Vascular endothelial growth factor (VEGF) is associated with renal pathogenesis of Fabry disease (FD). Proteomic studies have demonstrated that circulating levels of VEGF are higher in young FD patients compared with controls and an overexpression of tissue VEGF in animal models of Fabry nephropathy has been reported. In kidneys, VEGF-A is predominantly produced by podocytes, and its action on endothelial dysfunction is known. An alternative VEGF-A isoform, VEGF-A165b, confers benefit in microvascular disease states, and it was studied in diabetic nephropathy having demonstrated a protective function, acting on the restoration of the podocyte glycol-calyx.
MethodsCross-sectional design. Urinary mRNA was obtained by RT-qPCR.
Results48 subjects were included; 24 FD patients (17F/7M – 23.7±17.5 ys) and 24 healthy volunteers (17F/7M – 23.0±17.0 ys). 12 adults and 12 pediatrics in both populations Median uACR (p=0.999) and eGFR (p=0.999) were similar between both groups. FD genotypes included were R301Q, R363H, R227Q, del3&4exon, L106R, E398X, L415P and C238Y; 15 FD patients were treatment naïve and 9 receiving ERT (agalsidase-β); median time of ERT was 15.6±28.3 months. Comparative expression of urinary VEGF-165b-mRNA was higher among FD patients versus controls although without statistical significance (p=0.369). No significant correlations were found between urinary VEGF-165b-mRNA and variables “Age” (p=0.845), “Gender” (p=0.369), “αGal-A” (p=0.631), “Genotype” (p=0.142), “Phenotype” (p=0.898), “uACR” (p=0.744), “eGFR” (p=0.059) and “ERT or Naïve” (p=0.507). A significant correlation between urinary VEGF-165b-mRNA and “time of ERT treatment” was found (p=0.05).
ConclusionOverexpression of urinary VEGF-165b-mRNA (with known renal cyto-protective effects) is a probable response to injury in FD nephropathy. The only variable correlated with the highest urinary expression of VEGF-165b-mRNA was the time of ERT treatment. Probably in patients with longer treatment time there is a decrease in FD damage. A limitation of the present work is the low sample size and cross-sectional design.
El factor de crecimiento endotelial vascular (VEGF) está asociado a patogenia renal de la enfermedad de Fabry (EF). Los estudios proteómicos han demostrado que los niveles circulantes del VEGF son más altos en pacientes de EF jóvenes, en comparación con los controles, habiéndose reportado la sobreexpresión de VEGF tisular en modelos animales de nefropatía de Fabry. En los riñones, VEGF-A es producido predominantemente por los podocitos, desconociéndose su acción en la disfunción endotelial. Una isoforma alternativa de VEGF-A, VEGF-A165b, confiere beneficio en los estados microvasculares de la enfermedad, habiendo demostrado el estudio en nefropatía diabética una función protectora, actuando en la restauración del podocito glicol-cálix.
MétodosDiseño transversal. El ARNm de la orina se obtuvo mediante RT-qPCR.
ResultadosSe incluyeron a 48 sujetos; 24 pacientes de EF (17M/7V – 23,7±17,5 años) y 24 voluntarios sanos (17M/7V – 23±17 años). Doce pacientes adultos y 12 pacientes pediátricos en ambas poblaciones. Los valores medios de uACR (p=0,999) y eGFR (p=0,999) fueron similares entre ambos grupos. Los genotipos de EF incluidos fueron R301Q, R363H, R227Q, del3&4exon, L106R, E398X, L415P y C238Y; 15 pacientes de EF recibieron tratamiento por vez primera y 9 recibieron TREP (agalsidasa-β); el tiempo medio de la TREP fue de 15,6±28,3 meses. La expresión comparativa del ARNm del VEGF-165b en la orina fue superior entre los pacientes de EF frente a los controles, aunque sin significación estadística (p=0,369). No se encontraron correlaciones significativas entre el ARNm del VEGF-165b en la orina y las variables «Edad» (p=0,845), «Sexo» (p=0,369), «αGal-A» (p=0,631), «Genotipo» (p=0,142), «Fenotipo» (p=0,898), «uACR» (p=0,744), «eGFR» (p=0,059) y «TREP o sin tratamiento» (p=0,507). Se encontró una correlación significativa entre el ARNm del VEGF-165b en la orina y el «tiempo del tratamiento TREP» (p=0,05).
ConclusiónLa sobreexpresión del ARNm del VEGF-165b en la orina (con efectos citoprotectores renales conocidos) es una respuesta probable a la lesión en la nefropatía de la EF. La única variable correlacionada con la mayor expresión de ARNm del VEGF-165b en la orina fue el tiempo del tratamiento TREP. Probablemente en los pacientes con mayor tiempo terapéutico existe una reducción del daño de la EF. Una limitación del presente trabajo es el tamaño muestral pequeño y el diseño transversal.
Fabry disease (FD; OMIM 301500) is a chronic, progressive and multisystemic lysosomal storage disease.1 In FD, X-linked genetic variants of GLA gene cause alpha-galactosidase-A (αGal-A) activity deficiency and, as consequence, glycosphingolipid substrates, mainly globotriaosylceramide (Gb3) accumulates in lysosomes, all cells and tissues throughout the body, leading to organ dysfunction.1 Globotriaosylsphingosine (Lyso-Gb3), a deacylated molecule from Gb3, circulates in plasma and is capable of causing damage to multiple tissues in affected patients.2
Life expectancy among FD patients is shorter than the general population due to premature onset of renal disease, cardiomyopathy, malignant arrhythmias, and cerebrovascular events at an early age in both genders.1
FD renal damage is manifested by pathological albuminuria on childhood and subsequent estimated glomerular filtration rate (eGFR) decrease on adulthood2; end-stage kidney disease (ESRD) with requirement of dialysis or transplant occurs at age 40 in males with “classic” FD phenotype (or FD type 1).2,3 In women with “classic” FD, renal disease is more variable, usually onset later2; while in patients of both genders with the “late-onset” FD (or FD type 2), nephropathy may be the only manifestation or be absent.2
Pathological albuminuria is then the first clinical manifestation of FD nephropathy2,3 but some pathophysiological mechanisms are pre-albuminurics.4–6 Then, determining the mechanisms involved in Fabry nephropathy early stages is useful to: (i) delay progression of nephropathy, (ii) increase the probability of therapeutic efficacy, before renal fibrosis and (iii) develop new potential specific therapies.2,7
We previously reported miR-200 and miR-29 in early stages of Fabry nephropathy and the possible role of transforming growth factor-β (TGF-β) in podocyte damage and microRNA regulation in FD.8–11 While TGF-β effects in FD nephropathy have been described,4,12,13 other growth factors such as vascular endothelial growth factor (VEGF) do not have effects well-known in renal damage due to FD among humans. Existing reports regarding the role of VEGF in animal models of FD nephropathy14 and VEGF circulating levels in humans FD15 but the VEGF renal effect and VEGF urinary expression in humans FD have not been described. Then, VEGF was associated with renal pathogenesis of FD and, proteomic studies have demonstrated that circulating VEGF levels are higher in young FD patients compared with controls15 and an overexpression of tissue VEGF in animal models of Fabry nephropathy has been reported.14
In kidneys, VEGF-A is predominantly produced by podocytes, and its action on endothelial dysfunction is known. An alternative VEGF-A isoform, VEGF-A165b, confers benefit in microvascular disease states, and it was studied in diabetic nephropathy having demonstrated a protective function, acting on the restoration of the podocyte glycol-calyx.16–18
In this work, we presented results of a pilot study regarding urinary VEGF-A165b mRNA expression in FD patients versus controls.
MethodsCross sectional designSubjectsInclusion criteria: Patients with FD confirmed diagnosis of any age and gender. Exclusion criteria: (i) patients with a cause of kidney disease other than FD, (ii) patients who, at the moment of screening presented any clinical condition that potentially alters the results of biomarkers included in the study (diabetes mellitus, autoimmune diseases and systemic pathologies that can affect vascular structures were excluded, but not arterial hypertension because it is an accompanying manifestation of FD), and (iii) patients who fulfilled the inclusion criteria but refused to participate in the study. Elimination criteria were designed to ensure (i) the integrity of the results and (ii) the safety of the participants. Pre-existing medical conditions, concurrent treatments, or even being unfit to participate in the study at the discretion of the researchers were exclusion criteria.
In order to increase the internal validity of our study, and to increase the certainty of the effect of the variable “Fabry disease” on the variable “urinary excretion of VEFG-165b-mRNA”, a control group was included. Healthy subjects with similar demographic characteristics were included as a control group. In this group, as in the FD patient population, any condition that could modify the variable “urinary excretion of VEFG-165b-mRNA” was excluded.
All study subjects gave their informed written consent, authorizing the publication of their results. Adults signed consent and those of pediatric age agreed, and an adult representative also signed, according to local legislation.
Blood and urine samples were taken first thing in the morning on patients fasting. To classify GLA gene variants, www.omim.org/entry/301500 database was used. The quantification of αGAL-A activity was performed by the fluorometric method; values greater than >4.0nmol/h/l, were normal. Plasma and urinary creatinine were determined by electrochemiluminescence (Roche Diagnostics). Albuminuria was determined by a colorimetric method (Roche Diagnostics). The uACR was determined by the ratio albumin (mg)/creatinine (gr). eGFR was estimated by the CKD-EPI equation in adults and Schwartz 2009 equation in pediatric patients.19
mRNA extraction and RT-qPCRTotal RNA extraction was performed according to the MagNA Pure Compact RNA Isolation Kit protocol, Roche Diagnostics, Switzerland. The integrity of the RNA was confirmed by running on an agarose gel, which was found to be suitable for PCR. RNA purity was confirmed using an IMPLEN 330 relative absorbance ratio 260/280 (Implen, Germany). Samples with a radius greater than 1.8 were used for PCR. A Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics, Switzerland) was used for RT. Preparation, protocol, reaction conditions and storage were previously described by us.11 mRNA quantification was performed with an ABI Step One Plus system (Applied Biosystems, USA). Human β-actin was used as a reference housekeeping gene.11 A FastStart Universal SYBR Green Master/ROX system (Roche Diagnostics, Switzerland) was used for the q-PCR reaction. The quantification of each mRNA relative expression was performed by the ΔΔCt method.11
Statistics analysisDescriptive statistics: Qualitative variables were expressed as percentages and continuous (quantitative) variables as mean±standard deviation. Inferential statistics: parametric or non-parametric tests were used depending on the type of variable to be analyzed. A confidence interval of 95% was used. p<0.05 were considered statistically significant. Data was processed in IBM SPSS Statistics database.
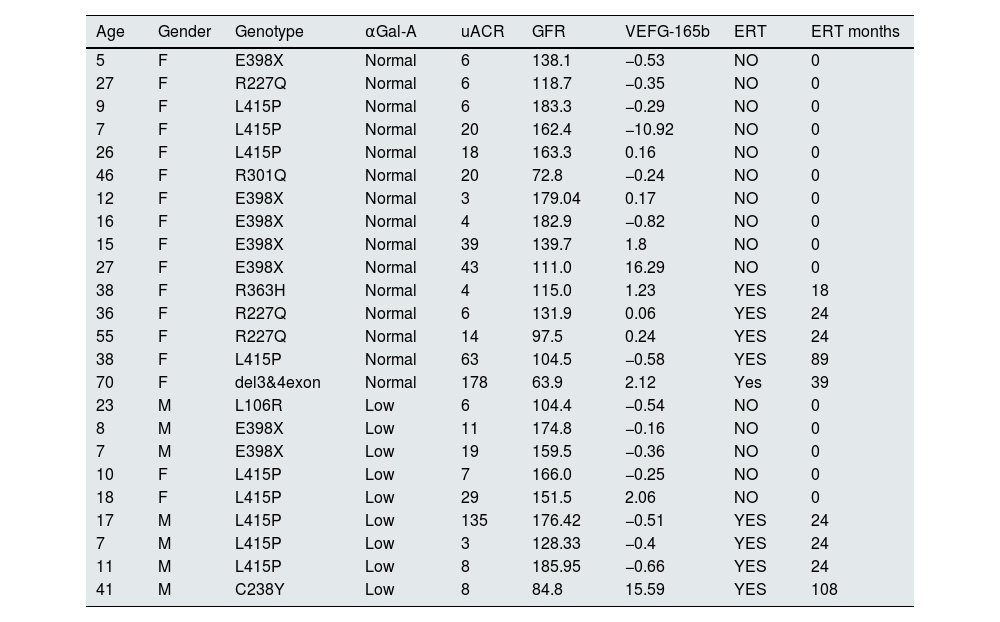
ResultsForty-eight subjects were included: 24 FD patients (17F/7M – 23.7±17.5 ys) and 24 healthy volunteers (17F/7M – 23.0±17.0 ys). 12 adults and 12 pediatrics in both populations. Median uACR (p=0. 999) and eGFR (p=0.999) were similar between both groups. FD genotypes included were: R301Q, R363H, R227Q, del3&4exon, L106R, E398X, L415P and C238Y; 15 FD patients were treatment naïve and 9 receiving ERT (agalsidase-β); median time of ERT was 15.6±28.3 months (Table 1).
Patient population with Fabry disease included.
| Age | Gender | Genotype | αGal-A | uACR | GFR | VEFG-165b | ERT | ERT months |
|---|---|---|---|---|---|---|---|---|
| 5 | F | E398X | Normal | 6 | 138.1 | −0.53 | NO | 0 |
| 27 | F | R227Q | Normal | 6 | 118.7 | −0.35 | NO | 0 |
| 9 | F | L415P | Normal | 6 | 183.3 | −0.29 | NO | 0 |
| 7 | F | L415P | Normal | 20 | 162.4 | −10.92 | NO | 0 |
| 26 | F | L415P | Normal | 18 | 163.3 | 0.16 | NO | 0 |
| 46 | F | R301Q | Normal | 20 | 72.8 | −0.24 | NO | 0 |
| 12 | F | E398X | Normal | 3 | 179.04 | 0.17 | NO | 0 |
| 16 | F | E398X | Normal | 4 | 182.9 | −0.82 | NO | 0 |
| 15 | F | E398X | Normal | 39 | 139.7 | 1.8 | NO | 0 |
| 27 | F | E398X | Normal | 43 | 111.0 | 16.29 | NO | 0 |
| 38 | F | R363H | Normal | 4 | 115.0 | 1.23 | YES | 18 |
| 36 | F | R227Q | Normal | 6 | 131.9 | 0.06 | YES | 24 |
| 55 | F | R227Q | Normal | 14 | 97.5 | 0.24 | YES | 24 |
| 38 | F | L415P | Normal | 63 | 104.5 | −0.58 | YES | 89 |
| 70 | F | del3&4exon | Normal | 178 | 63.9 | 2.12 | Yes | 39 |
| 23 | M | L106R | Low | 6 | 104.4 | −0.54 | NO | 0 |
| 8 | M | E398X | Low | 11 | 174.8 | −0.16 | NO | 0 |
| 7 | M | E398X | Low | 19 | 159.5 | −0.36 | NO | 0 |
| 10 | F | L415P | Low | 7 | 166.0 | −0.25 | NO | 0 |
| 18 | F | L415P | Low | 29 | 151.5 | 2.06 | NO | 0 |
| 17 | M | L415P | Low | 135 | 176.42 | −0.51 | YES | 24 |
| 7 | M | L415P | Low | 3 | 128.33 | −0.4 | YES | 24 |
| 11 | M | L415P | Low | 8 | 185.95 | −0.66 | YES | 24 |
| 41 | M | C238Y | Low | 8 | 84.8 | 15.59 | YES | 108 |
References: αGal-A: αGalactosidase-A; uACR: urinary albumin/creatinine ratio; GFR: glomerular filtration rate; VEFG-165b: vascular endothelial growth factor-165-b; ERT: enzyme replacement therapy; ERT months: months of enzyme replacement therapy.
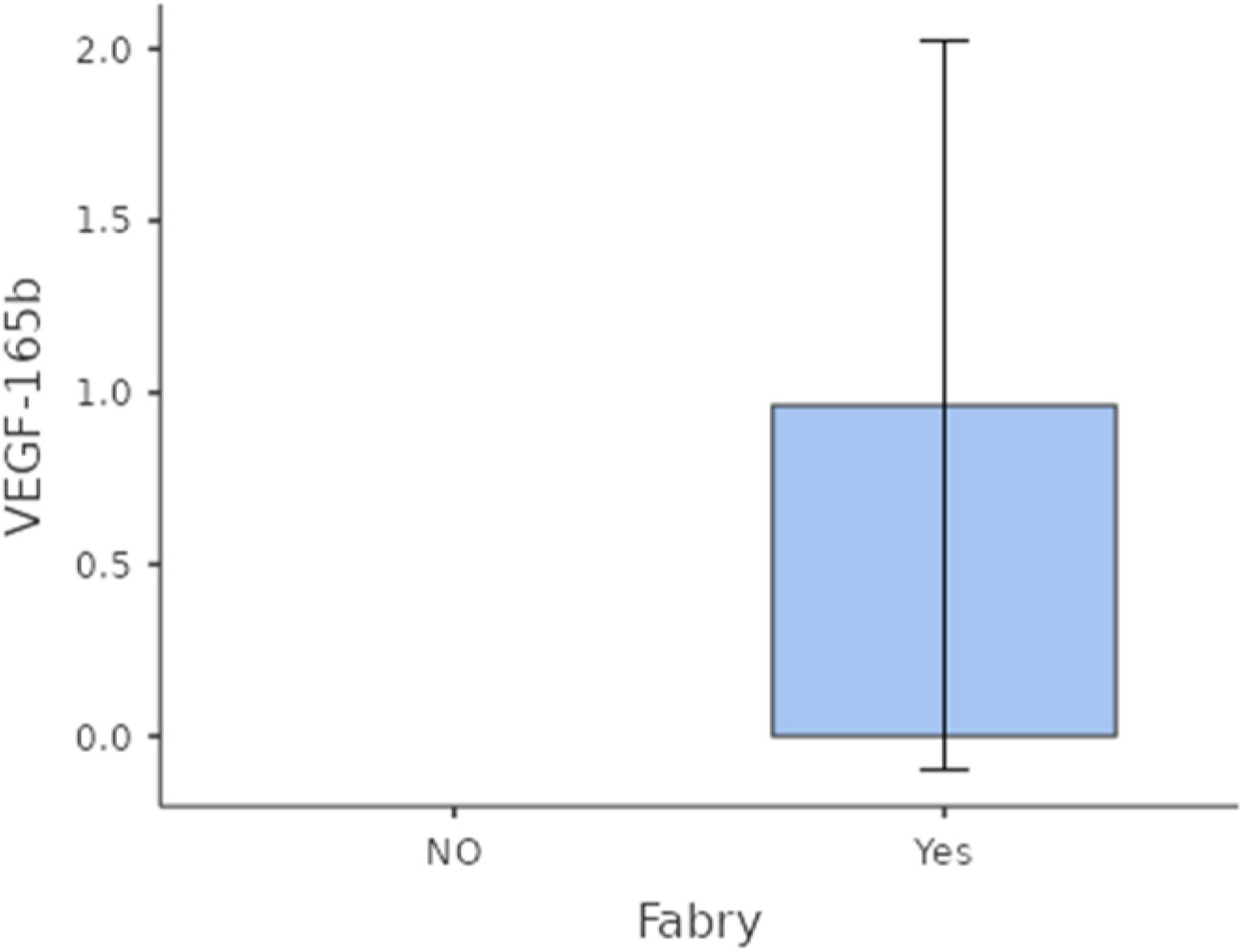
Comparative expression of urinary VEGF-165b-mRNA was higher among FD patients versus controls although without statistical significance (p=0.369) (Fig. 1).
No significant correlations were found between urinary VEGF-165b-mRNA and variables “Age” (p=0.845), “Gender” (p=0.369), “αGal-A” (p=0.631), “Genotype” (p=0.142), “Phenotype” (p=0.898), “uACR” (p=0.744), “eGFR” (p=0.059) and “ERT or Naïve” (p=0.507).
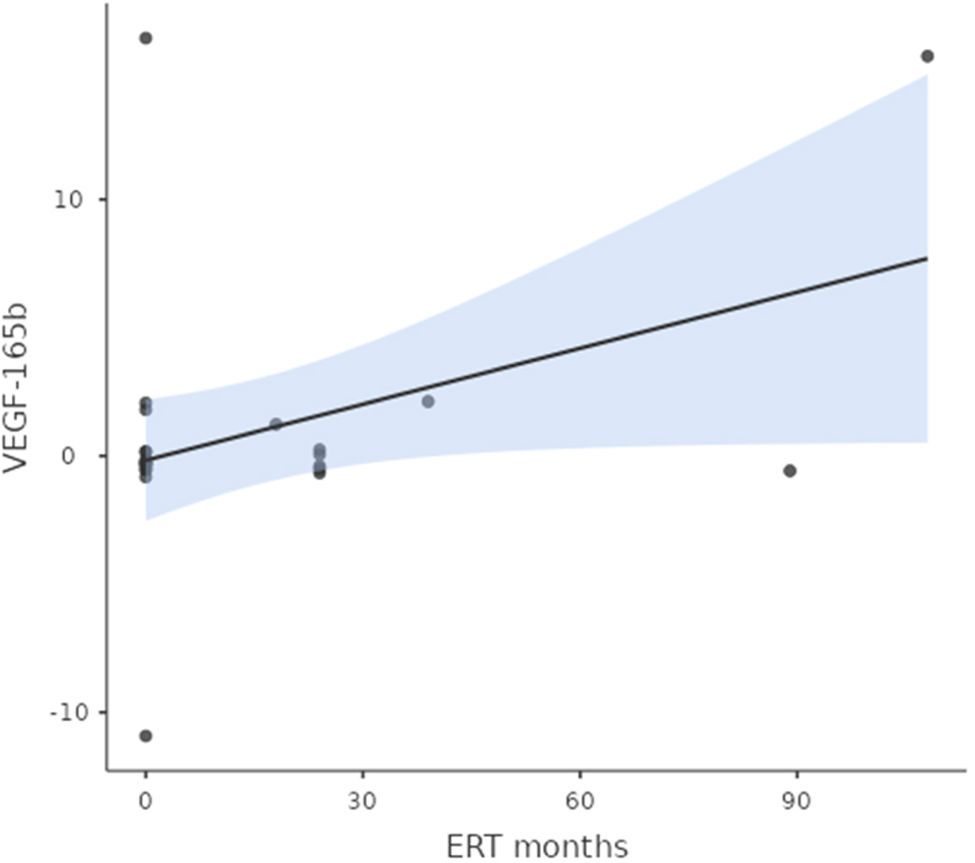
A significant correlation between urinary VEGF-165b-mRNA and “time of ERT treatment” was found (p=0.05 among Fabry population (Fig. 2).
There were no other systemic pathologies other than FD in the clinical history of the study subjects, nor did any new ones appear during the present study.
DiscussionProgression in chronic kidney diseases (CKD) causes the progressive loss of eGFR and move forward ESRD, which happens approximately 40–42 years in FD men classically affected in our geographic region, similar to others.3,7 Renal fibrosis is It is the underlying histological lesion of clinical progression in CKD of any cause20 and it is also not possible to reverse with any therapeutic intervention.20 TGF-β is the master molecule involved in renal fibrosis20,21 and in Fabry nephropathy its effect is known,4,12,13 however VEGF, another growth factor involved in renal physiopathology it has not been well studied in FD nephropathy.
VEGF-A is a group of secreted glycoprotein iso-forms that has been reported as an endogenous protective factor,22 mainly described in diabetes vasculopathy.23 VEGF-A is predominantly produced by podocytes, and its protective actions on the blood-glomerular barrier are known.24
While VEGF-A165a (a VEGF-A isoform) can accelerate diabetic nephropathy,25 VEGF-A165b is protective and restores endothelial glycocalyx.18
As mentioned previously, in FD nephropathy there is no evidence regarding the role of VEGF in humans. In 2007, a proteomic study of Moore et al., reported that circulant VEGF-A levels are high in FD affected patients15 and in 2012, Lee et al., showed evidence regarding high tissue VEGF expression in FD nephropathy animal models and a direct correlation between VEGF expression and increased extracellular matrix components.14 These works are the background on the topic of the present study.
Regarding the results obtained in this pilot study, our hypotheses are as follows: (i) overexpression of urinary VEGF-165b-mRNA (with known renal cyto-protective effects) is a probable response to injury in FD nephropathy, (ii) as the unique variable correlated with the highest urinary expression of VEGF-165b-mRNA was the time of ERT treatment, probably in patients with longer treatment time there is a decrease in FD damage.
A limitation of the present work is the low sample size and cross-sectional design. On the other hand, another main limitation of our work is the use of methods that, although standardized, are not validated for determinations in routine practice. This work is a basic science pilot study that should be followed up longitudinally in the future to be eventually translated into the clinic. In the meantime, our novel results only come from a basic science pilot study.
ConclusionOverexpression of urinary VEGF-165b-mRNA (with known renal cyto-protective effects) is a probable response to injury in FD nephropathy. The only variable correlated with the highest urinary expression of VEGF-165b-mRNA was the time of ERT treatment. Probably in patients with longer treatment time there is a decrease in FD damage. A limitation of the present work is the low sample size and cross-sectional design.
Authors’ contributionsAll authors made substantial contributions to each of the following aspects: (1) conception and design of the study, or acquisition of data, analysis and interpretation of data, (2) drafting the article or revising it critically for intellectual content, (3) final approval of the submitted version.
Ethical approval and consent to participate and consent for publicationProtection of human and animal subjects: The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki). The present study was approved by “Comité de Ética y Bioética del Instituto Universitario Italiano de Rosario” (Res: 23/17). The results of this work are derived from data of the Thesis called “Enfermedad de Fabry. Excreción urinaria de microRNAs relacionados a fibrosis renal”. Confidentiality of data: The authors declare that they have followed the protocols of their work center on the publication of patient data. Right to privacy and informed consent: The authors have obtained the written informed consent of the patients or subjects mentioned in the article. This document It is available and can be consulted at: investigacion@iunir.edu.ar.
Financial support and sponsorshipNot applicable.
Conflicts of interestAll authors declared that there are not conflicts of interest.
Availability of data and materialsData of this work is available and can be consulted at: investigacion@iunir.edu.ar.
Not applicable.