Graft outcomes in pancreas transplantation have improved in recent decades, but data are mainly derived from registries or prospective single-centre studies. This large epidemiological study was undertaken to investigate the impact of clinical and demographic factors on graft and patient survival in pancreas transplant recipients in Spain, and to provide robust, country-wide, practice-based data to complement registry findings.
Patients and methodsWe conducted a retrospective, longitudinal, epidemiological study to assess risk factors impacting patient and graft survival in pancreas transplant recipients in eight centres in Spain. All patients transplanted between 1 January 2008 and 31 December 2012 were included; data were collected until 31 December 2015. The Kaplan–Meier method was used for all time-to-event analyses, including patient survival, graft survival, acute rejection, and BPAR. For graft survival analysis, in cases of death with functioning graft, patients were censored without any event on the date of death. For acute rejection and BPAR, patients were censored without any event on the date of death or graft loss. Univariable and multivariable analyses (Cox proportional hazards model) were conducted to assess the association between baseline clinical and demographic characteristics and patient/graft survival.
ResultsData were included for 241 (80.1%) simultaneous pancreas-kidney transplants, 56 (18.6%) pancreas-after-kidney transplants and 4 (1.3%) pancreas transplants alone. Mean±standard deviation time from diagnosis until transplantation was 26.1±7.5 years. Nineteen patients died, mainly due to infections (n=10); the remaining 282 patients (93.7%) survived from transplantation until the end of the study. Among 55 patients (18.3%) with pancreas graft loss, the main reasons were vascular thrombosis (n=19), chronic rejection (n=10), acute rejection (n=6) and death with a functioning graft (n=5). The overall rate of vascular-related death was 1.3% at 5 years post transplant. Univariable analysis showed that patient age and weight, donor age, previous kidney transplantation, previous cardiovascular events and need for insulin more than 48h post transplantation were significantly associated with pancreas graft survival. Of these, in multivariable analyses pancreas graft survival was inferior in patients who had received a previous kidney transplant prior to pancreas transplantation (log-rank test, p=0.0002). Glucose metabolism, renal function and cardiovascular risk factors were generally stable following transplantation.
ConclusionsThe results of this multicentre study highlight the excellent patient and graft outcomes following pancreas transplantation, with a notably low incidence of cardiovascular events.
Los resultados del injerto en el trasplante de páncreas han mejorado en las últimas décadas, pero los datos provienen principalmente de registros o estudios prospectivos unicéntricos. Este estudio epidemiológico se llevó a cabo para investigar el impacto de los factores clínicos y demográficos en la supervivencia del injerto y del paciente en receptores de trasplante de páncreas en España, y proporcionar datos sólidos, basados en la práctica a nivel nacional, para complementar los hallazgos de los registros.
Pacientes y métodosRealizamos un estudio epidemiológico longitudinal, retrospectivo, para evaluar los factores de riesgo que influyen en la supervivencia del paciente y del injerto en receptores de trasplante de páncreas en 8 centros de España. Se incluyeron todos los pacientes trasplantados entre el 1 de enero de 2008 y el 31 de diciembre de 2012; los datos se recogieron hasta el 31 de diciembre de 2015. Se utilizó el método de Kaplan-Meier para todos los análisis del tiempo transcurrido hasta el evento, incluida la supervivencia del paciente, la supervivencia del injerto, el rechazo agudo y el BPAR. Para el análisis de la supervivencia del injerto, en los casos de muerte con injerto funcionante, los pacientes fueron censurados sin ningún evento en la fecha de la muerte. Para el rechazo agudo y BPAR, los pacientes fueron censurados sin ningún evento en la fecha de la muerte o pérdida del injerto. Se realizaron análisis univariables y multivariables (modelo de riesgos proporcionales de Cox) para evaluar la asociación entre las características clínicas y demográficas basales y la supervivencia del paciente/injerto.
ResultadosSe incluyeron datos de 241 (80,1%) trasplantes de páncreas-riñón simultáneos, 56 (18,6%) trasplantes de páncreas después de riñón y 4 (1,3%) trasplantes de páncreas aislados. El tiempo medio±desviación estándar desde el diagnóstico hasta el trasplante fue de 26,1±7,5 años. Diecinueve pacientes fallecieron, principalmente por infecciones (n=10); los 282 pacientes restantes (93,7%) sobrevivieron desde el trasplante hasta el final del estudio. De los 55 pacientes (18,3%) con pérdida del injerto de páncreas, las principales razones fueron trombosis vascular (n=19), rechazo crónico (n=10), rechazo agudo (n=6) y muerte con un injerto funcionante (n=5). La tasa global de muerte relacionada con eventos vasculares fue del 1,3% a los 5 años del trasplante. El análisis univariable mostró que la edad y el peso del paciente, la edad del donante, el trasplante renal previo, los eventos cardiovasculares previos y la necesidad de insulina durante más de 48h después del trasplante se asociaron significativamente con la supervivencia del injerto de páncreas. De estos, en los análisis multivariables, la supervivencia del injerto de páncreas fue inferior en los pacientes que habían recibido un trasplante de riñón previo al trasplante de páncreas (prueba de rango logarítmico, p=0,0002). El metabolismo de la glucosa, la función renal y los factores de riesgo cardiovasculares se mantuvieron en general estables después del trasplante.
ConclusionesLos datos obtenidos de este estudio multicéntrico destacan los excelentes resultados del paciente y del injerto después del trasplante de páncreas, con una incidencia notablemente baja de eventos cardiovasculares.
Pancreas (with or without kidney) transplantation is an established treatment option for patients with type 1 diabetes and end-stage renal disease, as well as for selected patients with type 2 or type 1 diabetes with labile glucose control.1,2 Simultaneous pancreas and kidney transplantation (SPK) is the most frequently performed procedure (80%)3; alternative approaches include pancreas-after-kidney transplantation (PAK) and pancreas transplantation alone (PTA), although PTA remains a minority procedure (<5%).3
While there have been considerable improvements in pancreas transplantation medicine in recent years, particularly in SPK,2,4 data have generally derived from single-centre studies5,6 and registries (e.g. United Network for Organ Sharing [UNOS], International Registry in Organ Donation and Transplantation [IRODaT], and the International Pancreas Transplant Registry [IPTR]).2,4,7 As a consequence, there are still gaps in knowledge in terms of efficacy, risks and long-term benefits of pancreas transplantation.4 The paucity of these data, together with the increasing prevalence of diabetes mellitus in Europe and worldwide,8 highlights a pressing need to identify factors that influence recipient and graft survival, as well as the metabolic control obtained after pancreas transplantation.
The number of pancreas transplants in Europe has increased over the last 12 years from 4069 in 2005–2009, to 4433 in 2010–2014.2 In Spain, 5249 solid organ transplants were performed in 2017, including 70 pancreas transplants at 12 pancreas transplant centres.7 As the number of pancreas transplants increases, evidence regarding factors that may influence mortality and comorbidity is emerging. We therefore undertook this large epidemiological study to investigate the impact of clinical and demographic factors on graft and patient survival in pancreas transplant recipients, and to provide robust, country-wide, practice-based data to complement the registry findings.
Patients and methodsStudy design and patientsThis was a retrospective, longitudinal, 5-year epidemiological study to evaluate clinical and demographic variables impacting survival in pancreas transplant recipients at eight pancreas transplantation centres in Spain. Although all 12 Spanish centres that perform pancreas transplantation were invited to participate, four centres declined to participate for administrative or other internal reasons. All patients transplanted between 1 January 2008 and 31 December 2012 were included; data were collected until 31 December 2015. Due to protracted development of the clinical study report and manuscript, the data were not available for publication until 2020.
The study was conducted in accordance with the Declaration of Helsinki, the International Council of Harmonisation Good Clinical Practice guidelines for observational studies, and local ethical committee regulations in all participating centres. Each patient provided informed consent for inclusion in this study. Retrospective data were collected and anonymized by investigators, preserving confidentiality according to local regulation. Data collection was performed from medical records if the patient met one of the following inclusion criteria: SPK transplantation performed in patients with diabetes mellitus and end-stage renal disease; PAK performed in patients with diabetes mellitus and a functioning kidney graft from a living-related or deceased donor; or PTA performed in patients with labile diabetes mellitus and preserved renal function. In patients undergoing PAK who had received more than one kidney transplant, the last kidney transplant was the reference for data collection.
EndpointsThe co-primary endpoints were patient survival and pancreas graft survival. Patient survival was calculated from the time of pancreas transplantation until either the date of death or study end. Pancreas graft survival was calculated based on the time from transplantation until pancreas graft loss, patient death with functioning pancreas graft, or study end. Pancreas graft failure was defined as C-peptide<1ng/mL and exogenous insulin requirements>0.5IU/kg/day.
Secondary endpoints included: kidney graft survival (defined as time from kidney transplantation until return to dialysis or kidney graft loss), acute pancreatic rejection and biopsy-proven acute rejection (BPAR), acute kidney rejection and BPAR, renal function, glucose metabolism, lipid levels and blood pressure. With the exception of kidney transplant outcomes (i.e. kidney graft survival, and acute kidney rejection and BPAR), secondary endpoints were assessed in all patients irrespective of transplant type.
Patient and donor parameters were assessed to determine possible associations with patient survival and pancreas graft survival. These included the recipient variables: age, sex, weight, body mass index (BMI), type of transplant (SPK, PAK, PTA) and cardiovascular events; and the donor variables: age, sex and pancreas ischaemic time. Safety events occurring during follow-up were also recorded.
Statistical analysesContinuous variables were summarized as mean±standard deviation (SD) or median [interquartile range (IQR)], as appropriate. Categorical variables were summarized as incidence counts or rates. The Kaplan–Meier method was used for all time-to-event analyses, including patient survival, graft survival, acute rejection, and BPAR. For graft survival analysis, in cases of death with functioning graft, patients were censored without any event on the date of death. For acute rejection and BPAR, patients were censored without any event on the date of death or graft loss. Univariable and multivariable analyses (Cox proportional hazards model) were conducted to assess the association between baseline clinical and demographic characteristics and patient/graft survival. Factors found to be associated with patient/graft survival in univariable analyses were included in the multivariable analysis and backward elimination applied to identify the variables that significantly affected patient survival. There were no imputations for missing data. All analyses were performed using SAS® Version 9.3, and p≤0.05 was considered statistically significant.
ResultsPatient characteristicsOf 304 patients recruited into the study, three were excluded from the analysis because no post-transplant data were available. Of the 301 patients included in the analysis, there were 241 (80.1%) SPKs, 56 (18.6%) PAKs and four (1.3%) PTAs. Nineteen patients died during the study period. The majority of patients (65.4%) were followed for 3 years after pancreatic transplantation, and 87 (28.9%) were followed for 5 years (Supplementary Fig. 1). The median follow-up period from pancreas transplantation was 3.2 years (maximum 5.6 years).
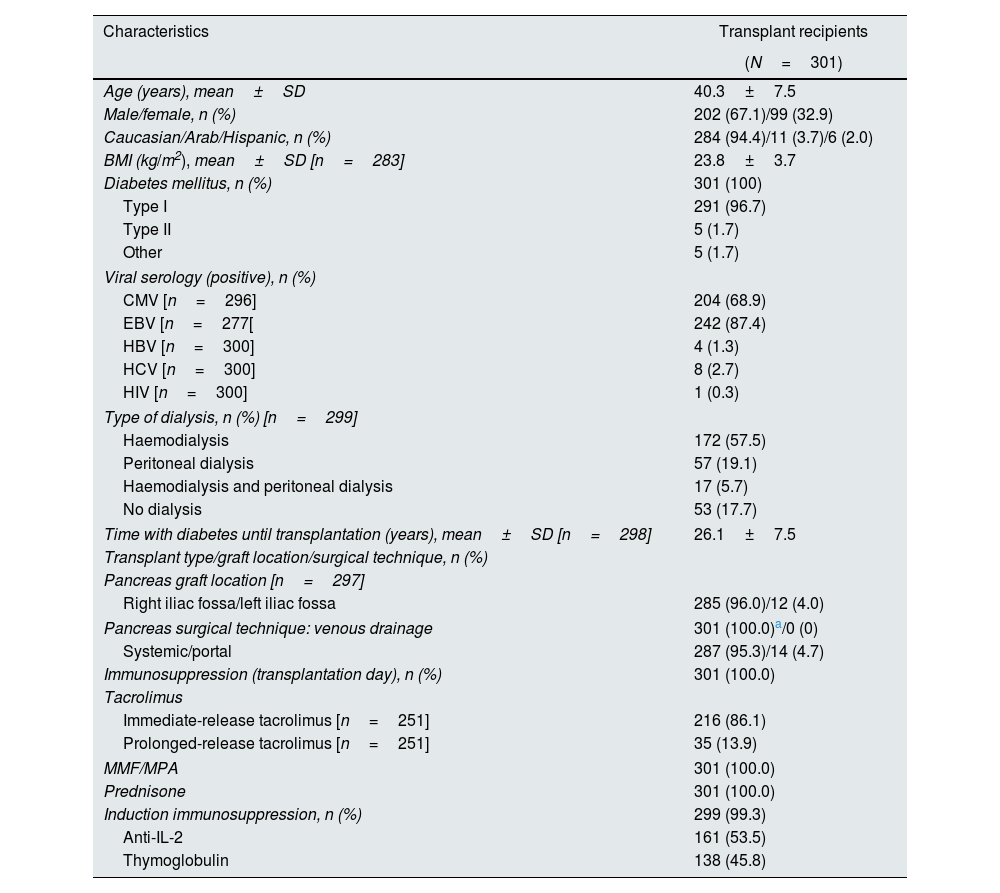
Patient demographics and baseline characteristics for all transplant recipients are presented in Table 1. The mean±SD age of patients at pancreas transplantation was 40.3±7.5 years; 67.1% were male and 94.4% were Caucasian. The majority of transplant recipients had diabetes mellitus type I (96.7%) and were receiving dialysis (82.3%). The mean±SD time from diagnosis of diabetes (including type I, type II and maturity onset diabetes of the young) until transplantation was 26.1±7.5 years. For the 56 transplant recipients in the PAK group, the median (range) time from kidney transplantation to pancreas transplantation was 2.7 (0.6–14.1) years.
Demographics and baseline characteristics for all transplant recipients (SPK, PAK, PTA).
| Characteristics | Transplant recipients |
|---|---|
| (N=301) | |
| Age (years), mean±SD | 40.3±7.5 |
| Male/female, n (%) | 202 (67.1)/99 (32.9) |
| Caucasian/Arab/Hispanic, n (%) | 284 (94.4)/11 (3.7)/6 (2.0) |
| BMI (kg/m2), mean±SD [n=283] | 23.8±3.7 |
| Diabetes mellitus, n (%) | 301 (100) |
| Type I | 291 (96.7) |
| Type II | 5 (1.7) |
| Other | 5 (1.7) |
| Viral serology (positive), n (%) | |
| CMV [n=296] | 204 (68.9) |
| EBV [n=277[ | 242 (87.4) |
| HBV [n=300] | 4 (1.3) |
| HCV [n=300] | 8 (2.7) |
| HIV [n=300] | 1 (0.3) |
| Type of dialysis, n (%) [n=299] | |
| Haemodialysis | 172 (57.5) |
| Peritoneal dialysis | 57 (19.1) |
| Haemodialysis and peritoneal dialysis | 17 (5.7) |
| No dialysis | 53 (17.7) |
| Time with diabetes until transplantation (years), mean±SD [n=298] | 26.1±7.5 |
| Transplant type/graft location/surgical technique, n (%) | |
| Pancreas graft location [n=297] | |
| Right iliac fossa/left iliac fossa | 285 (96.0)/12 (4.0) |
| Pancreas surgical technique: venous drainage | 301 (100.0)a/0 (0) |
| Systemic/portal | 287 (95.3)/14 (4.7) |
| Immunosuppression (transplantation day), n (%) | 301 (100.0) |
| Tacrolimus | |
| Immediate-release tacrolimus [n=251] | 216 (86.1) |
| Prolonged-release tacrolimus [n=251] | 35 (13.9) |
| MMF/MPA | 301 (100.0) |
| Prednisone | 301 (100.0) |
| Induction immunosuppression, n (%) | 299 (99.3) |
| Anti-IL-2 | 161 (53.5) |
| Thymoglobulin | 138 (45.8) |
A duodenoenterostomy enteric drainage procedure was performed in most pancreas transplant recipients (99.3% [298/300 patients]).
BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IL-2, interleukin-2; MMF, mycophenolate mofetil; MPA, mycophenolic acid; PAK, pancreas after kidney transplantation; PTA, pancreas transplantation alone; SD, standard deviation; SPK, simultaneous pancreas and kidney transplantation
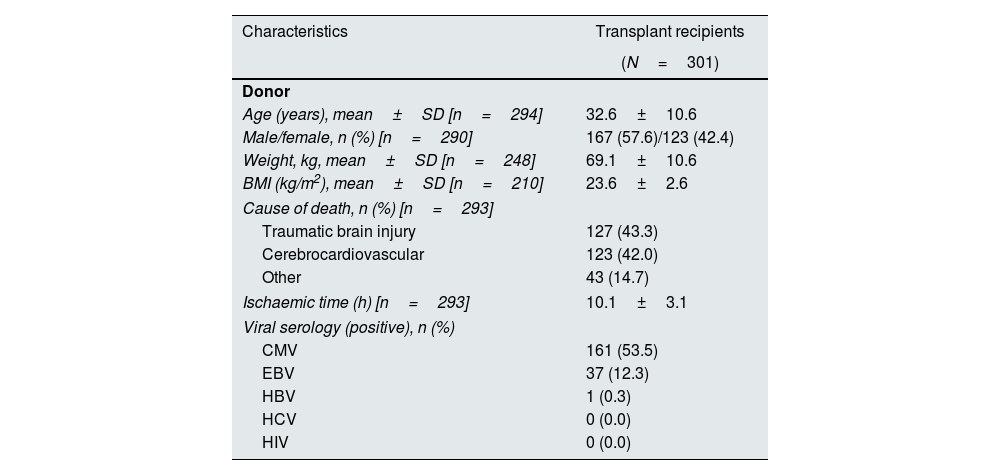
Pancreas donors had a mean±SD age of 32.6±10.6 years (range, 5–54 years), and the majority (57.6%) were male, with a mean±SD BMI of 23.6±2.6kg/m2. Most pancreas donors died as a result of traumatic brain injury (43.3%) or from cerebrocardiovascular causes (42.0%) (Table 2).
Pancreas donor transplantation data.
| Characteristics | Transplant recipients |
|---|---|
| (N=301) | |
| Donor | |
| Age (years), mean±SD [n=294] | 32.6±10.6 |
| Male/female, n (%) [n=290] | 167 (57.6)/123 (42.4) |
| Weight, kg, mean±SD [n=248] | 69.1±10.6 |
| BMI (kg/m2), mean±SD [n=210] | 23.6±2.6 |
| Cause of death, n (%) [n=293] | |
| Traumatic brain injury | 127 (43.3) |
| Cerebrocardiovascular | 123 (42.0) |
| Other | 43 (14.7) |
| Ischaemic time (h) [n=293] | 10.1±3.1 |
| Viral serology (positive), n (%) | |
| CMV | 161 (53.5) |
| EBV | 37 (12.3) |
| HBV | 1 (0.3) |
| HCV | 0 (0.0) |
| HIV | 0 (0.0) |
CMV, cytomegalovirus; EBV, Epstein–Barr virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; SD, standard deviation.
Of the 301 patients, all except two received induction therapy with either anti-interleukin-2 (IL-2) receptor (n=161; 53.5%) or thymoglobulin (n=138; 45.8%). All 301 patients received prednisone at baseline; by the end of the study, of 87 patients with available data at 5 years, 65 (74.7%) were receiving prednisone (Supplementary Table 1). All patients were treated with tacrolimus; at baseline, of the 251 patients with available formulation data, 216 (86.1%) received immediate-release tacrolimus. By the end of the study, of the 85 patients with available formulation data at 5 years, 57 (67.1%) were receiving prolonged-release tacrolimus (Supplementary Table 1). The dose of tacrolimus decreased over the 5 years of the study. All 301 patients received concomitant immunosuppression with mycophenolate at baseline [193 (64.1%) received mycophenolate mofetil (MMF) and 108 (35.9%) received mycophenolic acid (MPA)]. Of 85 patients with available data at the end of the study at 5 years, 80 (94.1%) were taking these therapies (Supplementary Table 1).
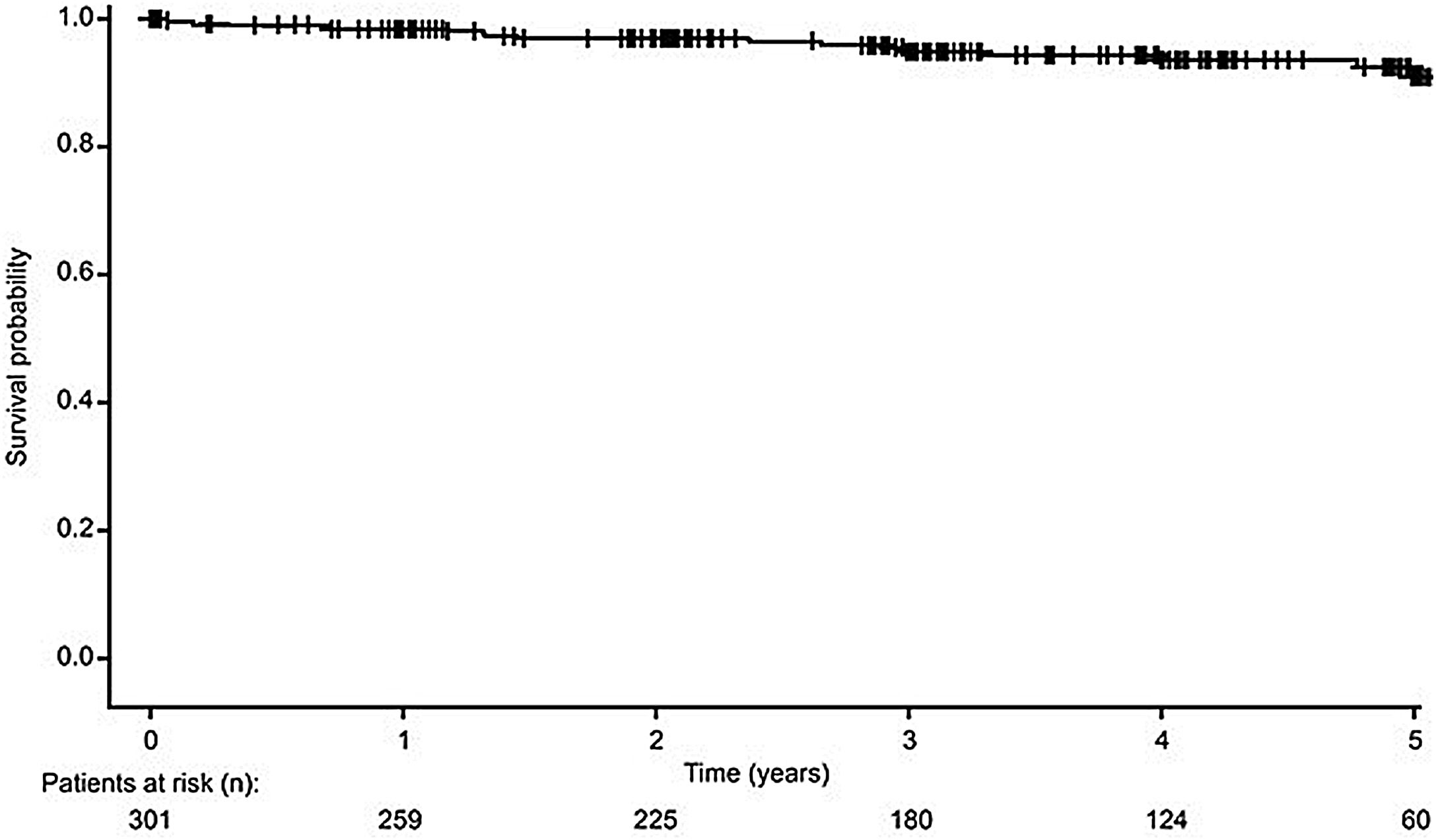
Patient survivalA total of 282 recipients (93.7%) survived from the date of transplantation until the study end, with estimated 12-month, 3-year and 5-year survival rates of 98.7%, 96.1% and 94.4%, respectively (Fig. 1). A total of 19 (6.3%) recipients died during the study period, due to infection (n=10, 52.6%), vascular disease (n=4, 21.1%), sudden death (n=2, 10.5%), gastrointestinal haemorrhage (n=2, 10.5%) and road traffic accident (n=1, 5.3%). The overall rate of vascular-related death was 1.3% at 5 years post-transplant.
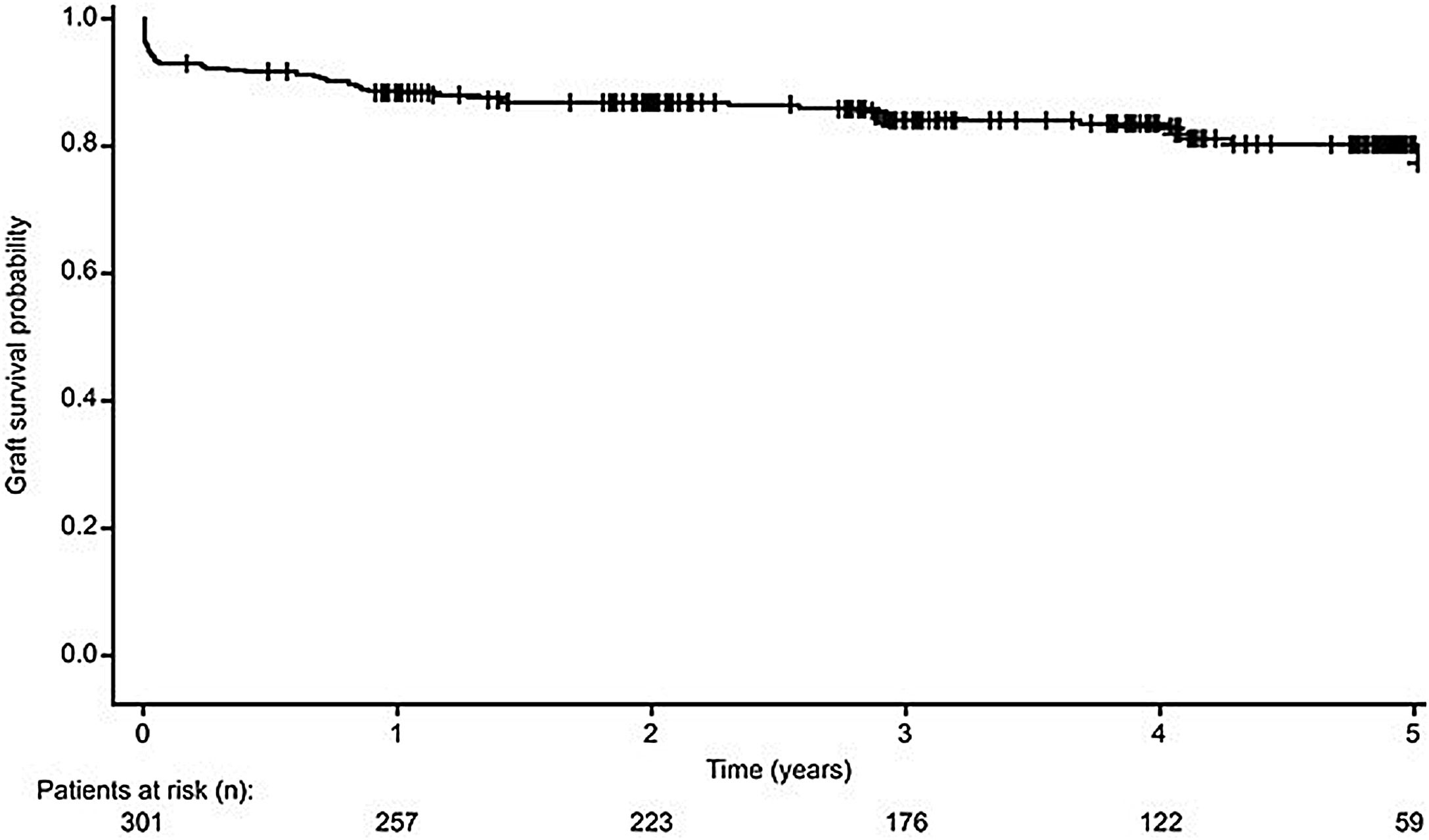
Pancreas graft survivalA total of 246 (81.7%) pancreas grafts survived from transplantation date to study end, with estimated 12-month, 3-year and 5-year pancreas graft survival rates of 87.8%, 84.9% and 83.0%, respectively (Fig. 2). Fifty-five (18.3%) patients experienced pancreas graft loss during the study. In these patients, primary non-function occurred in 22 transplants (40.0%), due to vascular thrombosis (n=19, 34.5%), poor graft perfusion (n=2, 3.6%) and bile leak (n=1, 1.8%). The remaining causes of graft loss were acute (n=6, 10.9%) and chronic rejection (n=10, 18.2%), infection (n=2, 3.6%), 10 (18.2%) due to other reasons, and death with a functioning graft (n=5, 9.1%).
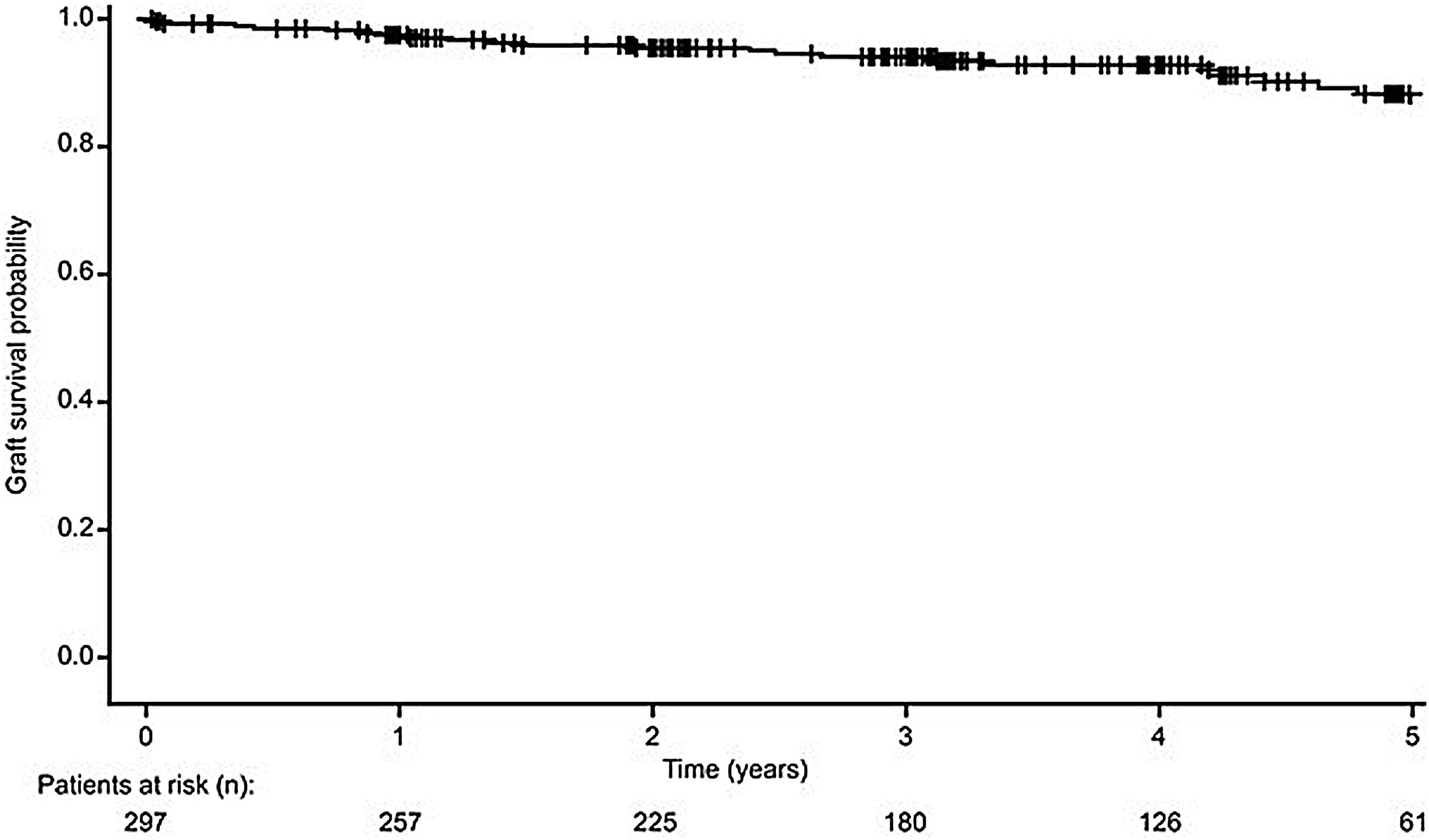
Kidney graft survivalOf the 297 recipients who received either SPK or PAK transplants, 271 kidney grafts (91.2%) survived from transplantation date until the end of the observation period (Fig. 3). For the 26 recipients (8.8%) who developed graft loss during the observation period, the reasons for graft loss included: death with a functioning graft (n=7, 26.9%), chronic humoral rejection (n=4, 15.4%), thrombosis (n=4, 15.4%), cellular rejection (n=3, 11.5%), acute humoral rejection (n=1, 3.8%), glomerular disease (recurrent or de novo) (each n=1, 3.8%), and other reasons (n=6, 23.1%).
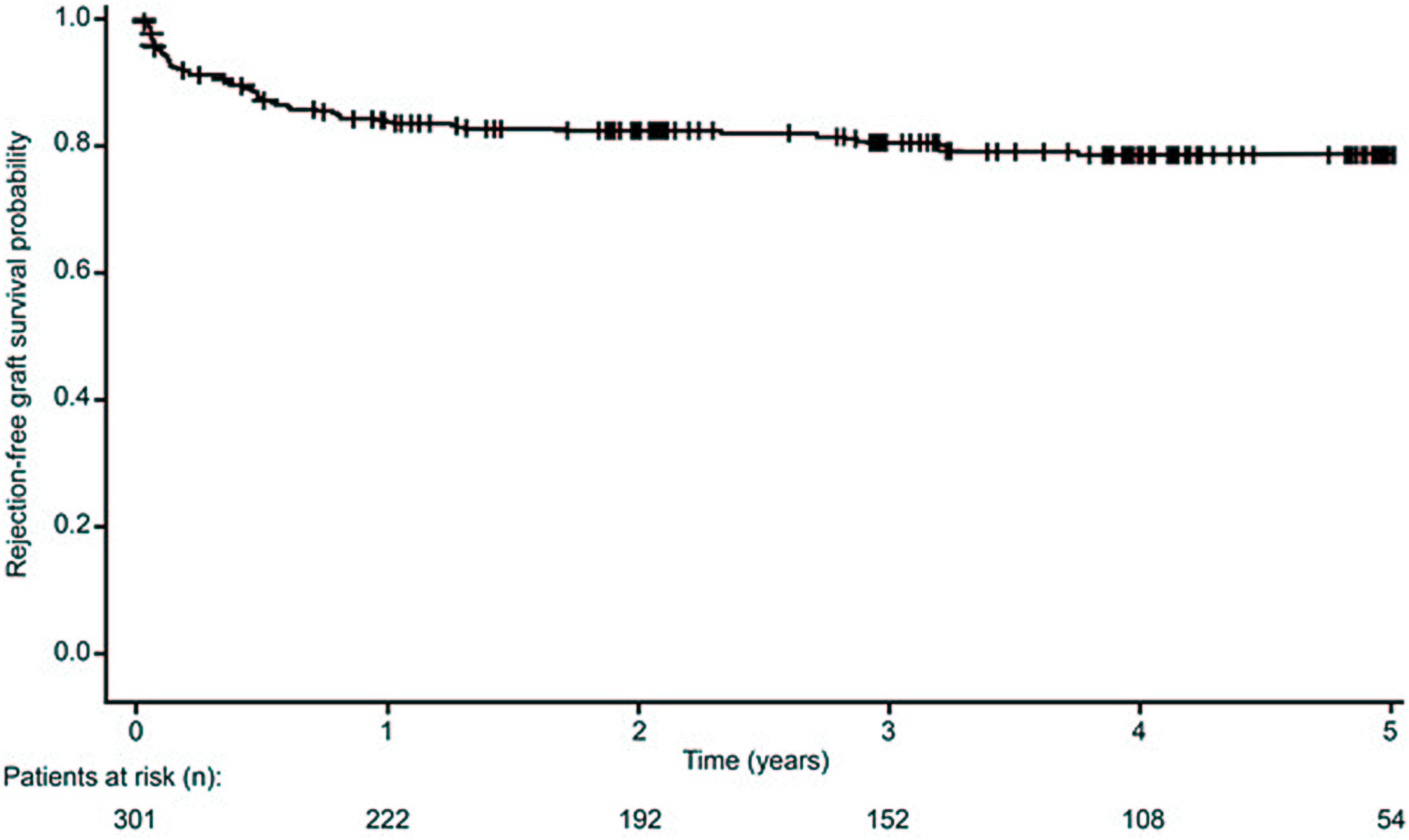
Acute pancreatic rejectionOf 77 episodes of acute pancreatic rejection in 55 recipients (18.3%), 29 were BPAR (37.7%). The estimated proportion of patients who had not experienced acute rejection was 83.2% at 12 months post-transplant, and 81.7% at 5 years (Fig. 4). At the end of the study, 91.7% of patients had not experienced a BPAR event.
Acute kidney rejectionOf 67 episodes of acute kidney rejection in 49 recipients (16.5%; based on clinical criteria and/or laboratory analyses), 49 were episodes of BPAR in 39 patients (13.1%). At the end of the study, 83.5% and 86.9% of patients had not experienced acute kidney rejection or BPAR, respectively.
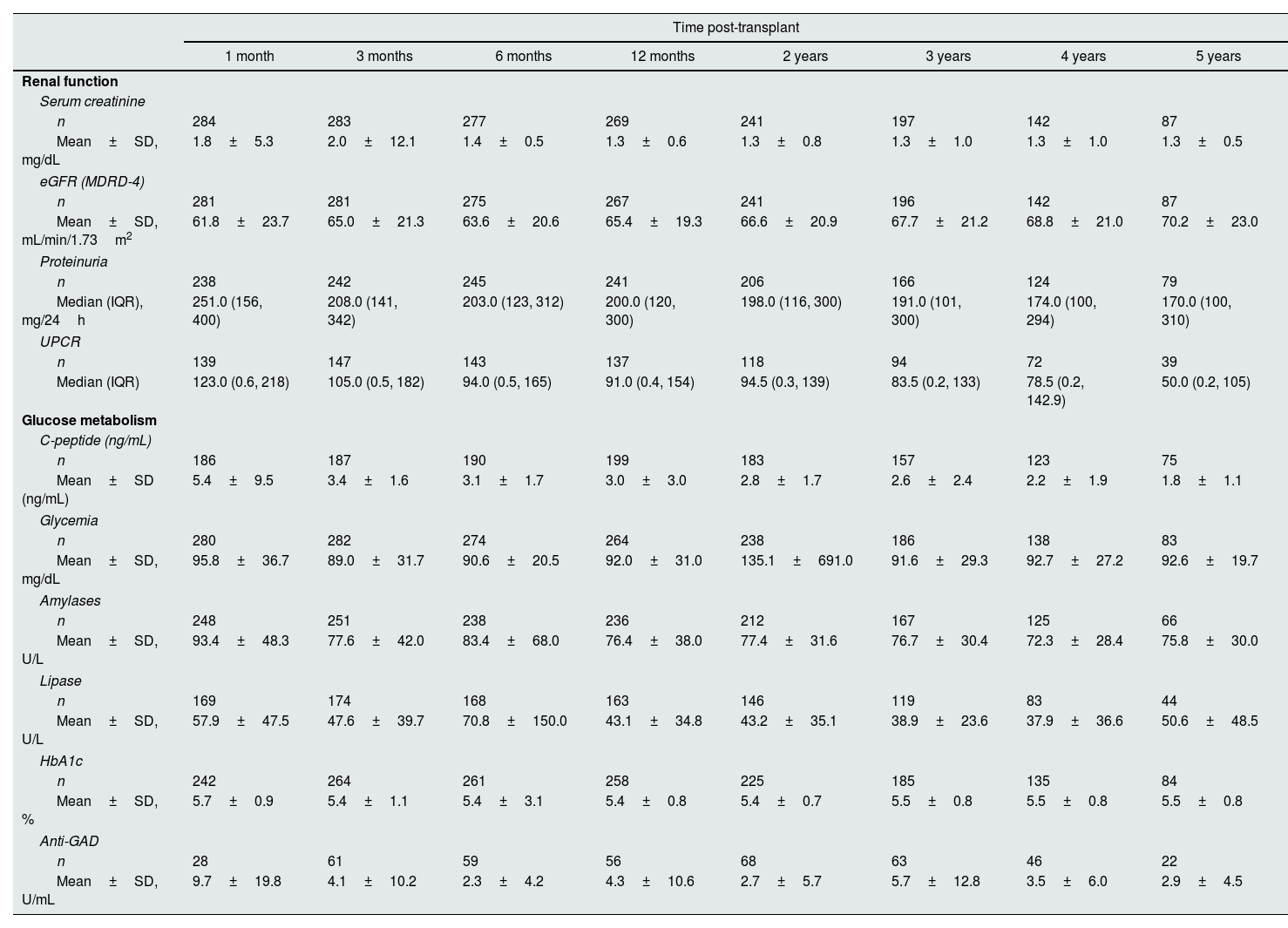
Renal function and glucose metabolismRenal function improved from the day of pancreas transplantation up to Month 6 and remained generally stable thereafter. At 5 years, mean±SD serum creatinine concentration was 1.3±0.5mg/dL and mean±SD estimated glomerular filtration rate (eGFR; Modification of Diet in Renal Disease-4 [MDRD-4]) was 70.2±23.0mL/min/1.73m2 (Table 3). Proteinuria remained stable throughout the study period, with a median (IQR) of 170.0 (100, 310)mg/24h at 5 years. Median urine protein-to-creatinine ratio decreased gradually throughout the study period. Glucose metabolism parameters generally remained stable over the 5 years of follow-up (Table 3), with the exception of C-peptide which decreased throughout the study period from 5.4±9.5ng/mL at Month 1 to 1.8±1.1ng/mL at 5 years.
Renal function and glucose metabolism from day of pancreas transplant to study end.
| Time post-transplant | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 12 months | 2 years | 3 years | 4 years | 5 years | |
| Renal function | ||||||||
| Serum creatinine | ||||||||
| n | 284 | 283 | 277 | 269 | 241 | 197 | 142 | 87 |
| Mean±SD, mg/dL | 1.8±5.3 | 2.0±12.1 | 1.4±0.5 | 1.3±0.6 | 1.3±0.8 | 1.3±1.0 | 1.3±1.0 | 1.3±0.5 |
| eGFR (MDRD-4) | ||||||||
| n | 281 | 281 | 275 | 267 | 241 | 196 | 142 | 87 |
| Mean±SD, mL/min/1.73m2 | 61.8±23.7 | 65.0±21.3 | 63.6±20.6 | 65.4±19.3 | 66.6±20.9 | 67.7±21.2 | 68.8±21.0 | 70.2±23.0 |
| Proteinuria | ||||||||
| n | 238 | 242 | 245 | 241 | 206 | 166 | 124 | 79 |
| Median (IQR), mg/24h | 251.0 (156, 400) | 208.0 (141, 342) | 203.0 (123, 312) | 200.0 (120, 300) | 198.0 (116, 300) | 191.0 (101, 300) | 174.0 (100, 294) | 170.0 (100, 310) |
| UPCR | ||||||||
| n | 139 | 147 | 143 | 137 | 118 | 94 | 72 | 39 |
| Median (IQR) | 123.0 (0.6, 218) | 105.0 (0.5, 182) | 94.0 (0.5, 165) | 91.0 (0.4, 154) | 94.5 (0.3, 139) | 83.5 (0.2, 133) | 78.5 (0.2, 142.9) | 50.0 (0.2, 105) |
| Glucose metabolism | ||||||||
| C-peptide (ng/mL) | ||||||||
| n | 186 | 187 | 190 | 199 | 183 | 157 | 123 | 75 |
| Mean±SD (ng/mL) | 5.4±9.5 | 3.4±1.6 | 3.1±1.7 | 3.0±3.0 | 2.8±1.7 | 2.6±2.4 | 2.2±1.9 | 1.8±1.1 |
| Glycemia | ||||||||
| n | 280 | 282 | 274 | 264 | 238 | 186 | 138 | 83 |
| Mean±SD, mg/dL | 95.8±36.7 | 89.0±31.7 | 90.6±20.5 | 92.0±31.0 | 135.1±691.0 | 91.6±29.3 | 92.7±27.2 | 92.6±19.7 |
| Amylases | ||||||||
| n | 248 | 251 | 238 | 236 | 212 | 167 | 125 | 66 |
| Mean±SD, U/L | 93.4±48.3 | 77.6±42.0 | 83.4±68.0 | 76.4±38.0 | 77.4±31.6 | 76.7±30.4 | 72.3±28.4 | 75.8±30.0 |
| Lipase | ||||||||
| n | 169 | 174 | 168 | 163 | 146 | 119 | 83 | 44 |
| Mean±SD, U/L | 57.9±47.5 | 47.6±39.7 | 70.8±150.0 | 43.1±34.8 | 43.2±35.1 | 38.9±23.6 | 37.9±36.6 | 50.6±48.5 |
| HbA1c | ||||||||
| n | 242 | 264 | 261 | 258 | 225 | 185 | 135 | 84 |
| Mean±SD, % | 5.7±0.9 | 5.4±1.1 | 5.4±3.1 | 5.4±0.8 | 5.4±0.7 | 5.5±0.8 | 5.5±0.8 | 5.5±0.8 |
| Anti-GAD | ||||||||
| n | 28 | 61 | 59 | 56 | 68 | 63 | 46 | 22 |
| Mean±SD, U/mL | 9.7±19.8 | 4.1±10.2 | 2.3±4.2 | 4.3±10.6 | 2.7±5.7 | 5.7±12.8 | 3.5±6.0 | 2.9±4.5 |
eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; GAD, glutamic acid decarboxylase; IQR, interquartile range; MDRD, Modification of Diet in Renal Disease; SD, standard deviation; UPCR, urine protein-to-creatinine ratio
BMI increased from Month 1 post-transplant to the end of the study (22.9±3.5 to 25.0±4.4kg/m2). Lipid profiles remained stable from the day of transplantation to the end of the study: total cholesterol (167.9±42.2–165.2±31.9mg/dL), high density lipoprotein cholesterol (47.5±16.2–54.3±12.3mg/dL), low density lipoprotein cholesterol (90.0±27.2–92.6±23.1mg/dL); triglycerides decreased from 135.4±71.3 on the day of transplantation to 103.1±108.2mg/dL at 5 years. A similar proportion of recipients were being treated with statins at transplant (51.2%) and at study end (51.7%).
Systolic and diastolic blood pressure (SBP and DBP) remained stable from Month 1 post-transplant to the end of the study (SBP: 127.5±18.4–128.2±16.6mmHg; DBP: 74.2±12.6–74.9±11.0mmHg). The proportion of patients with hypertension was 22.2% on the day of transplantation, which decreased and remained stable post-transplant (range, 5.3–8.4%). Post-transplant, the most commonly used hypertension treatments were beta blockers (range, 20.4–24.9%) and calcium antagonists (range, 18.4–25.4%) (Supplementary Table 2). Compared with 1 month post-transplant, the proportion of patients receiving an ACEi or ARB numerically increased by 5 years, while the proportion of patients receiving calcium antagonists decreased.
Association of risk factors with patient and graft survivalGiven the high patient survival rate, data for the association of risk factors with patient survival are not reported.
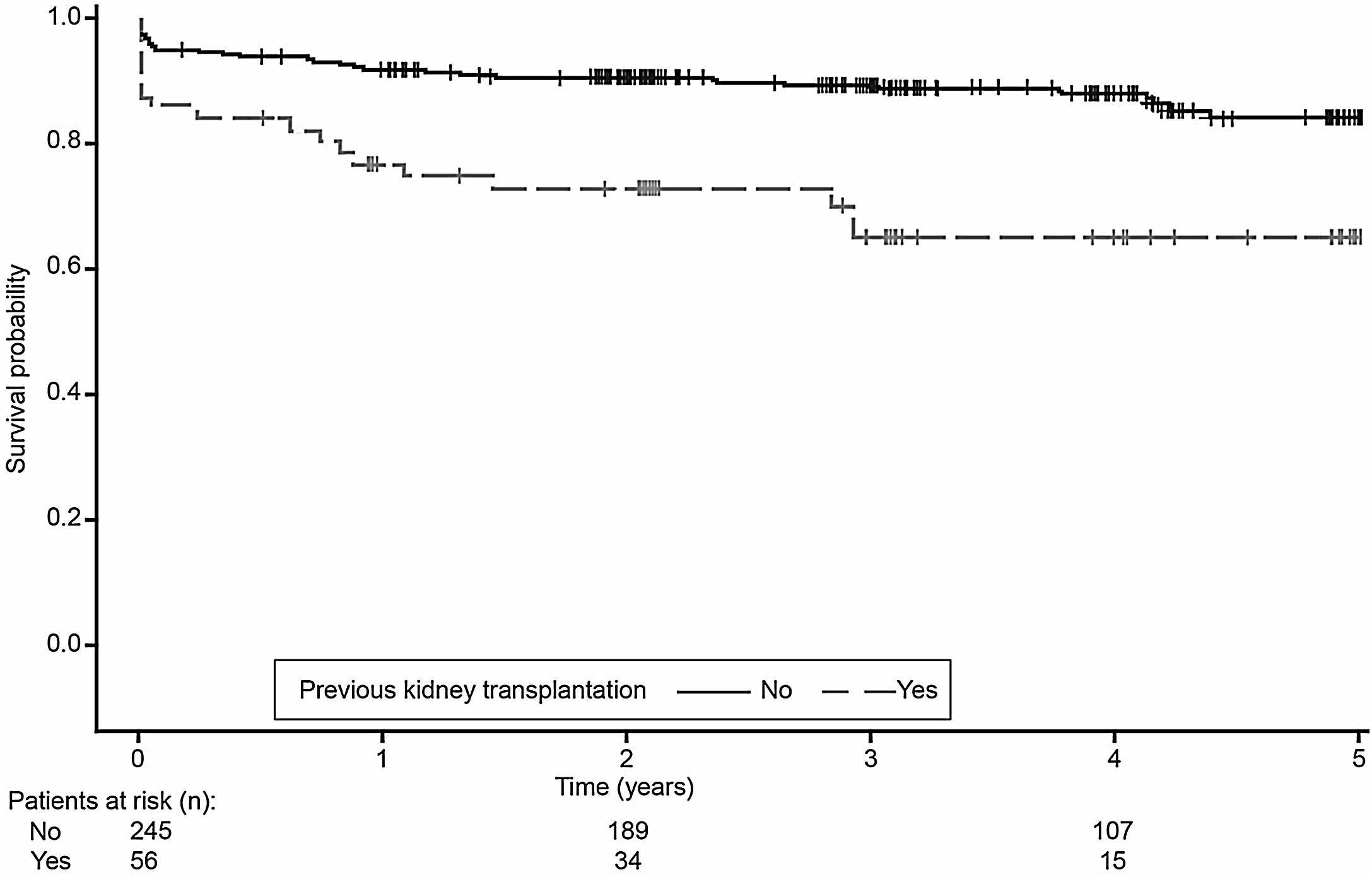
Univariable analysis showed that patient age and weight, donor age, previous kidney transplantation, previous cardiovascular events and need for insulin more than 48h post-transplantation were significantly associated with pancreas graft survival. Using a Cox proportional hazards model that included these variables, with backward elimination, previous kidney transplant was the only significant risk factor for graft failure (HR=3.3, 95% CI: 1.8, 5.9; p<0.0001). Kaplan–Meier analysis confirmed that pancreas graft survival was inferior in patients who had received a kidney transplant prior to pancreas transplantation compared with those who had not received a kidney transplant prior to pancreas transplantation (log-rank test, p=0.0002) (Fig. 5).
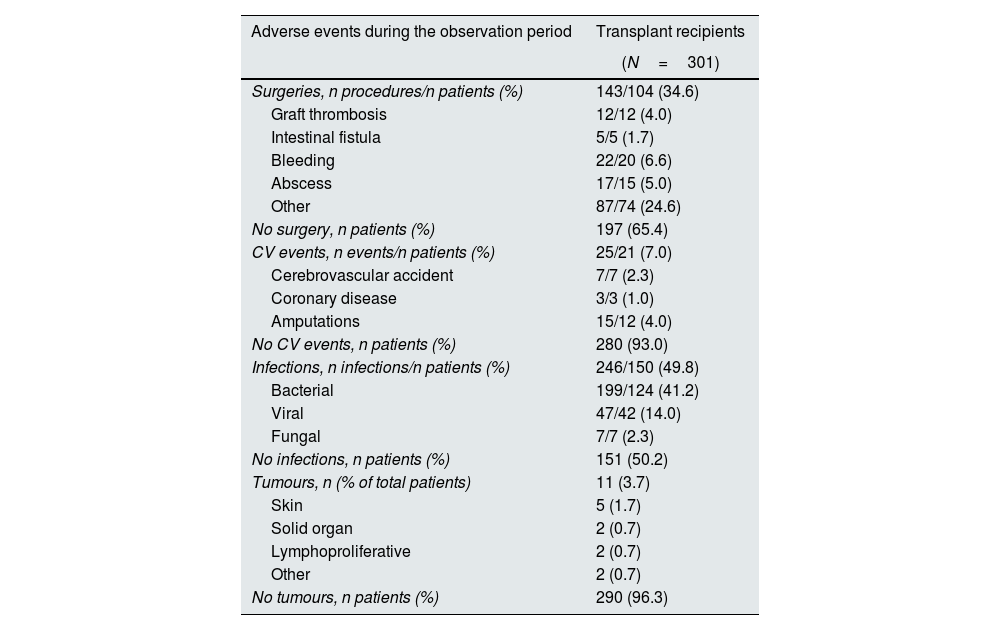
Safety observationsTable 4 shows the reports of post-transplant surgical intervention, cardiovascular events, infections and tumours occurring during the study. A total of 143 surgical procedures were required in 104 patients (34.6%); 25% of these patients required a surgical procedure during the first 6 months post-transplant. There were 25 cardiovascular events in 21 patients (7.0%), including cerebrovascular accident, coronary disease and amputations. There were 246 infections in 150 patients (49.8%), the majority of which were recorded as ‘bacterial’ in origin (n=199), occurring in 124 patients (41.2%). The median time from pancreas transplantation until the first infection was 2.3 years. Only eleven patients (3.7%) developed tumours during the study.
Adverse events during the observation period (transplant day to 5 years post-transplant).
| Adverse events during the observation period | Transplant recipients |
|---|---|
| (N=301) | |
| Surgeries, n procedures/n patients (%) | 143/104 (34.6) |
| Graft thrombosis | 12/12 (4.0) |
| Intestinal fistula | 5/5 (1.7) |
| Bleeding | 22/20 (6.6) |
| Abscess | 17/15 (5.0) |
| Other | 87/74 (24.6) |
| No surgery, n patients (%) | 197 (65.4) |
| CV events, n events/n patients (%) | 25/21 (7.0) |
| Cerebrovascular accident | 7/7 (2.3) |
| Coronary disease | 3/3 (1.0) |
| Amputations | 15/12 (4.0) |
| No CV events, n patients (%) | 280 (93.0) |
| Infections, n infections/n patients (%) | 246/150 (49.8) |
| Bacterial | 199/124 (41.2) |
| Viral | 47/42 (14.0) |
| Fungal | 7/7 (2.3) |
| No infections, n patients (%) | 151 (50.2) |
| Tumours, n (% of total patients) | 11 (3.7) |
| Skin | 5 (1.7) |
| Solid organ | 2 (0.7) |
| Lymphoproliferative | 2 (0.7) |
| Other | 2 (0.7) |
| No tumours, n patients (%) | 290 (96.3) |
CV, cardiovascular.
This study provides comprehensive, real-world data from a national patient series of routinely-monitored pancreas transplant recipients in Spain. Data from 301 pancreas transplant recipients were included, with the majority undergoing SPK transplantation, the most frequently performed pancreas transplant procedure.3 The Kaplan–Meier estimated 5-year patient survival rate (94.4%) was high and consistent with current 5-year survival rates in the US4 and also another European cohort.6 A total of 19 recipients died during the 5-year period; the main cause of death was infection, predominantly bacterial in origin. Complications due to bacterial infections continue to be a primary cause of morbidity and mortality in pancreas transplant recipients.9 In another study of 410 Spanish patients who received pancreas transplants, most of which were SPK, the most common complication was infection following surgery (experienced by 12% of patients), while the second most common complication was abdominal abscess (7.3% of patients).10
The pancreas graft survival rate at the end of this study (81.7%) was higher than the current rates reported in the US (SPK, 73%; PAK, 65%; PTA, 53%),4 but similar to that observed in two other Spanish studies (84.5% [SPK only] and 79.1% [SPK, PAK and PTA] at 5 years, respectively),11,12 and also the rate reported in the Netherlands (SPK, PAK, PTA, multivisceral and retransplant, 80.3%, following the introduction of modern induction therapy in 1999).6 The rate was lower than the rate of 95% reported by Gutiérrez et al. for a group of 20 Spanish patients who received SPK13; however, it should be noted that the follow-up period for those patients was 6 months, which is shorter than the 5-year observation period in the current study.
Our data show that pancreas graft survival was inferior in patients who had received a kidney transplant prior to pancreas transplantation compared with those who had not received a kidney transplant prior to pancreas transplantation. The higher rate of pancreas graft survival in SPK versus PAK during the first 3 years after pancreas transplant has been reported to be mainly due to an increased rate of immunologic graft loss in PAK.2,14 Nevertheless, it should be noted that PAK remains an appropriate option for some patients; for example, those on the SPK waitlist for whom a kidney becomes available. Results of a recent retrospective analysis of data from the US Organ Procurement and Transplantation Network (OPTN) show that PAK transplant recipients have an overall survival advantage compared with uraemic diabetic patients on the SPK waitlist who did not receive a transplant.15 PAK was also shown to be associated with increased kidney graft survival compared with that seen in patients with type 1 diabetes who received only a kidney transplant.15
Acute rejection as a cause of pancreas graft loss generally occurs 7–12 months post-transplant.16 As well as immunologic rejection, pancreatic allograft thrombosis is a major non-immunologic cause of pancreas graft loss, with a reported incidence of 23%,17 and accounting for a reported 29% of grafts lost during the first 6 months after pancreas transplant.18 Over this 5 year study, 55 patients (18.3%) experienced pancreas graft loss, mainly due to vascular thrombosis (34.5%) followed by chronic rejection (18.2%). These findings are consistent with a cumulative rate of graft loss of 14.0% at 5 years in a retrospective, single-centre US study conducted in 227 consecutive SPK, PAK and PTA patients, in which 57 recipients experienced 79 acute rejection episodes.19
Maintenance protocols after pancreas transplantation generally comprise tacrolimus and MMF with early or delayed corticosteroid withdrawal.20–22 The majority of recipients in this study received induction immunosuppression with immediate-release tacrolimus (86.1%), MMF (64.1%) and MPA (35.9%), and all patients received induction immunosuppression with prednisone. Overall, 25% of patients achieved corticosteroid withdrawal (prednisone) by the end of the study, with 94.1% of patients still receiving MMF or MPA, and 74.7% receiving prednisone.
Glucose metabolism and renal function were also maintained following transplantation. Although the urine protein-to-creatinine ratio dipped at 2 years and then increased at 5 years, this was not clinically significant given that proteinuria levels were low until 4 years and then increased, probably due to the increased chance of chronic histologic lesions at 5 years.
LimitationsWhile this study included a large number of pancreas transplant recipients, there was a considerable difference in the number and types of transplantations performed at the different centres, and four national transplant centres did not participate. Despite this, the setting reflects national clinical practice in Spain for this patient population. The broad inclusion criteria were necessary to ensure all three pancreas transplantation types were considered during the study. Given the high patient survival rate, data for the association of risk factors with patient survival were not reported. Additionally, kidney graft survival was not analyzed according to pancreas transplant type. Additionally, the proportion of patients with missing data increased over time, either due to a lack of follow-up assessments or to omission of results from the medical records. The data relating to renal function and glucose metabolism should therefore be interpreted with caution.
ConclusionThis real-world, retrospective study showed high patient and graft survival rates after PTA, SPK and PAK transplantation performed in Spain. Glucose metabolism, renal function and cardiovascular risk factors were generally stable following transplantation, and the rate of death was 6.3% at 5 years post-transplant. Risk factors were not assessed for patient survival due to the high patient survival rate; however, pancreas graft survival was higher in patients without a previous kidney transplant.
Data statementResearchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
FundingThis study was sponsored by Astellas Pharma, S.A.
Authors’ contributionAll authors made substantial contributions to the conception and design of the study, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; and provided final approval for submission.
Conflict of interestAll authors report non-financial support from Astellas Pharma Inc during the conduct of the study.
The authors would like to thank Professor Maria José Ricart for their wisdom, friendship, and leadership in the development of pancreas transplantation as an established treatment in Spain. Anisha Mehra, PhD, CMPP™ and Rachel Parratt, Ph.D. assisted in drafting the manuscript under the direction of the authors, and provided editorial support throughout its development for Cello Health MedErgy. Editorial support was funded by Astellas Pharma Inc.