Hyponatremia is a multifactorial disorder defined as a decrease in plasma sodium concentration. Its differential diagnosis requires an adequate evaluation of the extracellular volume (ECV). However, ECV determination, simply based on the clinical history, vital signs, physical examination, and laboratory findings can leads to misdiagnosis and inappropriate treatment. The use of Point-of-Care Ultrasound (POCUS), through the combination of Lung Ultrasound (LUS), Venous Excess UltraSound (VExUS) and Focused Cardiac Ultrasound (FoCUS), allows a much more accurate holistic assessment of the patient's ECV status in combination with the other parameters.
La hiponatremia es un trastorno multifactorial definido como una disminución en la concentración plasmática de sodio. Su diagnóstico diferencial requiere una evaluación adecuada del volumen extracelular. Sin embargo, la determinación del volumen extracelular, simplemente basada en la historia clínica, las constantes vitales, el examen físico y los hallazgos de laboratorio, conducen en ocasiones a un diagnóstico erróneo por lo que el enfoque terapéutico puede ser equivocado. El empleo de ecografía a pie de cama (Point-of-Care Ultrasound [PoCUS]), mediante la combinación de ecografía pulmonar (Lung Ultrasound [LUS]), Venous Excess UltraSound (VExUS) y la ecocardioscopia (Focused Cardiac Ultrasound [FoCUS]) permiten, en combinación con el resto de los parámetros, una valoración holística mucho más precisa del estado del volumen extracelular del paciente.
Hyponatremia is defined as a plasma sodium concentration≤135mEq/l. It affects about 6%–7% of the general population and up to 15%–30% of hospitalized adult patients.1–3
Its significance rests in the fact that it has been shown to be independently associated with increased mortality, increase in days of hospitalization, gait disturbances, falls, osteoporosis and a significant deterioration in quality of life.4,5 Therefore, its correct identification together with an adequate diagnosis and an appropriate therapeutic approach is essential; however and in some cases it continues to be an unmet medical demand.
Plasma sodium concentration depends on the content of exchangeable sodium in relation to total body water. Hyponatremia is related to a relative excess of water compared to extracellular sodium (most common) or an absolute loss of sodium (uncommon). Antidiuretic hormone (ADH) is the main regulator of renal water excretion, being elevated in most clinical scenarios of hyponatremia.6
Clinical caseA 71-year-old woman with clinical history tobacco use up to 2 years ago (exposure 29 pack-years), dyslipidemia, and bilateral gonarthrosis. Her treatment was only atorvastatin 20mg.
She went to the emergency room because during the last 2 months she had progressive asthenia, loss of appetite, weakness, and progressive swelling of both lower extremities. On physical examination, he was afebrile, the blood pressure was 135/86mmHg, heart rate of 85 beats per minute, and oxygen saturation of 96%. Hypoventilation at base of the right lung and bilateral pitting edema up to the root of the lower limbs. In the blood chemistry it was found hyponatremia of 122mEq/l (we do not have previous analysis controls in the last year, but it was known that somewhat earlier her serum sodium was normal), glucose an renal function were normal. Low plasma osmolality 252 mOsm/kg consistent with hypoosmolar hyponatremia. N-terminal B-type natriuretic peptide (NT-proBNP 745pg/mL. Urine Na and K were <20mmol/L, and 36mmol/l respectively with an urinary osmolarity of 295mOsm/kg. Chest x-ray showed the presence of right pleural effusion (no previous chest imaging studies were available) with a normal cardiothoracic index, without clear condensations, masses, or signs of vascular redistribution.
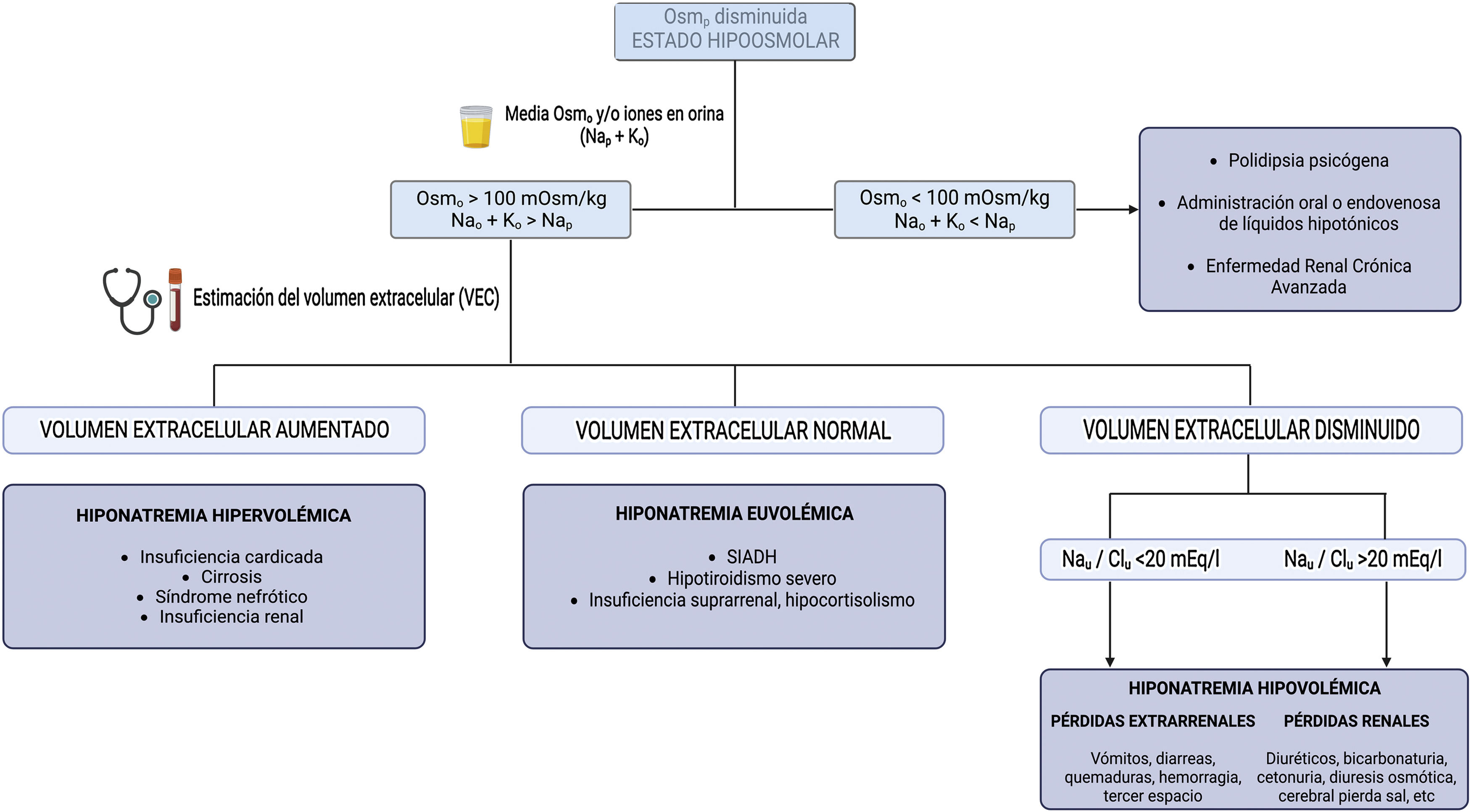
Diagnostic approachThe diagnostic approach most commonly used in clinical practice is based on 3 questions (Fig. 1):
- 1
What is the plasma osmolality? It allows differentiating hypotonic hyponatremias from pseudohyponatremias (hyperlipidemia or hyperproteinemia) and hyponatremias with normal or increased osmolality (hyperglycemia, administration of parenteral substances with osmolar power such as mannitol).
- 2
What is the urinary osmolality or electrolyte-free water clearance (Nau and Ko)? It allows to distinguish between hyponatremias in which the kidney eliminates free water adequately and hyponatremias in which the renal response is inadequate, usually due to an excess of circulating ADH.
- 3
How is the extracellular volume (ECV)? This provides an indication of the ultimate cause that has triggered hyponatremia.
For this purpose, it is essential to know the plasma Osm, urinary Osm, the main ions in urine (Nau, Ku) and to estimate ECV, this last point being sometimes a real challenge for the clinician. Unfortunately, isolated physical examination using parameters such as the degree of mucosal hydration or skin turgor, ocular tone, or the presence or absence of pulmonary crackles and edema, has a low sensitivity and in some cases it is associated with an incorrect diagnosis.7 Chung et al. demonstrated in a study including 58 patients, all with hyponatremia (Nap<130mEq/l), that they only adequately identified 47% of hypovolemic patients and 48% of euvolemic patients.8 This fact highlights the need for the use of other tools for a more accurate assessment of ECV.
In recent years, the concept of multiparametric assessment of congestion has emerged in which the use of serum biomarkers of congestion has been proposed, such as NT-proBNP produced in response to the stress to which myocardiocytes are subjected by volume and/or pressure overload, and is currently the most widely used biomarker for the diagnosis and prognosis of HF. Its elevation predominates in patients with left ventricular (LV) diastolic dysfunction. Factors such as age and glomerular filtration rate, among others, influence circulating levels of NT-proBNP, therefore its use is limited in a high number of patients. Another novel biomarker, recently described, is carbohydrate antigen 125 (CA 125), which is produced in serosal cells such as the pleura, peritoneum and pericardium in response to increased tissue hydrostatic pressures and/or inflammation, and which shows a stronger association than NT-proBNP with the rest of the congestion parameters in addition it is not influenced by glomerular filtration. Despite this, it seems reasonable that its combined use could provide complementary information, with NT-proBNP being a “biochemical window to the left heart”, while CA 125 both a “window to the right heart” in HF and a marker of congestion in other hydropic states.9–11
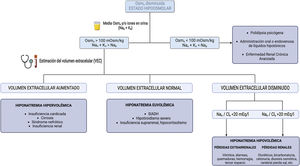
Including insonation, as the fifth pillar of the physical examination,12 improves the sensitivity and specificity in determining ECV. The ultrasound study includes echocardioscopy for the morphological and functional assessment of the heart, in addition it allows an assessment of the stroke volume and cardiac output. The Venous Excess UltraSound (VExUS) allows phenotyping and grading of venous congestion, and finally, it evaluates the presence of free fluid, both in lungs by lung ultrasound (Lung Ultrasound [LUS]) and in the abdomien.13 For this reason, in recent years different works have been published emphasizing the usefulness of bedside ultrasound (Point-of-Care Ultrasound [PoCUS]) in patients with hyponatremia.14–18
Thus, we suggest the implementation of PoCUS in the routine evaluation of the patient with hyponatremia, as well as an update in the diagnostic algorithm for hyponatremia (Fig. 2), bearing in mind that not all ultrasound findings are always present and that hyponatremia often has a multifactorial origin.
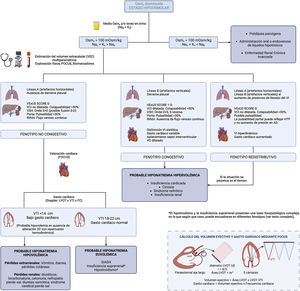
Furthermore, although a description of the basic principles of VExUS in the determination of ECV is not the subject of this review, it is important to recognize its main limitations in order to minimize errors both in the acquisition of images and in their correct interpretation (Fig. 3).
Non-congestive phenotypeIn the presence of hypovolemia it is common to identify an A-line pattern (horizontal artifacts, parallel to the pleural line) by LUS, accompanied by decreased systolic volume measured by determining the size LV outflow tract and the integral of the velocity with respect to time calculated at that point, in addition to a preserved right ventricle/LV ratio in the parasternal plane short axis and the presence of a non-dilated inferior vena cava (IVC) with collapsibility <50% (low right atrial pressure).
The presence of an IVC of normal diameter and collapsibility, in the absence of free fluid, indicates the absence of venous congestion. However, as a general rule, it does not allow per se to differentiate hypovolemic patients from those with euvolemia, although extreme findings such as a completely collapsed IVC should guide us to the first scenario of hypovolemia. In this situation, the estimation of stroke volume is helpful, being decreased in case of hypovolemia as mentioned above.
Congestive phenotypeIn case of congestion, depending on where this predominates, it will be possible to find pleural effusion or the presence of B lines or “pleural comets” on lung ultrasound and/or ascites on abdominal insonation, a dilated IVC with collapsibility <50%, plus presence of venous congestion with pathological VExUS and by echocardioscopy a LV systolic and/or diastolic dysfunction (if the etiology is HF) both with low cardiac output (if systolic HF), or normal-high cardiac output, right ventricular dilatation and/or flattening of the interventricular septum (predominance in diastole if volume overload and in systole-diastole if there is pressure overload).
Redistributive phenotypeAlthough congestion and volume overload often go hand in hand, they are not synonymous. Sometimes there is a redistribution of fluid from a venous reservoir such as the splanchnic bed, with increased secondary cardiac output and B lines (only in the case of increased LV filling pressures), with an adequate variability of ICV, without an initial increase in ECV, as can be seen in certain scenarios of heart failure, in the early phase of hepatorenal syndrome or in the septic patient. If the condition that has precipitated the development of this scenario is not reversed, the elevated sodium and water avidity in the renal tubule, as well as the imbalance between the hydrostatic and oncotic pressures of the intravascular and interstitial compartment, would lead to a more gradual increase in ECV.
Special scenariosBoth hypothyroidism and adrenal insufficiency have been discarded from the algorithm because they present a complex pathophysiological basis in which, depending on the predominant mechanism, congestion may or may not be observed, with its different phenotypes, as well as a variable systolic volume.
In patients with myxedema, the accumulation of mucopolysaccharides in the interstitium favors the accumulation of water at this level, a low intravascular volume and the consequent non-osmotic release of ADH.19 In acute severe hypothyroidism, as occurs after discontinuation of thyroid hormone treatment prior to iodine administration in patients with thyroid cancer, decreased glomerular filtration rate and the reduced capacity to excrete free water seem to be the predominant mechanism. In chronic hypothyroidism, non-osmotic ADH release secondary to decreased cardiac output is postulated as the main mechanism, although it is not the only one. However, hypothyroidism outside myxedema is being increasingly discussed as a cause of hypothyroidism.
In adrenal insufficiency, the low levels of aldosterone, a priori, would favor natriuresis with the development of hypovolemia and secondary release of ADH. However, many times the reduction of volemia is modest, added to the fact that other factors such as the increase in angiotensin II or norepinephrine increase renal sodium reabsorption. Furthermore, glucocorticoid deficiency induces a reduction in blood pressure and cardiac output with non-osmotic release of ADH; in addition, the increase in Corticotrophin-releasing hormone (CRH) levels will act, per se, as an ADH secretagogue.20–22
Resolution of the clinical caseThe PoCUS study demonstrated the presence of a collapsed, filiform IVC, with a VExUS SCORE of 0 points. At the lung level there was a right pleural effusion without significant B lines. Finally, we should highlight the presence of a discrete amount of free abdominal fluid, as well as a pelvic mass suggestive of tumor. Expanding the study with an urgent thoracic, abdominal and pelvic CT, the presence of a uterine mass with compression of both iliac territories was confirmed, as well as a right pleural effusion and a lung lesion at the right base suggestive of metastasis.
With all this and despite the presence of pleural effusion, edema and low sodium in urine, which could have been interpreted as hypervolemic hyponatremia with the establishment of diuretic treatment. Instead, fluid therapy was started with 0.9% physiological saline solution with normalization of the serum sodium levels 72h after admission.
This case illustrates the usefulness of PoCUS in situations in which clinical and biochemical data do not clearly guide the diagnosis. The clearance of electrolyte-free water estimated by the elimination of cations and the osmolar clearance estimated by urinary osmolality provide a contradictory information.
Key concepts- -
Hyponatremia is not a diagnosis, but the result of a gain of free water due to a reduce excretion, caused by a series of very varied pathologies that may coexist.
- -
The assessment of ECV is fundamental in the diagnostic approach of hyponatremia. However, the determination of ECV solely based of classical tools relatively often leads to erroneous diagnostic and an incorrect therapeutic approach.
- -
Bedside ultrasound or PoCUS has become a very useful tool to complement the physical examination and analytical parameters in the patient with hyponatremia.
- -
The use of PoCUS answers a specific question quickly, noninvasively and reproducibly. However, its performance and interpretation is not without limitations, so it is considered necessary to increase its presence in the different training programs of the specialty and in the scientific societies of Nephrology.
The authors declare that they have no conflict of interest.