Antecedentes: El estudio de Belgrado se realizó para detectar personas con marcadores de ERC en poblaciones de riesgo y formar a los especialistas de atención primaria sobre cómo realizar proyecciones de ERC. Métodos: El estudio fue realizado por especialistas de atención primaria de trece centros de salud en colaboración con nefrólogos de centros clínicos. Se incluyó a personas sin enfermedad renal previa conocida: 1316 pacientes con hipertensión sin diabetes, 208 pacientes con diabetes tipo 2 y 93 pacientes de más de 60 años sin hipertensión ni diabetes. El estudio consistía en una entrevista, determinación de la tasa de filtración glomerular estimada (TFGe-MDRD) y detección de proteinuria, hematuria, glucosuria y microalbuminuria con una única tira reactiva de orina. Resultados: Se detectó microalbuminuria con o sin proteinuria en combinación con una TFGe >60 ml/min/1,73m2 en el 17%, el 41% y el 24% de los pacientes con hipertensión, diabetes y mayores de 60 años, respectivamente. Se encontró una TFGe reducida (<60 ml/min/1,73m2 ) en el 23%, el 12% y el 22% de estos mismos grupos de pacientes. La prevalencia de los marcadores de ERC aumentaba cuanto mayor era el número de factores de riesgo. Conclusión: La elevada prevalencia de marcadores de ERC en una población de riesgo detectada por los médicos de atención primaria en este estudio de colaboración parece ser la mejor forma de motivar a estos especialistas para que realicen cribados de ERC con regularidad.
Background: Belgrade screening study was undertaken in order to detect persons with CKD markers in at risk populations and to educate primary care physicians how to carry out CKD screening. Methods: The study was performed by primary care physicians from thirteen Belgrade health centers in collaboration with nephrologists from clinical centers. Subjects without previously known kidney disease were enrolled: 1316 patients with hypertension without diabetes, 208 patients with type 2 diabetes and 93 subjects older than 60 years without hypertension or diabetes. The survey consisted of an interview, consisted of an interview, estimation of glomerular filtration rate (eGFR–MDRD), single urine dipstick detection of proteinuria, hematuria, glucosuria, microalbuminuria. Results: Microalbuminuria with or without proteinuria in combination with eGFR>60 ml/min/1.73m2 was detected in 17% , 41% and 24% of patients with hypertension, diabetes and those above 60 years, respectively. Reduced eGFR (<60 ml/min/1.73m2 ) was found in 23%, 12% and 22% of the same patient groups. The prevalence of CKD markers increased with increasing number of risk factors. Conclusion: High prevalence of CKD markers in at risk population detected by primary care physicians in this collaborative study seems to be the best way to encourage primary care physicians to carry out regular CKD screening.
INTRODUCTION
The steady increase in the incidence of patients on renal replacement therapy (RRT) was first noted in developed countries and thereafter all around the globe1-3. Over the same period diabetes and hypertension became the leading causes of chronic kidney disease (CKD) and the number of elderly patients in end-stage renal disease increased steadily3. It became obvious that more attention should be directed to prevention and early detection of CKD. Recent data indicated stabilization of incidence rates of RRT patients in many developed countries4-6. Although different factors might have led to this stabilization, greater emphasis on early detection and prevention of CKD is doubtless one of them. However, our country is among those European countries where the incidence rate of patients on RRT is continuing to increase7,8. The experience of developed countries in primary and secondary prevention of CKD has taught us that attention must be moved from treating advanced stages of CKD towards treatment of the early stages. As early stage of CKD in most patients is asymptomatic and undiagnosed, detection can be achieved only by active screening. It was proposed that prevention and early detection of CKD would be best managed in a partnership between primary and secondary care9. Therefore, we undertook the first study for early detection of CKD in Serbia in which primary care physicians from thirteen Belgrade health centers collaborated with nephrologists from Belgrade clinical centers. The aim was to detect persons with CKD markers in populations at risk and also to educate primary care physicians how to carry out screening for CKD and how to interpret the results and manage subsequent treatment alone or in collaboration with nephrologists.
SUBJECTS AND METHODS
The present paper presents the results of screening for CKD carried out in Belgrade under the leadership of the Academy of Medical Science of the Serbian Medical Society. The study included three steps: (1) Education. Educative meetings for primary care physicians on prevention and early detection of CKD were organized by Academy in 2008. (2) Organization of study for detection of persons with CKD markers. At the beginning of 2009 primary care physicians from all 13 Belgrade Health Centers were invited to participate in the screening study. After their positive response nephrologists from three clinical centers presented the study design to primary care physicians who carried out investigations from April to June 2009. (3) Results presentation and guideline distribution. In November 2009, the Academy organized a meeting where the results from each health center were presented by general practitioners and the overall findings were collated and reported by nephrologists, coordinators of the study. At the same time a guideline for early detection and treatment of chronic kidney disease, prepared by members of the Academy was presented and distributed to all participants10.
This study enrolled 1617 adult patients without previously known renal disease who came for regular check-ups to their primary care physicians in Belgrade Health centers over a three month period. The patients were enrolled in the study according to the following criteria: patients with hypertension for more than 5 years, patients with type 2 diabetes mellitus for more than 5 years regardless of the presence or not of hypertension, and persons older than 60 years without hypertension or diabetes. Exclusion criteria involved: previously known kidney diseases, malignant disease, congestive heart disease, pregnancy, any acute illness, as well as persons younger than 18 years.
Informed consent was obtained from all patients and the Ethics Committee of the Clinical Center of Serbia approved the study.
The survey started with an interview in which the participants answered a detailed questionnaire on demographic issues, personal medical and family history with special attention to duration and treatment of hypertension and diabetes and data on smoking. After the interview the primary care physicians examined all selected subjects physically, including measurement of body weight, height and blood pressure. In addition, they analyzed the medical records of each subject and calculated the average systolic and diastolic blood pressure as well as the average serum glucose level using all values registered in the year preceding the study. These data were also noted in the questionnaire.
All participants were sent to the laboratory where serum creatinine level was measured by a kinetic Jaffé method and glomerular filtration rate (eGFR) estimated using the original Modification of Diet in Renal Disease (MDRD) Study formula11. Proteinuria, hematuria, glycosuria and microalbuminuria were assessed semiquantitatively in spot urine samples using the urine dipstick test. Proteinuria, hematuria, glycosuria were defined when dipstick analysis quantified them as 1+ or more. Microalbuminuria (MAU) was detected with Micral-test® strips (ACCU-CHEK product, Roche Diagnostics). These immunoassay reagent strips (monoclonal antibodies to human albumin) reveal a distinctive color corresponding to a scale on the vial label giving a range of albumin concentrations as follows: negative, 20mg/L, 50 mg/L and 100 mg/L . According to the manufacturer’s instructions albumin concentrations detected as ≥ 20 mg/L are consistent with microalbuminuria ≥ 30 mg/day. This was confirmed in 100 patients with MAU detected by the Micral-dipstick test during the screening and subsequent determination of albumin in a 24h urine collection by immunonephelometry and Micral-dipstick. Therefore, the finding of MAU ≥ 20 mg/L was considered as the presence of MAU. Nephrologist coordinators collected the questionnaire lists and laboratory results from all health centers participating in the study and analyzed the results.
A person was considered as having arterial hypertension if systolic blood pressure was ≥140 mmHg and/or diastolic pressure was ≥90 mmHg or if antihypertensive treatment had been previously prescribed. Patients with known and treated type 2 diabetes were considered diabetic regardless of their glycemic control. Body mass index (BMI) was calculated using the formula: weight (kg) /height 2 (m2) and, depending on the obtained BMI value, patients were classified according to the WHO recommendations12. Smoking was defined as actual use of cigarettes (not ex-smokers).
The estimated GFR (eGFR) was used to classify subjects into Kidne Disease Outcomes Quality Initiative (K/DOQI) stages of CKD13. In addition, stage 3 CKD was divided into two sub-stages14: sub-stage 3a with a GFR between 45 mL/min/1.73m2 and 59 mL/min/1.73m2 and sub-stage 3b with a GFR between 30 mL/min/1.73m2 and 44 mL/min/1.73m2.
Descriptive statistics were presented as mean values ± standard deviation (SD) for the continuous variables, or as frequencies for categorical variables. Analysis of variance accompanied by Bonferroni multiple comparison tests and chi-square analysis were used for between-group comparisons of continuous and categorical variables, respectively. Pearson correlation coefficients were used to detect associations among eGFR and other variables. A p value of <0.05 was considered to be statistically significant.
All analyses were performed using the SPSS statistical software package (Version 10; SPSS).
RESULTS
Out of 1617 subjects enrolled into the study, 1316 were patients with hypertension without diabetes, 208 patients had type 2 diabetes and 93 were subjects older than 60 years who had neither hypertension nor diabetes. Thus, patients with hypertension formed the largest group, but the relatively low number of individuals with type 2 diabetes enrolled in the study could have been due to way their health care is organized in our health centers. Namely, the majority of patients with diabetes are not controlled by the general practitioners who recruited patients for our screening studies but in special advice centers for diabetics. In addition, the number of enrolled subjects older than 60 years without hypertension and diabetes was also low, because people of that age frequently had hypertension or a comorbidity excluding them from the present study.
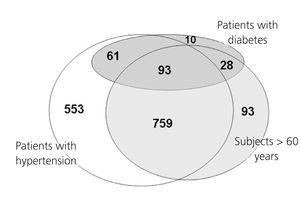
Inclusion criteria allowed patients with hypertension older than 60 years to be included in the hypertension group, while patients with type 2 diabetes regardless of having hypertension or not or being above 60 years old were included in the diabetes group. Therefore, some subjects could have had more than one of the three risk factors for CKD. The Venn diagram presented in figure 1 shows that 759 (57.6%) of the patients with hypertension were older than 60 years, while among patients with diabetes 93 (44.7%) were above 60 years old and had hypertension, 61 (29.3%) had hypertension but were younger than 60 years and only 10 (5%) diabetics were normotensive and younger than 60 years.
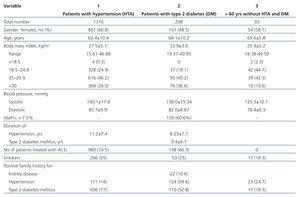
The demographic and clinical characteristics of the study population are shown in table 1. Patients with diabetes had significantly higher mean BMI and, as expected, the groups of patients with hypertension and diabetes had significantly higher systolic and diastolic blood pressure than the group of subjects older than 60 years.
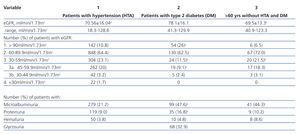
The results of laboratory analyses are presented in table 2. Patients with diabetes had significantly higher mean eGFR and most of them were in stage 1 and the least in stage 3 of CKD. When the patients in stage 3 of CKD were divided into two substages only one seventh of them belonged to substage 3b. Table 2 also shows that patients with diabetes had the highest prevalence of both MAU and proteinuria, but patients with hypertension had a significantly lower prevalence of MAU than the other two groups.
Calculation of the Pearson correlation coefficient revealed significant associations between eGFR and systolic blood pressure (r= - 0.163, p = 0.023), duration of hypertension (r= - 0.276, p = 0.0001) and age (r= -0.383, p = 0.0001).
Different combinations of laboratory findings were detected in the examined subjects (figure 2). The most frequent pathological finding was MAU with or without proteinuria in combination with eGFR above 60 ml/min/1.73m2. In patients with diabetes this was detected in the majority (41%) of patients with pathological laboratory findings, while in two other groups this percent was about two times lower. In the group with hypertension and with subjects older than 60 years the percentage of patients with MAU/proteinuria and eGFR above 60 ml/min/1.73m2 was similar to that of patients with normal urinary finding and eGFR below 60 ml/min/1.73m2.
As many of our subjects had more than one of the three risk factors for CKD (hypertension, diabetes, older age), the frequency of MAU and reduced eGFR was examined depending on the number of risk factors. The analysis showed that in subjects with one, two or three risk factors MAU was present in 135 (21.0%), 215 (25.2 %) or 44 (46.3%) subjects, but eGFR below 60 ml/min/1.73m2 in 101 (15.4%), 241 (28.4%) or 26 (26.8%) subjects, respectively.
DISCUSSION
The present study aimed to detect persons with CKD markers in at risk population was carried out in Belgrade through the collaboration of nephrologists and primary care physicians and involved 1617 subjects. Among them there were 1316 patients with hypertension without diabetes, 208 patients with type 2 diabetes and 93 subjects older than 60 years without either hypertension or diabetes. Pathological findings were revealed in 46-56% of the subjects the most frequent being MAU with or without proteinuria in combination with eGFR above 60 ml/min/1.73m2 that was found in 17%, 41% and 24% of the subjects with hypertension, diabetes and those older than 60 years, respectively
The dramatic increase of CKD prevalence has directed the attention of nephrologists towards prevention and early detection, so numerous screening programs have been running all over the world. Nevertheless, the methods used in these studies have differed widely. In some the screening was limited to determination of serum creatinine level and eGFR15-17 but, with time, the opinion prevailed that, besides GFR, screening should also include a measure of proteinuria or even better microalbuminuria18-20. In addition, low eGFR and albuminuria are two basic markers for CKD classification proposed by the Kidney Disease Outcomes Quality Initiative of the National Kidney Foundation13. In the present study dipstick measurement of urine protein and albumin was used together with estimation of GFR by MDRD equation and different combinations of these markers were found in the examined persons.
The second methodological question is who should be included in screening for CKD. Universal screening of unselected populations not already known to be at risk has not been shown to be cost-effective, so targeted screening for CKD was proposed as more economical than universal screening21-23. In different guidelines diverse at risk populations have been proposed for screening but patients with hypertension or diabetes were generally accepted as relevant ones13,24. Therefore, these two at risk groups were included here in addition to subjects older than 60 years without hypertension or diabetes as also proposed as a target population by KDOQI guidelines11. Our inclusion criteria enabled some subjects to have more than one of these three risk factors for CKD. More than a half of the patients with hypertension or diabetes were above 60 years old and majority of patients with diabetes had hypertension. Analysis of the results revealed that subjects with two or three risk factors had almost double the prevalence of reduced eGFR and MAU compared with those with only one risk factor.
Reduced eGFR (<60 ml/min/1.73m2), a criterion for CKD stage 3 according to KDOQI guidelines13, was found in 15% to 23% of subjects in the three at risk groups. These values are comparable to those obtained in other studies that targeted different at risk populations25-27. However, as GFR declines with normal ageing, it was indicated that using KDOQI guidelines classification large numbers of the elderly and females would be classified in CKD stage 3 without other objective evidence of kidney disease28. In addition, several epidemiological studies showed that the risk of progressive decline in renal function or of cardiovascular events was not equal for all subjects with stage 3 CKD, being low for subjects with eGFR29,30 between 30 to 59 ml/min/1.73m2. Therefore, it was proposed to subdivide CKD 3 into stage 3a (eGFR between 45–59 ml/min/1.73 m2) and stage 3b (eGFR between 30–44 ml/min/1.73 m2)14,29,31. This classification was used in the present study and most of the 348 subjects with stage 3 CKD belonged to substage 3a (298; 85.6%). If the subjects with eGFR in this range had neither MAU nor proteinuria, they were considered to be not at risk for renal disease progression31. Figure 2 showed that in the group of subjects older than 60 years without hypertension and diabetes there were six times more subjects with eGFR below 60 ml/min/1.73m2 and normal urinary findings than with pathological urinary findings. In the group with hypertension in which 56.6% of the patients were older than 60 this ratio was 2.3:1. Nevertheless, there are many arguments that the decline in kidney function with age cannot be considered as normal physiology. Therefore, although the subdivision of CKD 3 is useful to focus the attention of health care professionals on patients in substage 3b with worse cardiovascular and CKD outcomes, it does not mean that persons in stage 3a, even with normal urinary findings, could be excluded from the measures for prevention of CKD progression. Also, eGFR, regardless of classification into two substages, should be taken into account in medication dosing, so avoiding drug-induced kidney toxicity.
As CKD in its earliest stages is usually asymptomatic, it is often overlooked and most patients, even in at risk populations, were unaware of the CKD diagnosis32-34.
One exclusion criterion in our study was previously known kidney disease and all patients denied any knowledge of kidney disease. Also, their primary care physicians had no data on previous kidney disease, although more than 80% of the patients had checked serum creatinine level and urine analysis in the year preceding the study35. However, examination of MAU is not available in our health center laboratories, while estimation of GFR has not been introduced in the regular practice of general practitioners, so, despite laboratory control, many CKD patients might remain undetected. Therefore, one of the aims of this screening study was to educate primary care physicians about screening for CKD. The educative meeting that preceded the study, the collaboration of nephrologists and primary care physicians during the study, and especially the results obtained clearly suggested the general practitioners on the necessity of regular screening in at risk populations. As already stressed by other authors, prevention and early detection of CKD cannot be managed by an insufficient number of nephrologists and in nephrology outpatient clinics and it would be best managed in a partnership arrangement between primary and secondary care9,36. However, it was shown that primary care physicians did not have enough knowledge for this task37,38. Our results also indicated insufficient attention of primary care physicians to CKD prevention and management. Although, most patients with hypertension used ACEi, average blood pressure in the year preceding the study was above the target value proposed by guidelines. At inclusion in the study 54.5% of patients had systolic blood pressure above 140 mmHg and 40.2% had diastolic blood pressure above 90 mmHg. A similar state was found concerning control of glycemia in diabetics and 60.6% of these patients had average HbA1c above 7.5%. In addition, the majority of patients, especially those in the hypertension and diabetes groups, were overweight. Therefore, additional education of primary care physicians on risk factors, prevention, early detection and management of CKD is necessary and nephrologists should have the main role in this education. That directed us to organize the presented project and prepare guidelines for prevention, early detection and treatment of CKD patients for primary care physicians10. Nevertheless, guidelines alone are not sufficient and they can be implemented only in close collaboration between primary care physicians and nephrologists.
This study has some limitations. First, it was a cross-sectional study and CKD markers were measured just once. That might cause false positive and false negative errors. Secondly, both proteinuria and MAU were detected by urine dipstick test with limited accuracy. The use of a single measurement of dipstick proteinuria might lead to overestimation as described for a study population in the United States, where in a repeat measurement only 63% of subjects with proteinuria had a positive result39.
Despite these limitations, the present study carried out in Belgrade is the first study for early detection of CKD in Serbia. Moreover, the study was carried out in collaboration between primary care physicians and nephrologists with the aim to encourage primary care physicians to carry out regular control of CKD markers in patients at risk for CKD and to established better collaboration between primary care physicians and corresponding nephrologists. At the same time similar screening studies are being prepared in several other Serbian towns in order to spread knowledge about the significance and necessity of early detection of CKD as a task of primary care.
Conflict of interest
The authors declare they have no potential conflicts of interest related to the contents of this article.
Figure 1. Venn diagram presenting the distribution of patients depending on the presence of three risk factors
Figure 2. Distribution of subjects according to eGFR and urinary findings
Table 1. Demographic and clinical characteristics of the study groups
Table 2. Kidney function and urinary findings in the three studied groups