Se presentan dos casos de proteinuria en jóvenes obesos no diabéticos, con histología de Glomeruloesclerosis Focal y Segmentaria (GFS). La efectiva reducción de peso corporal mediante cirugía bariátrica se siguió de una remisión sostenida de la proteinuria, permitiendo una significativa reducción o suspensión de las dosis de fármacos bloqueadores del sistema renina-angiotensina.
2 cases of proteinuria in obese non-diabetic young males, both corresponding to focal segmental glomerulosclerosis are presented. Effective reduction of body weight by bariatric surgery was followed by sustained remission of proteinuria allowing significant reduction or total removal of blockers of the reninangiotensin-system.
INTRODUCTION
Obesity, defined as a Body Mass Index (BMI) ≥ 30, has become a worldwide health issue of epidemic proportions. In the United States, its prevalence reached 65% in 2003 and as a disease it is becoming increasingly relevant in developing countries like Chile, where a National Health Survey in 2003 indicated a prevalence of 23%. This condition is associated with other cardiovascular risk factors like high blood pressure, insulin resistance/type 2 diabetes mellitus, dyslipidaemia and coronary heart disease and can lead to progressive renal failure.
The first descriptions of nephropathy associated with obesity were published 30 years ago, being followed by numerous reports of kidney disease in obese subjects without diabetes.
Nephrotic syndrome associated with obesity has been described as a glomerulopathy that presents variable proteinuria. Occasionally nephrotic range proteinuria is present, but without the full blown nephrotic syndrome1, and with histopathological findings of FSGS. Diferences with the primary form of FSGS involves less foot process effacement of podocytes, perihilar predominance and the presence of glomerulomegaly. It progresses naturally into renal failure in half of cases2 if left untreated.
Taking into consideration the fact that developing countries are experiencing the epidemic spread of chronic kidney failure and that this could increase due to the concomitant raise in obesity incidence, we have decided to communicate two cases of FSGS affecting young obese males who were not diabetics, and who, following bariatric surgery experienced a significant remission of proteinuria in one case and total disappearance of the condition in another, which made it possible to reduce or suspend antiproteinuric treatment.
CLINICAL CASES
Case 1
This case involves a male patient who was obese from the age of 17, with some type 2 diabetic (DM2) relatives on his father’s side and who was taking atorvastatin for mild hyperlipidaemia. During a routine check-up at 19 years of age, the patient weighed 102kg (BMI 36.6), proteinuria was 3.8g/day, and there were no abnormalities in the urine sediment. Fasting glycaemia, albumin, thyroid profile and lipid levels were normal, blood pressure was 140/75, blood creatinine was 1.4mg/dl and the serology was negative for systemic diseases that could potentially affect the kidneys.
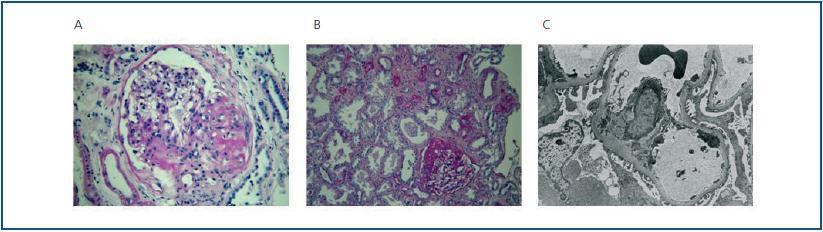
A kidney biopsy obtained 10 glomeruli, one was generally sclerotic and one of the others showed segmental sclerosis, significant hyalinosis and adherence to Bowman’s capsule which was perihilar in location (figure 3a); moderate interstitial fibrosis and tubular atrophy affecting the sclerotic glomerulus and efferent arteriolar hyalinosis were observed. The Electron Microcope (EM) revealed foot process effacement of podocytes with microvillous transformation and segmental changes in the Glomerular Basement Membrane (GBM), mainly in the paramesangial area, without any electron-dense deposits or tubulo-reticular inclusions. The immunofluorescence assay (IFA) only showed unspecific traces of IgM and C3. It was concluded that the patient presented the perihilar variant of FSGS.
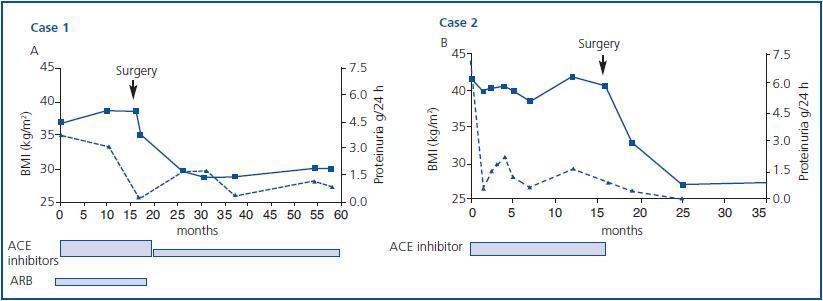
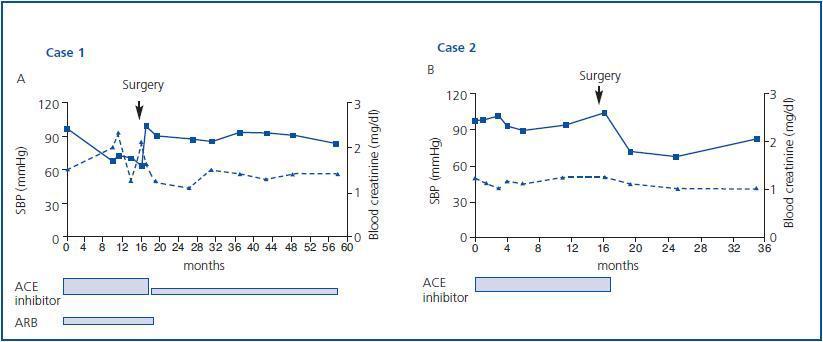
Antiproteinuric treatment with 20mg/day of lisinopril and 160mg/day of valsartan induced a moderate reduction in proteinuria to 1.5g/day. Efforts to lose body weight through exercise and diet were ineffective and open gastric bypass surgery was performed 17 months later which resulted in a weight loss of 80kg and the significant remission of proteinuria, which made it possible to reduce the dose of lisinopril to 5mg/day, suspend valsartan and atorvastatine treatment altogether and maintain renal function (blood creatinine 1.4mg/dl) after 58 months of follow up (figures 1a and 2a).
Case 2
This case involves a male patient who was obese from the age of 20. On examination, hypothyroidism and hyperlipidaemia were detected (total cholesterol 351mg/dl, HDL 49mg/dl and LDL 280mg/dl), both were quickly treated with thyroid hormone replacement therapy but there was no change in weight. One uncle had been diagnosed with DM2. During a routine check-up at 35 years of age the patient weighed 124kg (BMI 41.4), proteinuria was 7.14g/day, and there were no abnormalities in the urine sediment. Thyroid hormone levels were normal, blood pressure was normal (130/80mmHg), fasting glycaemia and albumin were normal and blood creatinine was 1.27mg/dl. The serology was also normal.
The kidney biopsy showed 16 glomeruli; two were generally sclerotic, three showed segmental sclerosis which was perihilar in location and adherence to Bowman’s capsule; the rest were about 50% larger in size. There was moderate interstitial fibrosis, tubular atrophy (figure 3b), interstitial lymphocyte infiltration and mild intimal fibrosis with moderate arteriolar hyalinosis. The EM revealed foot process effacement of podocytes (figure 3c), segmental changes in the GBM without electro-dense deposits or tubulo-reticular inclusions. The IFAdid not provide any additional information and it was concluded that patient presented the perihilar variant of focal glomerulosclerosis and glomerulomegaly.
Treatment with 10mg/day of fosinopril was administered which resulted in a reduction in proteinuria to 1.0g/day, without any change in weight despite modifying the patient’s diet for 17 months. Subsequently, open gastric bypass surgery was performed which resulted in a significant reduction in weight (85kg). His proteinuria disappeared, which made it possible to suspend all treatment with ACE inhibitors. Kidney function remained stable, with blood creatinine at 1.0mg/dl, after 35 months of observation (figures 1b and 2b).
DISCUSSION
Our two cases graphically illustrate the most typical glomerular disease associated with severe obesity and the positive response to the effective reduction in weight that was the result of bariatric surgery. Although it is clear that our patients reduced protein excretion after the use of RASblocking agents, the high initial levels of proteinuria and the failure of measures to reduce weight prompted the decision to perform bariatric surgery. In this respect, the beneficial effects of RAS-blocking agents could potentially have been lost if the obesity was not radically dealt with.2 The protracted remission following the effective weight loss confirms the importance of obesity in the pathogenesis of this condition. It is clear that a control kidney biopsy would have been useful in order to identify histological changes that associated with remission; however this possibility was ruled out for ethical reasons.
The physiopathological analysis of kidney disease associated with obesity is well supported with experimental examples of obese rats that present early podocyte damage and macrophage infiltration associated with hyperlipidaemia and hyperglycaemia, which precedes the development of glomerulosclerosis and tubulo-interstitial damage. Cases of spontaneous obesity in rhesus monkeys develop into full blown metabolic syndrome with progressive weight gain, followed by a sustained increase in blood insulin without hyperglycaemia, and finally leads to significant hyperglycaemia and a drop in insulin levels.3 Both our patients had a family history of DM2 but did not present it themselves, and although unfortunately we did not record insulin levels, they never presented hyperglycaemia when fasting plasma glucose tests were carried out on several occasions. Furthermore, the hyperlipidemia in one of the patients quickly normalized after thyroid hormone replacement and the same occurred in the other following the weight loss, making possible to eliminate statins.
The pathogenic mechanisms of renal damage associated with obesity are related to the adverse effects of the body adapting to the increase in the excretory load, salt retention and the direct or indirect effects of hyperinsulinaemia/insulin resistance and renal lipotoxicity. Haemodynamic changes are very important given that an increase in intraglomerular pressure associated with hyperinsulinaemia has been observed in experimental conditions and that obese patients without DM2 present greater glomerular filtration compared with control subjects with a normal BMI.4 The pathogenic role of RAS and the therapeutic use of RAS- blockers is significant because an increase in BMI is associated with an augment in vascular sensitivity to angiotensin II, which is confirmed by the regression of intraglomerular hypertension and structural damage after using ACE inhibitors.5
The importance of fatty tissue has also been evaluated since adipocytes not only act as an energy store but also they secrete hormones, cytokines and growth factors for the systemic vascular and renal bed, including renin, angiotensinogen, IL-6, leptin, PAI-1, adiponectin and TGFbeta. This is how animals with overexpression of angiotensin II in adipocytes develop hypertension which directly or indirectly affects the kidney. Leptin, a peptide produced by fatty tissue reduces food intake and increases sympathetic nerve activity in both thermogenic and non-thermogenic tissue and is considered an anti-obesity hormone. However, for reasons that have not yet been clarified, it has been observed that obese subjects develop a resistance to the satiety and loss of weight induced by leptine, conserving the sympathetic outflow to non-thermogenic tissues like the kidney, heart and adrenals. Therefore, leptine may modify the production of nitric oxide and induce sodium retention, systemic vasoconstriction and hypertension.6
At present, the management of nephropathy associated with obesity focuses on the use of antiproteinuric agents, with ACE inhibitors and angiotensin II receptor blockers being the most commonly used treatments, which also improve insulin sensitivity and protect the kidneys and cardiovascular system. Statins, metformin and PPARg have also been tested. Notwithstanding the foregoing, the only reduction in body weight has been effective in reducing proteinuria7. Bariatric surgery is considered a viable method for achieving it, involving a reasonable risk, and has already been suggested for the management of proteinuria in obese diabetic patients8 and other glomerulopathies. Therefore, it should form part of the therapeutic arsenal for treating renal diseases associated with obesity9 when the weight loss objective cannot be reached using conventional medical treatments or when the degree of proteinuria does not allow a long wait.10
Figure 1.
Figure 2.
Figure 3.