Primary hyperparathyroidism (PHPT) is a common endocrine disorder characterised by hypercalcaemia and parathormone increase. Decreased glomerular filtration rate (<60ml/min) continues to be a parathyroidectomy (PTX) criterion in asymptomatic PHPT. The influence of PTX on renal function evolution is the subject of debate.
ObjectiveTo analyse the clinical, laboratory and histological characteristics of patients undergoing PHPT, as well as renal function evolution after PTX.
Material and methodsRetrospective study of 297 patients diagnosed with PHPT and referred to surgery in a single centre between 1998 and 2016. Laboratory parameters were determined at baseline, one week and one year after PTX.
ResultsThe Incidence of PTX was 38cases/million/year. Mean age was 60±14 years and 80.5% of the patients were female. Approximately 65.3% were asymptomatic. Nephrolithiasis was the most common clinical finding (33%), followed by bone involvement (29.5%). PTX indications were: clinical symptoms (34.7%), hypercalcaemia >11.2mg/dl (27%), nephrolithiasis (13%), low bone mass (12%), age <50 years (11%) and decreased glomerular filtration rate <60ml/min (2.3%).
For diagnostic localisation, spect-MIBI had a sensitivity of 92% and cervical ultrasound of 70%. A total of 94.3% of PHPT cases were due to a parathyroid adenoma.
After PTX, normalisation of PHPT-related parameters was observed. We found a significant increase in serum creatinine levels (0.81 vs 0.85mg/dl, p<.001) from the first week post-PTX until the end of the first year. The renal function was only found to be significant in patients with glomerular filtration rate >60ml/min (baseline serum creatinine levels 0.77mg/dl vs serum creatinine levels after one year 0.81mg/dl, p<.001).
ConclusionsPHPT was asymptomatic in most patients who underwent surgery. Hypercalcaemia and nephrolithiasis were the most common indications of parathyroidectomy in asymptomatic patients. MIBI scan was the most useful localisation method. Surgical treatment of PHPT is followed by renal function impairment, which persists after the first week post-PTX.
El hiperparatiroidismo primario (HPTP) es un trastorno endocrino frecuente, caracterizado por hipercalcemia y elevación de la parathormona. La disminución del filtrado glomerular (<60ml/min) se mantiene en la guías como un criterio para la realización de la paratiroidectomía (PTX) en el HPTP asintomático. La influencia que tiene la PTX sobre la evolución de la función renal es controvertida.
ObjetivosAnalizar las características clínicas, analíticas e histológicas de los pacientes intervenidos por HPTP, así como la evolución de la función renal tras la PTX.
Material y métodosEstudio retrospectivo de 297 pacientes con HPTP remitidos a cirugía en un único centro entre 1998 y 2016. Los parámetros analíticos se determinaron en situación basal, a la semana y al año de la PTX.
ResultadosLa incidencia de PTX fue de 38 casos/millón/año. La edad media fue 60±14 años y el 80,5% de los pacientes eran mujeres. El 65,3% estaban asintomáticos. La nefrolitiasis fue el hallazgo clínico más frecuente (33%) seguido de la afectación ósea (29,5%). Las indicaciones de PTX fueron: síntomas clínicos (34,7%), hipercalcemia >11,2mg/dl (27%), litiasis renal (13%), baja masa ósea (12%), edad <50 años (11%) y disminución del filtrado <60ml/min (2,3%).
En el diagnóstico de localización el spect-MIBI presentó una sensibilidad del 92% y la ecografía cervical del 70%. El 94,3% de los casos de HPTP eran debidos a un adenoma paratiroideo.
Tras la PTX se objetivó normalización de los parámetros relacionados con el HPTP. Objetivamos un incremento significativo de la creatinina sérica (0,81 vs. 0,85mg/dl, p<0,001) desde la primera semana del postoperatorio y que se mantiene al año. Cuando comparamos los pacientes según el filtrado glomerular basal, encontramos que el deterioro de la función renal solamente fue significativo en pacientes con filtrado glomerular >60ml/min (creatinina sérica basal 0,77mg/dl vs. creatinina sérica al año 0,81mg/dl, p<0,001).
ConclusionesEl HPTP cursó asintomático en la mayoría de los pacientes intervenidos. La hipercalcemia y la nefrolitiasis fueron las indicaciones más frecuentes de paratiroidectomía en los pacientes asintomáticos. El scan-MIBI fue el método de localización más útil. La curación quirúrgica del HPTP se sigue de un deterioro de la función renal, que se mantiene desde la primera semana de la cirugía.
Primary hyperparathyroidism (PHPT) is a common mineral metabolism disorder caused by the excessive secretion of parathyroid hormone (PTH) by one or more parathyroid glands. Autonomic secretion of PTH acts on its target organs (bone and kidney) and increases the concentrations of calcium in the extracellular space.1,2
There are two eras in the history of PHPT. The first was when the condition was discovered, with florid signs and symptoms of renal lithiasis, bone disease and frank hypercalcaemia. The second era began about 40 years ago after the introduction of autoanalysers, when PHPT could be detected at the stage of mild hypercalcaemia, without the classic clinical characteristics. We may now be entering a third era, in which patients have serum concentrations of total and ionised calcium within normal ranges, but persistently elevated PTH levels, known as normocalcaemic PHPT.1,2
In the era of symptomatic PHPT, patients often had renal lithiasis and stone-related complications, such as urinary tract infections, hydronephrosis and impaired renal function. Nowadays, these problems are rare, as most PHPT are asymptomatic; in terms of the kidneys, they may present with hypercalciuria, renal microlithiasis and slightly impaired renal function.2,3
The kidney is a classic target organ of PTH, and the relationship between severe PHPT and kidney damage was first described many years ago.3–7 Moreover, in PHPT the progressive deterioration of renal function secondarily elevates PTH levels.8 It has traditionally been assumed that resolving PHPT through surgery could preserve renal function in these patients.4 A decreased glomerular filtration rate (<60ml/min) remains in the guidelines as a criterion for parathyroidectomy (PTX).9,10
However, the influence that PTX has on any changes in renal function is open to debate. Most of the retrospective studies which have looked at this problem have found no improvement in renal function after PTX.11–16 Moreover, randomised controlled trials carried out mainly in patients with mild asymptomatic PHPT have not shown PTX to have any effect on renal function.17–20 Other studies have shown PTX to have a negative effect on renal function,21 particularly in patients with creatinine clearance >60ml/min.22
Although it cannot be denied that surgical cure of PHPT prevents further deterioration of renal function, an important question is whether or not PTX in patients with renal failure is capable of slowing down the decrease in the glomerular filtration rate.4,22 The aim of our study was to analyse a large series of patients with treated PHPT and identify changes in renal function after PTX.
Material and methodsDesign and patientsWe reviewed the records of all patients diagnosed with PHPT who underwent PTX at our centre from 1998 to 2016 who had over a year of postoperative follow-up. This was an observational, retrospective study.
We collected epidemiological data (age, gender) and the medical history of each patient (time since onset of PHPT, presence of renal lithiasis, hypertension, associated symptoms and biochemical parameters).
DefinitionsHypercalcaemia: serum calcium higher than 11.2mg/dl (serum calcium >1mg/dl above the upper limit of normal). The measurement of blood calcium was corrected for albumin: corrected calcium (mg/dl)=(4.0−serum albumin [g/dl])×0.8+total Ca (mg/dl).
Symptomatic PHPT: patients who presented symptoms in relation to recurrent renal lithiasis or bone manifestations (bone or joint pain or fractures) or general symptoms attributable to PHPT (gastrointestinal or neuromuscular).
Hypertension: patients who were receiving blood pressure-lowering treatment, regardless of the blood pressure values recorded, were considered hypertensive.
PHPT-related bone disease: Bone mineral density was measured by dual-energy X-ray absorptiometry (DEXA) in the lumbar spine (L2–L4) and femur. A patient was considered to have bone involvement when DEXA showed a decrease in bone mineral density (T score<−2.5SD in the spine or femur).
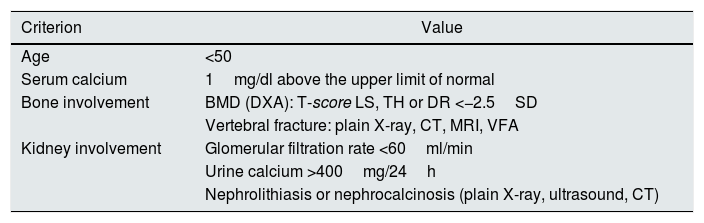
The surgical indication for PTX was identified in asymptomatic patients according to international guidelines.9 A patient might have more than one indication, so a hierarchical order was established for the criteria: renal lithiasis, bone involvement, hypercalcaemia, age <50 and decrease in glomerular filtration rate (<60ml/min) (Table 1).
Criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism.
| Criterion | Value |
|---|---|
| Age | <50 |
| Serum calcium | 1mg/dl above the upper limit of normal |
| Bone involvement | BMD (DXA): T-score LS, TH or DR <−2.5SD |
| Vertebral fracture: plain X-ray, CT, MRI, VFA | |
| Kidney involvement | Glomerular filtration rate <60ml/min |
| Urine calcium >400mg/24h | |
| Nephrolithiasis or nephrocalcinosis (plain X-ray, ultrasound, CT) |
BMD: bone densitometry; CT: computed tomography; DR: distal radius; DXA: dual-energy X-ray absorptiometry; LS: lumbar spine; MRI: magnetic resonance imaging; SD: standard deviation; TH: total hip; VFA: vertebral fracture assessment.
Source: Bilezikian et al.9
We also collected the results of our patients’ imaging techniques, including parathyroid ultrasound, computed tomography of neck and chest with intravenous contrast, with tomographic slices every 5mm, and pre-operative parathyroid scintigraphy with Tc99m-Sestamibi at a dose of 25mCi using a planar gamma camera. Scintigraphy records were made at 10min and at 2h post-injection, using parallel-hole pin-hole collimator. Persistence of the tracer in the recorded images at 2h was considered pathological.
Surgical procedureA simple adenomectomy was performed for the single adenomas. In patients with multiple-gland involvement, cases of repeat surgery for persistent PHPT or when there was concomitant thyroid pathology or a negative localisation study, we used the traditional approach of a full neck exploration. During surgery, intact PTH (iPTH) was monitored and a decrease of more than 50% from the pre-operative value was considered satisfactory. In the cases of hyperplasia, a subtotal PTX was performed or reimplantation in the forearm.
Analytical determination methodsAnalytical determinations were performed by HPLC autoanalyser (ADAMS A1c analyser, HA-8160, by Menarini) and iPTH by chemiluminescence (Roche diagnostics GmbH, Mannheim).
The glomerular filtration rate was estimated according to the CKD-EPI equation,23 which includes serum creatinine, age, gender and race as variables. Serum creatinine was measured using a compensated kinetic Jaffé method (Roche Diagnostics) traceable to the IDMS reference method. Urinary calcium was measured in a 24h urine sample and the urinary Ca/creatinine ratio calculated.
The normal laboratory ranges considered were: calcium 8.2–10.2mg/dl; phosphorus 2.3–4.6mg/dl; plasma creatinine in males 0.7–1.2mg/dl and in females 0.5–0.9mg/dl; and iPTH 15.0–51.0pg/ml. In urine, urinary calcium <200mg/day and the Ca/creatinine ratio <0.20.
The analytical tests were performed at baseline prior to surgery, one week after PTX (PTX-1 week) and 12 months after (PTX-12 months). No significant changes were found in serum creatinine from the time of diagnosis of PHPT until the baseline determination.
Statistical analysisThe statistical analysis used the SPSS 20 software program (IBM SPSS Statistics® 20). The Kolmogorov–Smirnov test was used to analyse the normal distribution of variables. The categorical variables were expressed as frequencies (%) and the continuous variables as means and standard deviation where they had normal distribution, or medians (p25–p75) otherwise. The Chi-square test was used to assess the association between the different types of PHPT and the categorical variables. The Student's t test was used for parametric continuous variables. In the case of non-parametric continuous variables, the Mann–Whitney test was used for independent samples or the Wilcoxon test for paired samples.
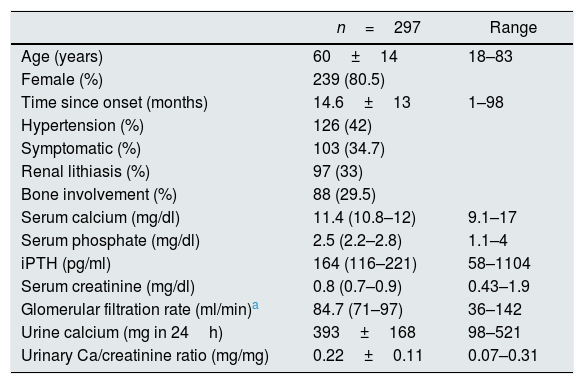
ResultsA total of 297 patients with PHPT had a PTX. The incidence of PTX was 39/million population/year. The mean age of the patients was 60±14. The female-to-male ratio was 4.1:1; with the males being younger at the time of the PTX. The mean time from diagnosis to surgery was 14.6±14 months. A total of 126 patients (42%) had hypertension. PHPT was asymptomatic in 194 patients (65.3%). The other clinical and analytical characteristics are shown in Table 2.
Baseline clinical and analytical characteristics.
| n=297 | Range | |
|---|---|---|
| Age (years) | 60±14 | 18–83 |
| Female (%) | 239 (80.5) | |
| Time since onset (months) | 14.6±13 | 1–98 |
| Hypertension (%) | 126 (42) | |
| Symptomatic (%) | 103 (34.7) | |
| Renal lithiasis (%) | 97 (33) | |
| Bone involvement (%) | 88 (29.5) | |
| Serum calcium (mg/dl) | 11.4 (10.8–12) | 9.1–17 |
| Serum phosphate (mg/dl) | 2.5 (2.2–2.8) | 1.1–4 |
| iPTH (pg/ml) | 164 (116–221) | 58–1104 |
| Serum creatinine (mg/dl) | 0.8 (0.7–0.9) | 0.43–1.9 |
| Glomerular filtration rate (ml/min)a | 84.7 (71–97) | 36–142 |
| Urine calcium (mg in 24h) | 393±168 | 98–521 |
| Urinary Ca/creatinine ratio (mg/mg) | 0.22±0.11 | 0.07–0.31 |
iPTH: intact parathyroid hormone; SD: standard deviation.
The laboratory values are expressed as median (p25–p75) or mean±SD.
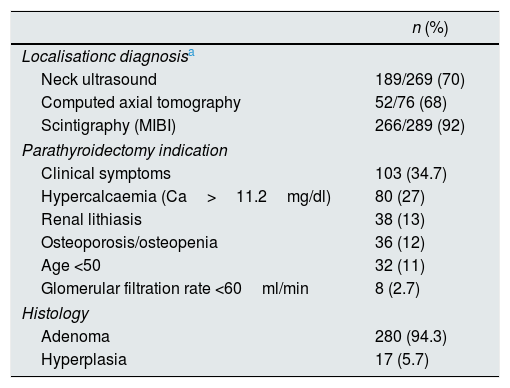
In terms of localisation diagnosis, sestamibi scintigraphy was the best method for locating the gland involved; this was performed in 289 patients and was diagnostic in 266 (92%). Ultrasound of the neck was performed in 269 patients and was positive in 189 (70%). Computed tomography had similar results, being positive in 52 (68%) of the 76 patients who had a CT scan of the neck (Table 2). With scintigraphy, 22 false negatives were found. Of these, the diagnosis was made with ultrasound in 11 and computed tomography in nine; no localisation images were available prior to surgery for two patients. In 34.7% of the patients the indication for PTX was symptomatic PHPT. Of the asymptomatic patients, hypercalcaemia (Ca>11.2mg/dl) was the most common cause (27%), followed by renal lithiasis (13%), bone involvement (12%) and being under the age of 50 (11%). In 280 cases (94.3%), the histology of the surgical specimen was compatible with parathyroid adenoma (Table 3).
Parathyroidectomy: localisation, indications and histology.
| n (%) | |
|---|---|
| Localisationc diagnosisa | |
| Neck ultrasound | 189/269 (70) |
| Computed axial tomography | 52/76 (68) |
| Scintigraphy (MIBI) | 266/289 (92) |
| Parathyroidectomy indication | |
| Clinical symptoms | 103 (34.7) |
| Hypercalcaemia (Ca>11.2mg/dl) | 80 (27) |
| Renal lithiasis | 38 (13) |
| Osteoporosis/osteopenia | 36 (12) |
| Age <50 | 32 (11) |
| Glomerular filtration rate <60ml/min | 8 (2.7) |
| Histology | |
| Adenoma | 280 (94.3) |
| Hyperplasia | 17 (5.7) |
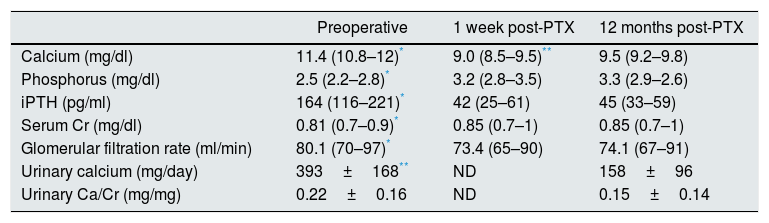
After PTX, laboratory tests showed a significant decrease in calcium, iPTH and urinary calcium, with an increase in phosphorus from that in the first week, all of which were maintained over time. Serum creatinine increased and the glomerular filtration rate (GFR) decreased in the first week after surgery, with similar values at 12 months post-PTX (Table 4).
Analytical changes in primary hyperparathyroidism (n=297).
| Preoperative | 1 week post-PTX | 12 months post-PTX | |
|---|---|---|---|
| Calcium (mg/dl) | 11.4 (10.8–12)* | 9.0 (8.5–9.5)** | 9.5 (9.2–9.8) |
| Phosphorus (mg/dl) | 2.5 (2.2–2.8)* | 3.2 (2.8–3.5) | 3.3 (2.9–2.6) |
| iPTH (pg/ml) | 164 (116–221)* | 42 (25–61) | 45 (33–59) |
| Serum Cr (mg/dl) | 0.81 (0.7–0.9)* | 0.85 (0.7–1) | 0.85 (0.7–1) |
| Glomerular filtration rate (ml/min) | 80.1 (70–97)* | 73.4 (65–90) | 74.1 (67–91) |
| Urinary calcium (mg/day) | 393±168** | ND | 158±96 |
| Urinary Ca/Cr (mg/mg) | 0.22±0.16 | ND | 0.15±0.14 |
Cr: creatinine; iPTH: intact parathyroid hormone; ND: not determined; PTX: parathyroidectomy; SD: standard deviation.
The laboratory values are expressed as median (p25–p75) and mean±SD.
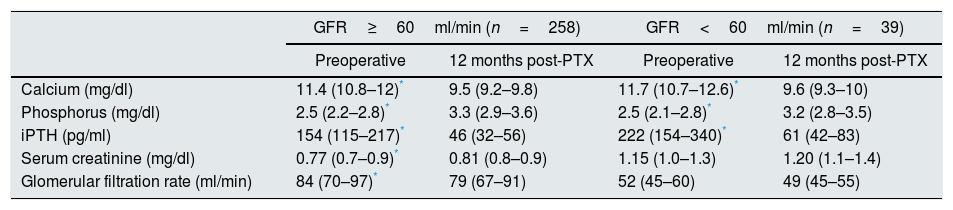
We divided the patients into two groups according to their baseline GFR (greater or less than 60ml/min). The patients with GFR <60ml/min were older (70±12 vs 59±14, p<0.001) and more likely to have hypertension (72% vs 39%; p<0.001). We found that the elevation in creatinine and the decrease in the GFR at 12 months post-PTX were statistically significant in cases with creatinine clearance ≥60ml/min; renal function also deteriorated in patients with GFR <60ml/min one year post-PTX, although this did not reach statistical significance (Table 5).
Analytical changes in primary hyperparathyroidism after PTX.
| GFR≥60ml/min (n=258) | GFR<60ml/min (n=39) | |||
|---|---|---|---|---|
| Preoperative | 12 months post-PTX | Preoperative | 12 months post-PTX | |
| Calcium (mg/dl) | 11.4 (10.8–12)* | 9.5 (9.2–9.8) | 11.7 (10.7–12.6)* | 9.6 (9.3–10) |
| Phosphorus (mg/dl) | 2.5 (2.2–2.8)* | 3.3 (2.9–3.6) | 2.5 (2.1–2.8)* | 3.2 (2.8–3.5) |
| iPTH (pg/ml) | 154 (115–217)* | 46 (32–56) | 222 (154–340)* | 61 (42–83) |
| Serum creatinine (mg/dl) | 0.77 (0.7–0.9)* | 0.81 (0.8–0.9) | 1.15 (1.0–1.3) | 1.20 (1.1–1.4) |
| Glomerular filtration rate (ml/min) | 84 (70–97)* | 79 (67–91) | 52 (45–60) | 49 (45–55) |
GFR: estimated glomerular filtration rate (CKD-EPI); iPTH: intact parathyroid hormone; PTX: parathyroidectomy.
The values are expressed as median (p25–p75).
PHPT is a common disorder. The prevalence varies in different analyses depending on the method of detection and the population studied. Population-based screening studies which included the measurement of serum calcium helped define the epidemiology of PHPT. The incidence has changed in the last forty years; in the United States, the annual incidence of PHPT in 1974 was 78cases/million/year and one year later, after routinely measuring serum calcium, it rose to 277cases/million/year. From 1983 to 1992, despite the more widespread and continuous use of the calcium measurement, the incidence decreased to 208cases/million/year.24 The situation has been similar in Europe, where the incidence is currently estimated at 100–150cases/million/year.25 In Spain, a recent study estimated an incidence of 99.5cases/million/year.26
Few studies have analysed the incidence of PTX in the general population, and they go back to before 2000; in the USA there was an estimated incidence of 44/million/year and in Sweden 50–100/million/year,24 while in our series the incidence was 38/million/year.
PHPT is more common in women. In our study, women accounted for 80.5% of cases (female-to-male ratio 4.1:1), slightly higher than that reported in the literature, with estimates of around 75%.27–29
The localisation techniques are parathyroid Tc99m-sestamibi scintigraphy (with or without SPECT) and ultrasound of the neck. Tc99m-sestamibi scintigraphy has sensitivity of 74–90% according to the different series10,30; in our series it was 92%. The profitability of neck ultrasound depends on the examiner's expertise, and varies from 70% to 87%31; in our study it was diagnostic in 70% of patients.
The clinical manifestations of PHPT have changed over the years, and 80% of patients in developed countries are now asymptomatic.10,32 Of our patients undergoing PTX, 65% were asymptomatic, but it is important to remember that this was a selected population which met criteria for parathyroid surgery.
PTX is the definitive treatment and provides a complete cure for PHPT. Blood calcium levels return to normal, the risk of lithiasis decreases and bone mass increases, with a reduction in the fracture risk. The effect on cardiovascular and neurocognitive symptoms is less certain. The cure rate is over 95%, with few complications and low morbidity and mortality rates.33 Another important aspect is that surgery reduces costs in PHPT; when performed soon after diagnosis it is 2.5 times more cost-effective than after a period of follow-up with medical treatment, and 1.5 times more cost-effective than in patients who only receive medical treatment.34
In the last three editions of the consensus on management of asymptomatic PHPT, renal failure (GFR <60ml/min/1.73m2) has been included among the surgical criteria.9
In mild forms of PHPT, observational studies are not very consistent, and there is therefore no definitive evidence that asymptomatic/mild PHPT has a negative impact on renal function.18,32,35,36 The recent, prospective PEARS study compared 1424 patients with PHPT with 7120 controls and found that individuals with PHPT had a 13.83-times higher risk of developing renal failure at ten years than controls.36
Despite the fact that the decrease in GFR is an indication for PTX,9 there are virtually no studies demonstrating a beneficial effect of PTX on renal function. When we searched the literature for evidence that PTX can change the course of renal failure, we found that most retrospective studies showed no change in renal function (whether estimated with serum creatinine, creatinine clearance, or GFR) during follow-up periods ranging from six months to 11 years.11,37,38
Two recent studies designed to assess the effect of PTX found that renal function became worse after PTX.21,22 The Egan et al.21 study analysed changes in renal function in 62 patients and found that creatinine rose postoperatively (1.09 vs 1.15mg/dl) and GFR decreased from 78 to 73ml/min at 8–12 weeks.
Tassone et al.22 analysed changes in renal function at six months post-PTX in 109 patients (14 with clearance <60ml/min). Serum creatinine increased significantly in patients with creatinine clearance >60ml/min (0.82 vs 0.87mg/dl, p<0.001); serum creatinine also worsened in the 14 patients whose GFR decreased (1.32 vs 1.41mg/dl, p=0.5) although this was not statistically significant. Of the published studies, our series includes the largest number of patients, and the results are superimposable. Renal function worsens in all patients from the first week and this situation is maintained one year after surgery. This is particularly alarming in patients who previously had normal renal function. Renal function also worsened in patients with creatinine clearance <60ml/min, but probably due to the sample size, this did not reach statistical significance. Our patients with renal failure were older and had a higher prevalence of hypertension, so these factors probably do not explain the worsening.
In a study performed in kidney transplant patients, comparing them with a control group with similar characteristics, a significant deterioration in serum creatinine was detected six months after PTX.39 The transplant patients had renal failure (mean clearance of 47ml/min) and the control group had no elevation of iPTH. The authors attributed this to a haemodynamic effect mediated by the decrease in PTH levels, but what was not explained was that the deterioration continued beyond the first months.
We included a large number of patients in our study, but due to its retrospective observational nature, we were unable to establish a direct cause-and-effect relationship between renal function and PTX. Another limitation of the study is the CKD-EPI method used in the estimation of GFR which, although it underestimates less than the MDRD in patients with GFR above 60ml/min/1.73m2, is not an exact measurement.40
We can only hypothesise that in the patients who did not have renal failure had a renal function deteriorated after PTX, this may have been due to factors unrelated to PHPT. One possible mechanism would be that PTH has a pre-glomerular vasodilatory effect; another would be the hypertensive effect of hypercalcaemia, although that would only explain an acute deterioration, not one maintained over time.41 Yet another proposed mechanism is that PTH increases the tubular secretion of creatinine by increasing permeability in the proximal tubule,42 and the normalisation of PTH would then raise serum creatinine levels.
Although a GFR <60ml/min is a criterion for surgery, we need prospective studies on the changes in renal function in PHPT which compare patients with PTX to a control group (observational) and measure GFR with more accurate methods that do not use creatinine as a marker.4
ConclusionsPHPT is a common endocrine disorder in older women and is asymptomatic in more than half of patients. PHPT should be ruled out when a patient presents with hypercalcaemia and renal lithiasis. Taking into account all the limitations mentioned above, our data show that surgical cure of PHPT is not accompanied by improvement in renal function, and this effect is evident from the first week and sustained over time.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: García-Martín F, Guadalix S, García-Boyano F, Melón Peña N, Martínez Pueyo JI, Callejas Martínez R, et al. ¿Mejora la función renal tras la paratiroidectomía en el hiperparatirodismo primario? Nefrologia. 2019;39:160–167.