clinical trials.43,44
In diabetic patients, dextrose-containing PD solutions may exacerbate metabolic abnormalities and increase cardiovascular risk. The IMPENDIA-EDEN trial sought to evaluate the effect of a low-dextrose regimen in improvement of metabolic parameters in diabetic DP patients. A total of total of n=251 patients were either assigned to a control group (using dextrose solutions only) or to the intervention group with the use of low-dextrose solutions (combinations of dextrose, icodextrine and amino-acid solutions) for a follow-up period of 6 months. Results showed improved metabolic indices with low dextrose solutions however association with adverse events due to extracellular volume expansion, raising the concern for uncontrolled volume status in the intervention group.45
In another trial, randomization to icodextrin (n=30) or dextrose (29) based PD solutions for the long dwell in diabetic patients on CAPD with high-average peritoneal transport rate lead to reduced glucose absorption, insulin necessity, as well as fasting blood glucose and triglyceride levels, suggesting that reduced peritoneal absorption of glucose improved metabolic control. In this study, patients on icodextrin had fewer adverse events related to hypervolemia.46 Other trials have supported advantages of icodextrin use in fluid management in the diabetic population however failing to show such improvements on glycemic indices follow up of 24 months.47
Peritoneal membraneDiabetic patients have thicker, poorly vascularized peritoneal membranes. A study in Japan evaluated peritoneal membrane biopsy specimens from 173 patients before and after PD. Through evaluation of the pre-peritoneal dialysis peritoneum, uremia and diabetes was shown to contribute to the pathogenesis of peritoneal sclerosis. Diabetic patients presented thickened vascular walls. These changes seem to be attributed to a greater number of advanced glycation end products (AGE) and glucose degradation products (GDP) present in this population.1,48
In an attempt to study the characteristics of peritoneal water transport in diabetic patients, a prospective, single-center design cross-sectional and longitudinal compared peritoneal water transport between diabetic (n=59) and non-diabetic (n=120) PD patients using 3.86/4.25% dextrose-based peritoneal equilibration tests (PET). A trend towards increased free water transport was observed in non-diabetics when compared to diabetic patients (p=0.033).49
High peritoneal transport status according to PET (including high and high average transport groups) had adverse influence on nutritional status of diabetic patients and reported as a significant independent risk factor for death-censored PD discontinuation or transfer to hemodialysis.50
Peritoneal dialysis associated complications and technique survivalInfections complications associated with PD technique include exit-site infections; tunnel infections and PD associated peritonitis.
Although peritonitis still remains the major cause of PD discontinuation in the general, diabetic patients with poor glycemic control seem to have a increased risk of catheter tunnel and exit-site infections, but not peritonitis.6,51 PD patients with DM are however more predisposed to Coagulase negative staphylococci infections but not Escherichia coli.52
Recent studies have showed that diabetes has not been associated with lower time on PD, and in fact increased in the more recent cohorts.53 Icodextrin-containing solutions seem to have a beneficial effect in this population, leading to reduction of PD discontinuation or transfer to hemodialysis due to volume overload as observed a randomized controlled trial (technique survival rate 71% with icodextrin vs. 45% in dextrose containing solutions).47
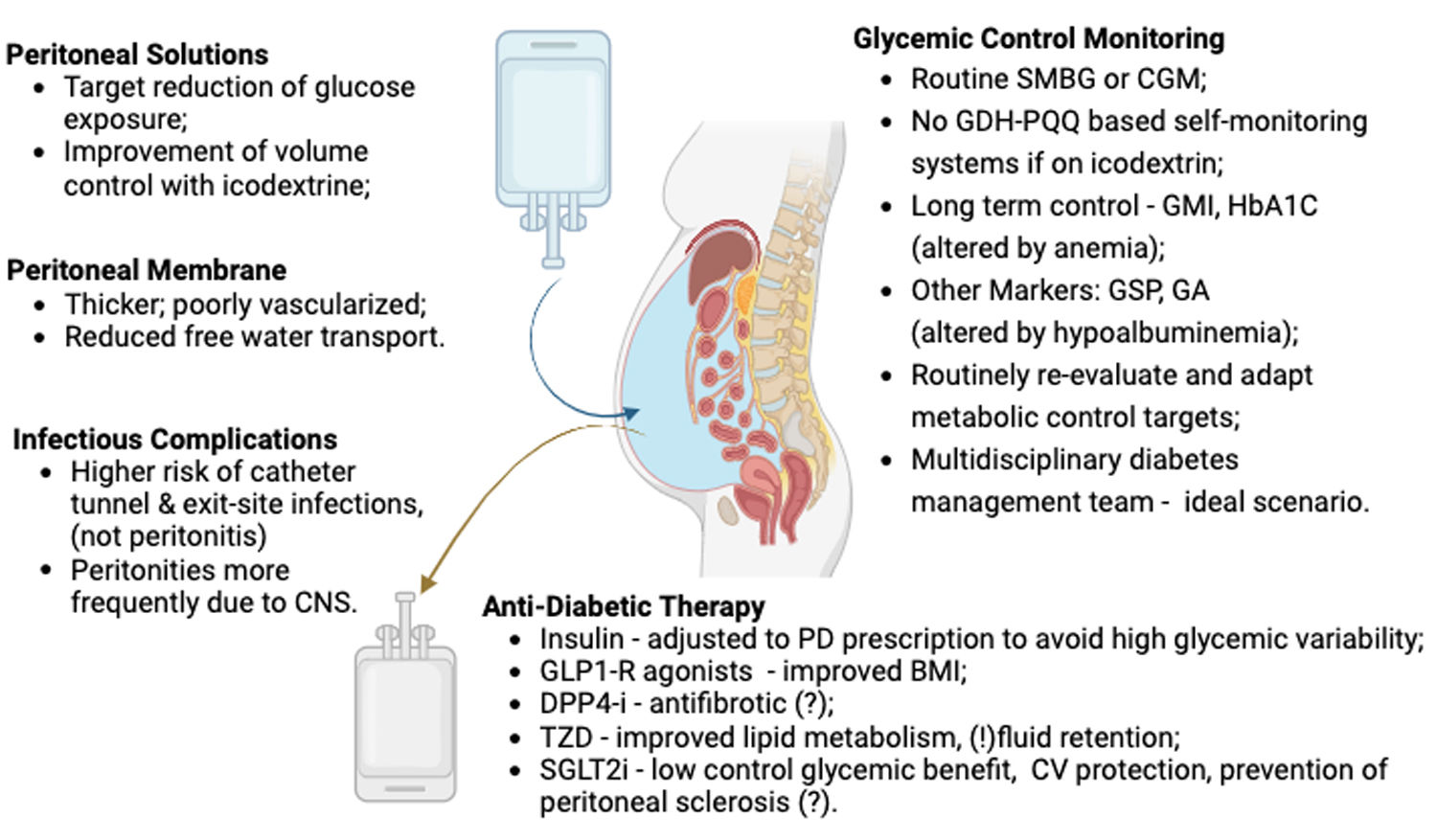
ConclusionThe review highlights the complexities in managing diabetic patients undergoing PD summarized in Fig. 2. Peritoneal dialysis’ role is well established however is accompanied by challenges in a population with increased cardiovascular risk due to underlying disease.
Particularities of technique management and diabetic control in peritoneal dialysis patients. SMBG – self monitoring blood glucose; CGM – continuous glucose monitoring; GDH-PQQ – glucose dehydrogenase pyrroloquinoline quinone; GMI – glucose management indicator; GSP – glycated Serum Protein; GA – glycated albumin; GLP1-R – glucagon-like peptide 1; DPP4-i – Dipeptidyl peptidase-4; TZD – thiazolidinediones; SGLT2i – sodium-glucose co-transporter 2 inhibitors; CNS – coagulase negative staphylococci.
Theoretical advantages of PD for diabetic patients are argued by multiple authors. These include improved hemodynamic tolerance, residual kidney function preservation, preservation of vascular capital. On the other hand, disadvantages include an adverse metabolic profile with dextrose-based solutions, glucose overload and malnutrition due to protein loss in PD fluid.
Regarding long-term glycemic control, HbA1c remains the most used and available method in PD patients. It is subject to careful interpretation and should be used with caution until a better biomarker is readily available. Further investigations on appropriate target levels of HbA1c and other monitoring parameters on PD are warranted.
Anti-diabetic therapies such as insulin require tailored adjustments and others, such as SGLT2i may have beneficial effects beyond their glucose lowering mechanism.
Dextrose-based solutions, may worsen metabolic parameters and hence CV risk in diabetic patients. Prescriptions with icodextrin have shown to benefit volume control in diabetic patients whose peritoneal membrane characteristics may affect peritoneal water transport. Special preventive strategies should be employed to avoid increase risk of exit site and catheter tunnel infections.
Overall, individualized treatment plans and multidisciplinary care is essential for optimizing outcomes in diabetic PD patients.
Authors’ contributionsVitória Paes de Faria, Joana Dias, Rute Carmo, Daniela Lopes, Ana Marta Gomes researched literature. Vitória Paes de Faria and Joana Dias wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Funding sourcesThis study was not supported by any sponsor or funder.
Conflicts of interestThe authors have no conflicts of interest to declare.
None.