The ESHOL study showed that post-dilution online hemodiafiltration (OL-HDF) reduces all-cause mortality versus hemodialysis. However, during the observation period, 355 patients prematurely completed the study and, according to the study design, these patients were censored at the time of premature termination.
MethodsThe aim of this study was to investigate the outcome of patients who discontinued the study.
ResultsDuring follow-up, 207 patients died while under treatment and 47 patients died after discontinuation of the study. Compared with patients maintained on hemodialysis, those randomized to OL-HDF had lower all-cause mortality (12.4 versus 9.46 per 100 patient-years, hazard ratio and 95% CI: 0.76; [0.59 to 0.98], P=0.031). For all-cause mortality by time-dependent covariates and competing risks for transplantation, the time-dependent Cox analysis showed very similar results to the main analysis with a hazard ratio of 0.77 (0.60 to 0.99, P=0.043).
ConclusionThe results of this analysis of the ESHOL trial confirm that post-dilution OL-HDF reduces all-cause mortality versus hemodialysis in prevalent patients. The original results of the ESHOL study, which censored patients discontinuing the study for any reason, were confirmed in the present ITT population without censures and when all-cause mortality was considered by time-dependent and competing risks for transplantation.
El estudio ESHOL ha demostrado que la hemodiafiltración on line (HDF-OL) posdilución reduce la mortalidad por todas las causas respecto a la hemodiálisis (HD) en pacientes prevalentes. Sin embargo, durante el periodo de observación, 355 pacientes finalizaron prematuramente el estudio, de acuerdo con el diseño del mismo. Estos pacientes fueron censurados en el momento de la finalización prematura.
ObjetivosEl objetivo de este estudio fue investigar los eventos de los pacientes que abandonaron el estudio.
MétodosReanalizar los datos de supervivencia utilizando la población por intención de tratar en los 3 años de seguimiento. Los datos fueron analizados considerando también el trasplante renal como evento competitivo de la muerte del paciente.
ResultadosDurante el seguimiento, 207 pacientes fallecieron durante el tratamiento y 47 después de abandonar el estudio. Comparados con aquellos pacientes que se mantuvieron en HD, los que fueron aleatorizados a HDF-OL tuvieron una mortalidad total menor (12,4 vs. 9,46 por 100/pacientes/año, hazard ratio [HR] e IC 95%: 0,76 [0,59-0,98]; p=0,031). La mortalidad total por todas las causas, teniendo en consideración el riesgo competitivo del trasplante renal y tiempo-dependiente, mostró en el análisis de Cox tiempo-dependiente resultados similares al análisis principal con un HR de 0,77 (0,60-0,99; p=0,043).
ConclusionesLos resultados del reanálisis del estudio ESHOL se confirman cuando se aplica el análisis en la población por intención de tratar sin censurar ninguna observación y considerando la mortalidad por todas las causas dependiente del tiempo y del riesgo competitivo del trasplante renal.
The “On-Line Hemodiafiltration Survival Study” [Estudio de Supervivencia de Hemodiafiltración On-Line’ (ESHOL)]1 showed that high-efficiency post-dilution OL-HDF reduces all-cause mortality versus conventional hemodialysis (HD) in prevalent chronic dialysis patients. The inferential analysis of the main variable, time to occurrence of any event, defined as all-cause mortality (overall survival), was estimated from the unadjusted Cox model with no imputation by means of the log-rank test for the between-treatment comparison and hazard ratios (HR) with their 95% confidence intervals. The study provided a detailed description of the time and causes of censoring to rule out any potential biases. However, during the observation period, 355 patients prematurely finished the study and, according to the study design, all these patients were censored at the time of premature termination. Most studies conducted in hemodialysis patients (the Hemodialysis (HEMO) study,2 the Membrane Permeability Outcome (MPO) study,3 the Turkish HDF study4) have been performed with this method, given that a high percentage of patients could be excluded mainly due to renal transplantation.
In randomized clinical trials (RCT), it is recommended that data be analyzed by an intention-to-treat (ITT) analysis, which compares outcomes according to the initial random allocation, regardless of which intervention the patients actually received. ITT is recommended as the method of choice for analysis in trials investigating the superiority of an intervention.5–7 Among studies comparing OL-HDF with HD, only the CONTRAST study8 used ITT to analyze the primary endpoint. This kind of analysis avoids various misleading artifacts that can arise in intervention research, such non-random attrition of participants from the study. Importantly, a non-ITT analysis may lose the benefits of randomization, as the groups may no longer be balanced with regard to factors influencing the outcome.9,10 In the ESHOL study,1 164 patients were censored in the HD arm and 191 patients in the OL-HDF arm. As expected, renal transplantation was the main cause of censoring (79 in the HD arm and 101 in the OL-HDF arm). In survival analysis conducted to analyze the risk of death in dialysis patients, renal transplantation is a well-known competing risk because, after transplantation, patients will no longer be on dialysis and, therefore, will not be at risk of dying on dialysis. In this setting, the competing event, i.e. kidney transplantation, hinders the occurrence of the event of interest. Different approaches have been proposed to overcome this difficulty, but the best option is to conduct a full ITT analysis after completion of follow-up for censored observations for any reason in order to evaluate the risk of death for censored observations from both arms of the trial.
The aim of this study was to investigate the outcome of patients who discontinued the ESHOL study and to re-analyze survival data by using the ITT population with a 3-year follow-up. We also performed a sensitivity analysis using the ITT population. Finally, we analyzed our data, considering renal transplantation as a competing event to patient death.
Patients and methodsGeneral methods and patientsThe design and methods of the ESHOL study have previously been reported.11 Briefly, the ESHOL study was a prospective, randomized, open-label clinical trial in patients with end-stage renal disease under hemodialysis in Catalonia (Spain). The registered protocol number is NCT00694031.12
The primary objective was to assess the effect of postdilution OL-HDF compared with hemodialysis on all-cause mortality. The primary outcome variable was the time to the occurrence of death from any cause. Key secondary outcomes were cardiovascular mortality and other causes of mortality.
Study populationThe study population has been previously described.1,11 Essentially, the inclusion criteria consisted of patients older than 18 years with end-stage renal disease receiving thrice-weekly standard hemodialysis for more than 3 months. Exclusion criteria were as follows: active systemic diseases, liver cirrhosis, malignancy, immunosuppressive therapy, inadequate dialysis dose (single pool Kt/V<1.3), single-needle dialysis and the use of temporary non-tunneled catheters.
Randomization and dialysis treatment parametersPatients were randomized 1:1 to continue on thrice weekly hemodialysis or to start OL-HDF 3 times a week. The length of the recruitment period was 16 months and the study was completed to provide a complete follow-up until patient death or 3 years for all surviving patients.
Treatment proceduresBoth OL-HDF and hemodialysis were performed with ultrapure dialysis fluids and the length of dialysis sessions in each treatment modality was not modified. For patients on postdilution OL-HDF, a minimum of 18L/session of replacement volume was requested. Patients not receiving the allocated treatment modality for more than 2 consecutive months were withdrawn from the study.
Censored observationsIn the original study, patients were observed until each enrolled patient completed 3 years of follow-up, until premature termination, or until death. In the original study, 355 out of 906 patients (39.2%) with premature termination were censored before completing the 3-year follow-up. For the present study, the principal investigators at each participating center were contacted to provide information on survival status at 3 years for each censored observation. Each principal investigator contacted the renal transplant unit where patients received a kidney transplant or other hemodialysis facilities where the patients were receiving treatment from the time of censoring. The date and cause of death for each participating patient censored during the study were recorded to calculate patient survival in the ITT population.
Statistical analysisAll-cause mortality, as well as cardiovascular death, cachexia, infection, tumors, sudden death and death from other causes were described by means of the Kaplan–Meier method. The log-rank test was used for hypothesis testing, and the hazard ratio and its 95% confidence interval (95% CI) were estimated from the unadjusted Cox model. Additional multivariate Cox regression sensitivity analyses were conducted with adjustment by age, gender, diabetes, the Charlson comorbidity index (the original scale and also excluding diabetes) and the type of vascular access. Time-dependent Cox analysis, which included the time of transplantation13 as well as the cumulative incidence curves of progression in a competing risks framework, with transplantation without death as a competing event,14,15 were also assessed to check the robustness of the study results.
Two-sided significance tests were used throughout, and a P-value of <0.05 was considered significant. All statistical analyses were performed using the SAS 9.2 statistical package.
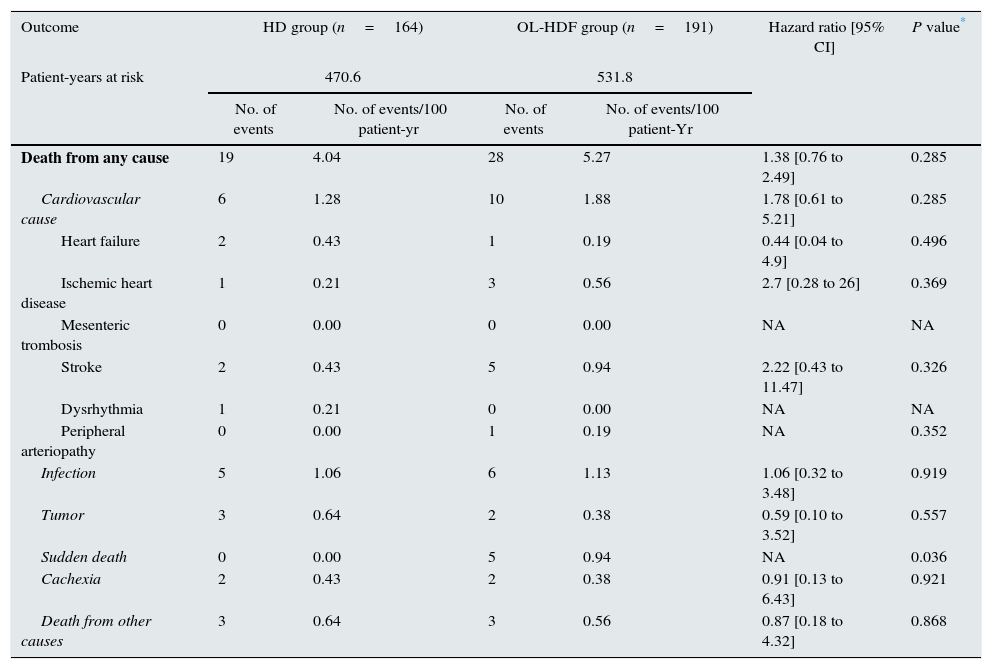
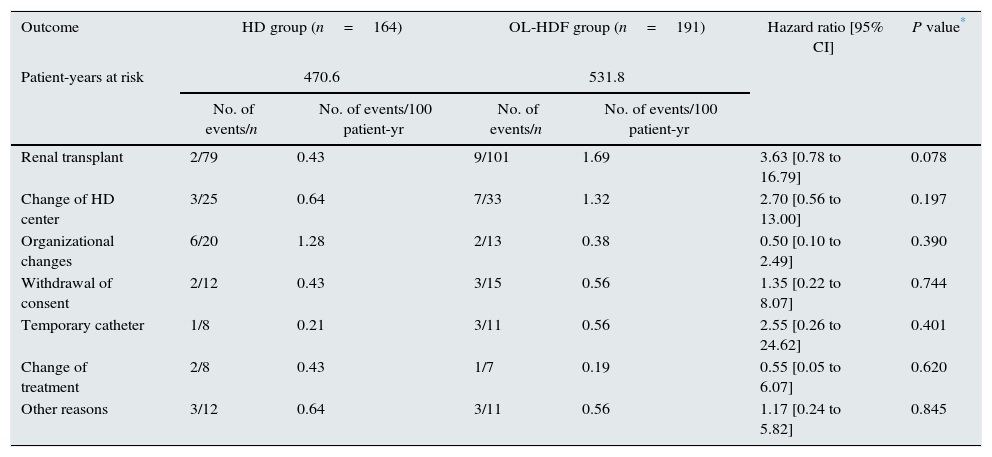
ResultsOutcome of censored observationsOf 906 patients included in the randomization, 355 (39.18%) prematurely discontinued the study because of kidney transplantation (n=180, 19.87%), change of dialysis unit (n=58, 6.40%), organizational changes (n=33, 3.64%), withdrawal of consent (n=27, 2.98%), need for a temporary catheter (n=19, 2.10%), change of treatment (n=15, 1.66%) or other, non-predefined reasons (n=23, 2.54%). In the present analysis, a 3-year follow-up was completed for all these patients with a premature termination. At 3 years, 305 patients were alive, 47 patients had died and only 3 patients were censored prematurely on the last date when known to be alive. Thus, the mean follow-up was 2.6±0.8 years and the median observation time for censored patients was 3.0 years. Mortality after discontinuation of the study was similar in both groups: 4.04 per 100 patient-year in the HD arm and 5.27 per 100 patient-year in the OL-HDF arm. The causes of mortality in discontinued patients in each arm are described in Table 1. There were no significant differences in the death rate in the 2 arms of the trial according to the causes of patient discontinuation (Table 2).
Mortality of the 355 patients that discontinued study.
| Outcome | HD group (n=164) | OL-HDF group (n=191) | Hazard ratio [95% CI] | P value* | ||
|---|---|---|---|---|---|---|
| Patient-years at risk | 470.6 | 531.8 | ||||
| No. of events | No. of events/100 patient-yr | No. of events | No. of events/100 patient-Yr | |||
| Death from any cause | 19 | 4.04 | 28 | 5.27 | 1.38 [0.76 to 2.49] | 0.285 |
| Cardiovascular cause | 6 | 1.28 | 10 | 1.88 | 1.78 [0.61 to 5.21] | 0.285 |
| Heart failure | 2 | 0.43 | 1 | 0.19 | 0.44 [0.04 to 4.9] | 0.496 |
| Ischemic heart disease | 1 | 0.21 | 3 | 0.56 | 2.7 [0.28 to 26] | 0.369 |
| Mesenteric trombosis | 0 | 0.00 | 0 | 0.00 | NA | NA |
| Stroke | 2 | 0.43 | 5 | 0.94 | 2.22 [0.43 to 11.47] | 0.326 |
| Dysrhythmia | 1 | 0.21 | 0 | 0.00 | NA | NA |
| Peripheral arteriopathy | 0 | 0.00 | 1 | 0.19 | NA | 0.352 |
| Infection | 5 | 1.06 | 6 | 1.13 | 1.06 [0.32 to 3.48] | 0.919 |
| Tumor | 3 | 0.64 | 2 | 0.38 | 0.59 [0.10 to 3.52] | 0.557 |
| Sudden death | 0 | 0.00 | 5 | 0.94 | NA | 0.036 |
| Cachexia | 2 | 0.43 | 2 | 0.38 | 0.91 [0.13 to 6.43] | 0.921 |
| Death from other causes | 3 | 0.64 | 3 | 0.56 | 0.87 [0.18 to 4.32] | 0.868 |
HD denotes hemodialysis, and OL-HDF on-line hemodiafiltration.
Revison of deaths of patients that discontinued study in relation to discontinued cause.
| Outcome | HD group (n=164) | OL-HDF group (n=191) | Hazard ratio [95% CI] | P value* | ||
|---|---|---|---|---|---|---|
| Patient-years at risk | 470.6 | 531.8 | ||||
| No. of events/n | No. of events/100 patient-yr | No. of events/n | No. of events/100 patient-yr | |||
| Renal transplant | 2/79 | 0.43 | 9/101 | 1.69 | 3.63 [0.78 to 16.79] | 0.078 |
| Change of HD center | 3/25 | 0.64 | 7/33 | 1.32 | 2.70 [0.56 to 13.00] | 0.197 |
| Organizational changes | 6/20 | 1.28 | 2/13 | 0.38 | 0.50 [0.10 to 2.49] | 0.390 |
| Withdrawal of consent | 2/12 | 0.43 | 3/15 | 0.56 | 1.35 [0.22 to 8.07] | 0.744 |
| Temporary catheter | 1/8 | 0.21 | 3/11 | 0.56 | 2.55 [0.26 to 24.62] | 0.401 |
| Change of treatment | 2/8 | 0.43 | 1/7 | 0.19 | 0.55 [0.05 to 6.07] | 0.620 |
| Other reasons | 3/12 | 0.64 | 3/11 | 0.56 | 1.17 [0.24 to 5.82] | 0.845 |
HD denotes hemodialysis, and OL-HDF on-line hemodiafiltration.
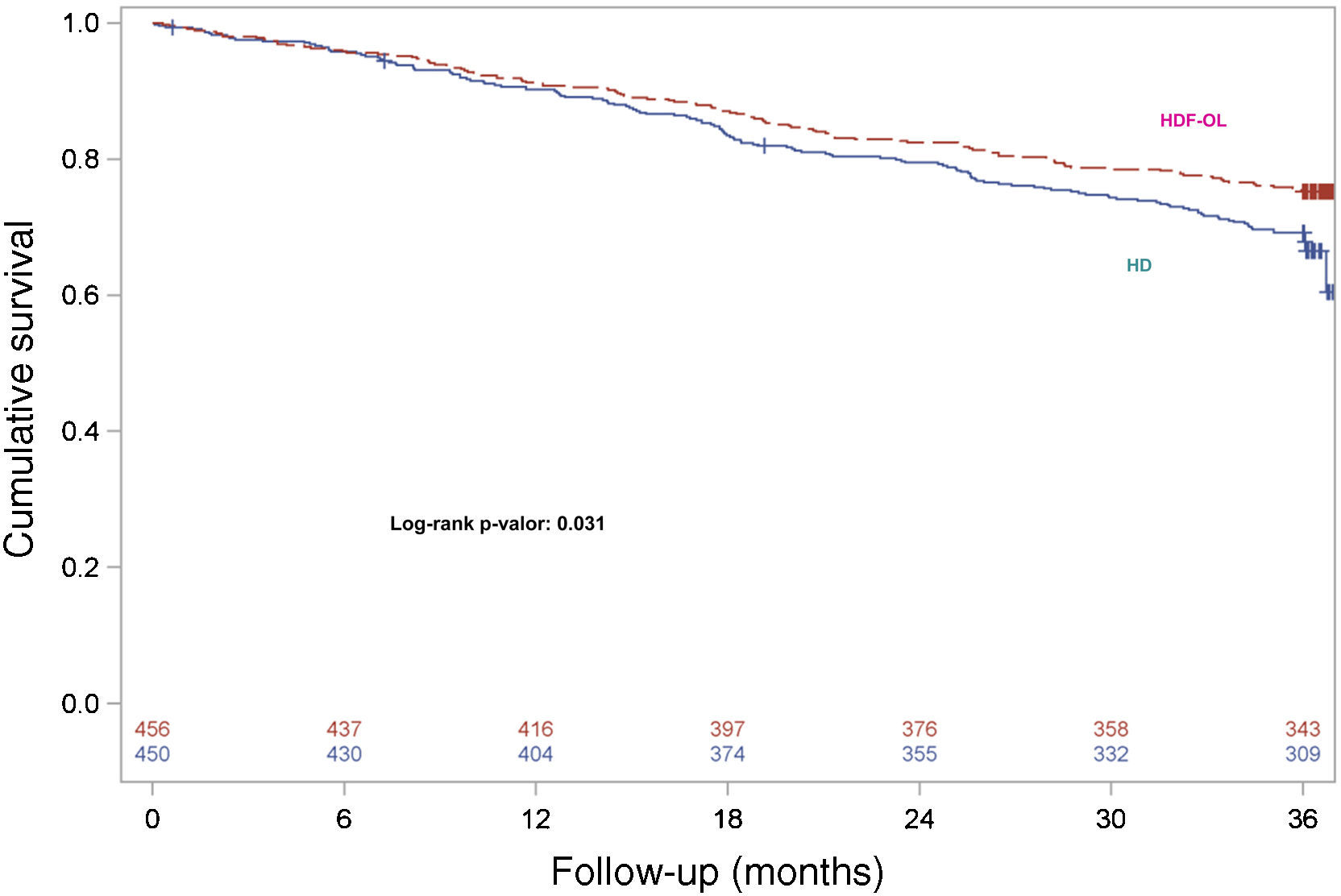
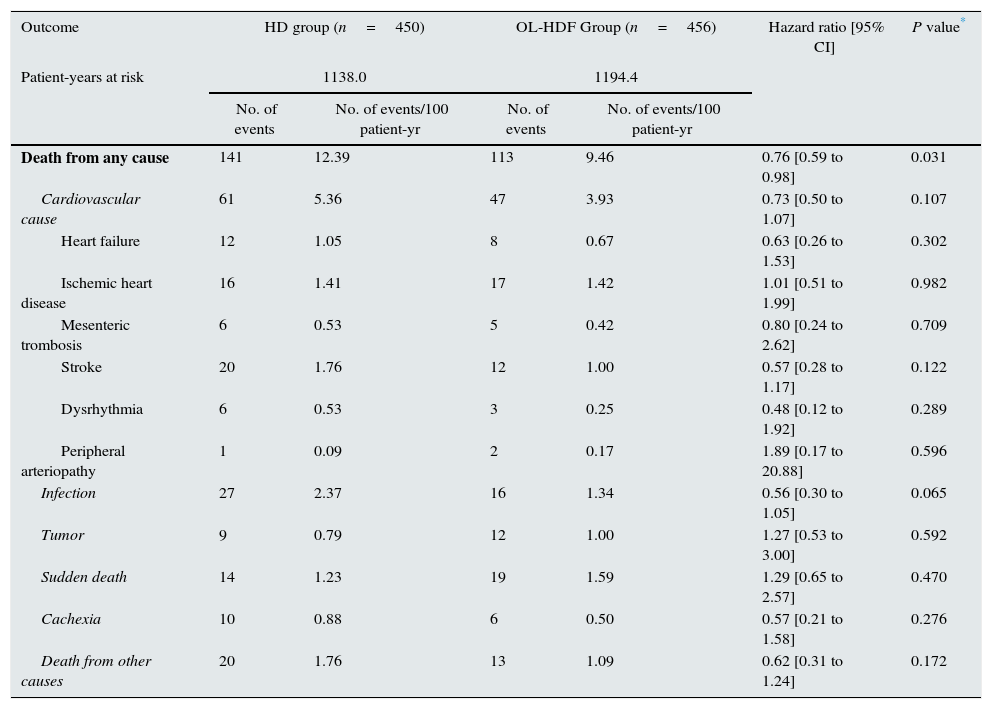
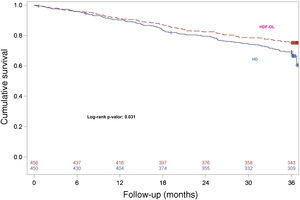
During the 3-year follow-up, 207 patients died on treatment and 47 patients died after discontinuation of the study; thus, 254 out of 906 patients died (28.03%) during follow-up, with a 3-year all-cause mortality rate of 24.78% and 31.33% in the OL-HDF and the HD groups, respectively. Kaplan–Meier survival curves with the ITT population with only 3 censored patients showed that patient survival was lower in patients allocated to the HD arm than in those allocated to OL-HDF arm (log-rank P=0.031) (Fig. 1). Univariate Cox regression analysis showed that patients allocated to the OL-HDF group had a 24% risk reduction (hazard ratio [95% CI]: 0.76 [0.59 to 0.98]; P=0.031) for mortality for any cause (Table 3). The main causes of death were cardiovascular diseases (42.5%) and infectious diseases (16.9%) and there were no significant differences between groups (Table 3).
Primary outcome. Mortality (ITT).
| Outcome | HD group (n=450) | OL-HDF Group (n=456) | Hazard ratio [95% CI] | P value* | ||
|---|---|---|---|---|---|---|
| Patient-years at risk | 1138.0 | 1194.4 | ||||
| No. of events | No. of events/100 patient-yr | No. of events | No. of events/100 patient-yr | |||
| Death from any cause | 141 | 12.39 | 113 | 9.46 | 0.76 [0.59 to 0.98] | 0.031 |
| Cardiovascular cause | 61 | 5.36 | 47 | 3.93 | 0.73 [0.50 to 1.07] | 0.107 |
| Heart failure | 12 | 1.05 | 8 | 0.67 | 0.63 [0.26 to 1.53] | 0.302 |
| Ischemic heart disease | 16 | 1.41 | 17 | 1.42 | 1.01 [0.51 to 1.99] | 0.982 |
| Mesenteric trombosis | 6 | 0.53 | 5 | 0.42 | 0.80 [0.24 to 2.62] | 0.709 |
| Stroke | 20 | 1.76 | 12 | 1.00 | 0.57 [0.28 to 1.17] | 0.122 |
| Dysrhythmia | 6 | 0.53 | 3 | 0.25 | 0.48 [0.12 to 1.92] | 0.289 |
| Peripheral arteriopathy | 1 | 0.09 | 2 | 0.17 | 1.89 [0.17 to 20.88] | 0.596 |
| Infection | 27 | 2.37 | 16 | 1.34 | 0.56 [0.30 to 1.05] | 0.065 |
| Tumor | 9 | 0.79 | 12 | 1.00 | 1.27 [0.53 to 3.00] | 0.592 |
| Sudden death | 14 | 1.23 | 19 | 1.59 | 1.29 [0.65 to 2.57] | 0.470 |
| Cachexia | 10 | 0.88 | 6 | 0.50 | 0.57 [0.21 to 1.58] | 0.276 |
| Death from other causes | 20 | 1.76 | 13 | 1.09 | 0.62 [0.31 to 1.24] | 0.172 |
HD denotes hemodialysis, and OL-HDF on-line hemodiafiltration.
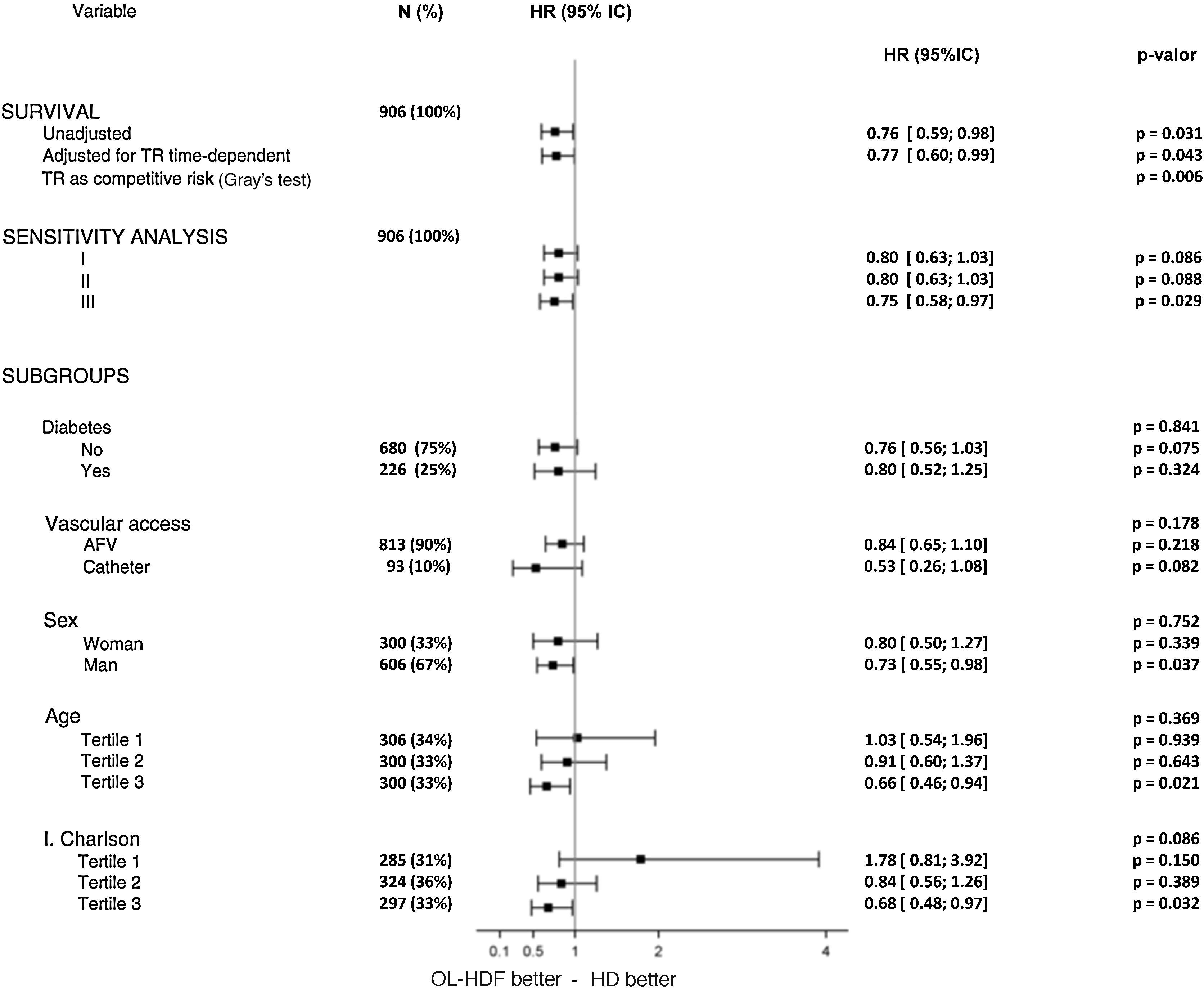
Sensitivity analyses were performed on the basis of the following variables, which were found to be independent predictors for all-cause mortality: age, gender, diabetes, the Charlson comorbidity index and vascular access. These variables were included in 3 different multivariate analyses to assess the covariate-adjusted risk estimates for the intervention (Fig. 2). When these covariates were included, the effect of treatment was on the verge of significance (model I). In model II, renal transplantation was added to model I as a time-dependent covariate and the adjusted risk estimate for the intervention was identical to model I. Finally, in model III, an adjusted risk estimate was done considering renal transplantation as the only cause for censoring. The treatment risk estimates were also calculated in all subgroups arising from these variables, using the original categories for nominal variables and tertiles for continuous variables. All hazard ratios were consistent for both types of analysis and the statistical tests for interaction were not significant (Fig. 2). In this analysis, male and older (upper tertile of age) patients, as well as patients in the upper tertile of the Charlson comorbidity index, obtained significant benefit from the intervention.
Sensitivity analyses for the main outcome showing hazard ratios [95% CI] for the intervention based on relevant variables that were found to be independent predictors for all-cause mortality. Multivariate I: age, gender, diabetes and vascular access. Multivariate II: age, gender, diabetes, vascular access and the Charlson comorbidity index. Multivariate III: age, gender, diabetes, vascular access, Charlson comorbidity index and censoring for transplantation. 1T, 2T and 3T: first, second and third tertiles.
Since the underlying risk of death is known to change after transplantation, we modeled all-cause mortality taking into account the time of transplantation in a time-dependent approach. The time-dependent Cox analysis (Fig. 2) showed very similar results to the main analysis with a hazard ratio [95% CI] of 0.77 [0.60 to 0.99] (P=0.043).
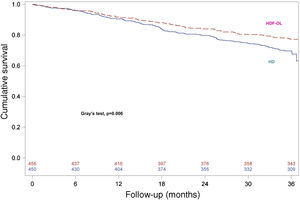
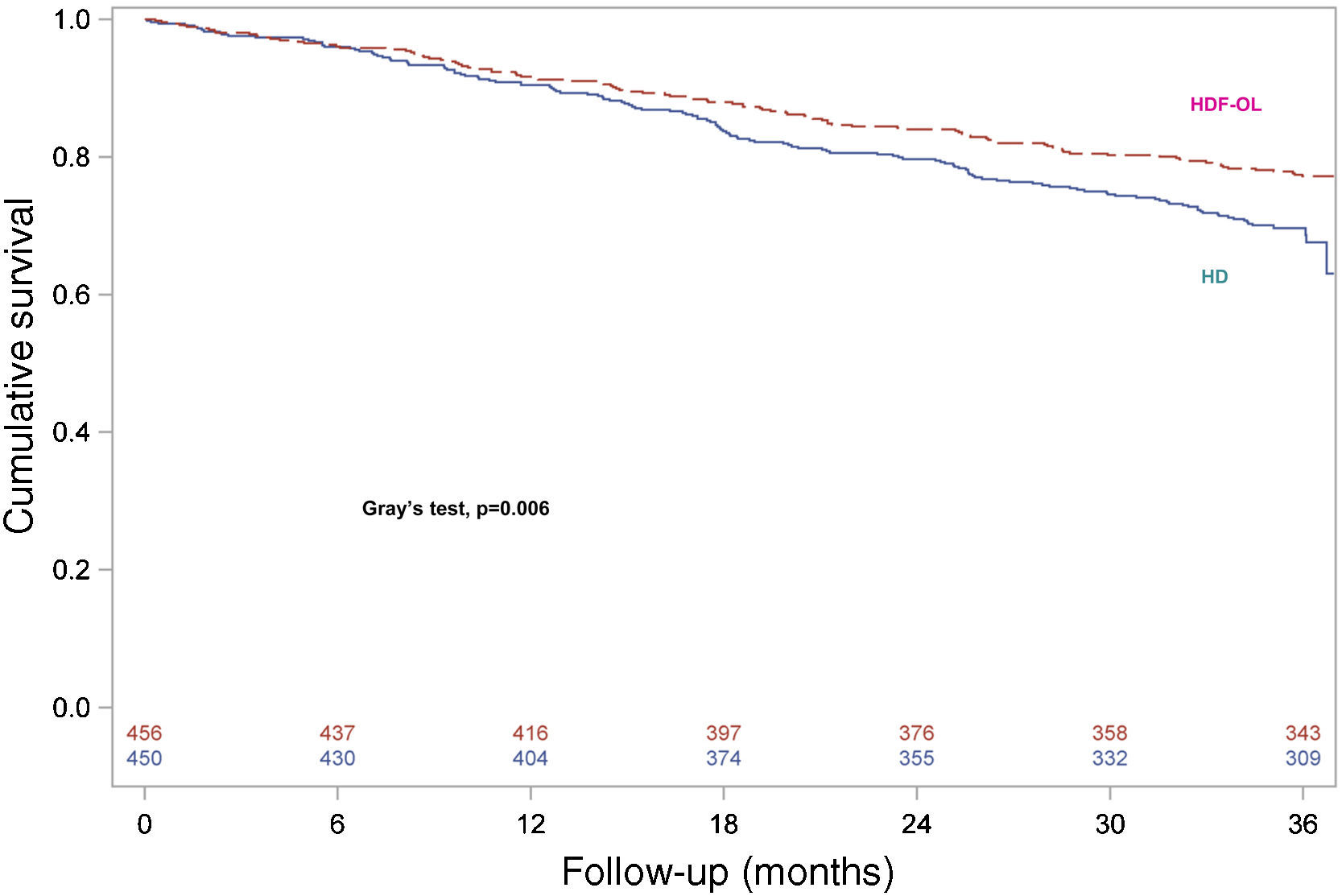
Survival analysis was repeated to assess the consistency of the results by considering renal transplantation as a competing event and consequently death after transplantation was not considered. For the other patients discontinuing the study, complete follow-up until 3 years was considered and events occurring after discontinuation of the study were included. Survival curves for both treatment groups are shown in Fig. 3, confirming that patients allocated to OL-HDF group had a higher survival than patients allocated to the HD arm (Gray's test, P=0.006).
DiscussionIn the present study, we conducted a new analysis of the ESHOL-study with the ITT population with a complete follow-up for 3 years after trial enrollment. In the previous study,1 patients who were alive when they discontinued the study for any reason were censored at the time of withdrawal. As in other studies conducted in patients on hemodialysis, there was a high rate of censored observations before 3 years (39.2%) and the main reason for censoring was renal transplantation, accounting for more than 50% of cases. Additionally, the proportion of patients receiving a kidney transplant was slightly higher in the HDF-OL arm than in the HD arm (101 out of 456 patients versus 79 out of 450 patients), suggesting that, despite the randomized allocation of patients to both arms, there may have been an unbalanced risk between the 2 groups. In survival analyses, all subjects at risk of experiencing an event constitute the risk set. Standard survival analytical methods such as the Kaplan–Meier method assume that censoring is “independent”, this is, that patients who are censored at a certain time point should be representative of those still at risk (and, thus, in the risk set) at that time point. In the ESHOL study, we observed that censoring was not independent, since censored patients had a lower risk of death than those continuing in the trial (the risk set). These results could be expected in hemodialysis patients who were censored mainly because of renal transplantation, since it is well known that the risk of death decreases after renal transplantation in chronic hemodialysis patients. Nevertheless, according to the randomized design of the trial, the risk of death in censored patients in both arms of the trial was not different (4.04 per 100 patients-year in the HD arm and 5.27 per 100 patients-year in the HDF-OL arm).
“Intention to treat” is a strategy to analyze randomized controlled trials comparing patients according to the initial random allocation. This is generally interpreted as including all patients, regardless of whether they actually satisfied the entry criteria, the treatment actually received, and subsequent withdrawal or deviation from the protocol. The ITT approach maintains treatment groups that are similar apart from random variation and allows for non-compliance and deviations from policy by clinicians.16 This approach characterizes the effectiveness of the intervention and offers information on the potential benefit observed in clinical practice. If an ITT analysis is not done, clinical effectiveness may be overestimated.17
In the ESHOL study, an ITT analysis was not originally planned since we expected a significant percentage of withdrawals (15% per year) and assumed that it would not affect the main conclusions of our study. Indeed, other studies conducted in hemodialysis patients to evaluate the benefit of different interventions were performed with a similar design. To overcome this limitation and to gain further insight into the effectiveness of our intervention, we decided to conduct the present study with a complete ITT analysis of the ESHOL study. In the univariate analysis of the present study, we were able to demonstrate that patients allocated to the OL-HDF arm had a significant 24% risk reduction of death compared with patients allocated to the HD arm. Importantly, the death rate was lower in this ITT population than in patients continuing under hemodialysis. Thus, this new analysis is less powered to detect a difference between groups. This loss of statistical power partly explains why, after adjustment for age, gender, diabetes, the Charlson comorbidity index and vascular access in the multivariate analysis, the 20% risk reduction was only on the threshold of significance (P=0.086).
Despite the usefulness of ITT analysis to analyze the ESHOL study, the main reason for withdrawal was renal transplantation. This is a classic example of competing risk in nephrology and, in this situation, it has been proposed that an alternative way to analyze data is by a proportional cause-specific hazards model with application of Cox regression analysis for each of the specific event types.7 In each of these models, the competing events are treated as censored observations. We have also performed this analysis (model III of the sensitivity analysis) and we observed a statistically significant 25% reduction in the risk of death by adjusting for confounders in patients allocated to the OL-HDF arm. Finally, the results of the 2 approaches to assess the potential effect of transplantation on the results, the time-dependent Cox analysis and the competing events approach, both yielded similar results to that of the primary raw analysis and confirmed the positive conclusions of the trial.
In the last year, 4 meta-analyses have been published that analyze the effect of convective against diffusive therapies.18–21 Susantitaphong et al.18 evaluated randomized controlled trials comparing the effect of convective therapies including high-flux dialysis, hemofiltration or hemodiafiltration versus low-flux dialysis. The 3 other most recent meta-analyses19–21 that included all 3 RCT with mortality as the primary endpoint1,4,8 to compare convective techniques versus low- or high-flux hemodialysis were positive in terms of survival, but the results were inconclusive, probably due to the disparity of criteria and confounding factors. Only the meta-analysis of the EuDial working group,21 including only RCT comparing OL-HDF versus hemodialysis (hemofiltration and AFB were excluded) showed the superiority of OL-HDF to HD on overall and cardiovascular mortality. The discrepancies between these meta-analyses can be explained by the different research questions, different selection criteria of clinical trials and potential confounding factors (delivered dose of convective therapy, pre- or postdilution infusion or treatment modality). A revised definition of HDF was published by the EuDial group,22 in which HDF is a blood purification therapy combining diffusive and convective solute transport using a high-flux membrane characterized by an ultrafiltration coefficient greater than 20mL/h/mmHg/m2 and a sieving coefficient for β2-microglobulin greater than 0.6; convective transport is achieved by an effective convection volume of at least 20% of the total blood volume processed. If these criteria were applied when selecting the RCT for inclusion in meta-analyses, none of the 4 published meta-analyses would meet them. It can therefore be questioned whether these meta-analyses are valid to answer the question of whether high-volume HDF improves survival compared with hemodialysis.
In summary, the results of this reanalysis of the ESHOL trial confirm that high efficiency postdilution OL-HDF reduces all-cause mortality versus conventional hemodialysis in prevalent patients. These results are consistent independently of the statistical analysis employed. The original results observed in the ESHOL study, which censored patients discontinuing the study for any reason, were confirmed in the present study, which considered all-cause mortality in the ITT population without censures and also considered all-cause mortality by time-dependent and competing risks for transplantation.
Conflicts of interestThe authors have no conflicts of interest to declare.
The ESHOL study has had the endorsement of the Catalan Society of Nephrology. This study was partly supported by grants from Fresenius Medical Care and Gambro through the Catalan Society of Nephrology.
The following institutions and investigators participated in the ESHOL study:
CETIRSA, Barcelona: M. Pons, B. Insensé, C. Perez, T. Feliz; Hospital San Antonio Abad, Vilanova i la Geltru: R. Ramos, M. Barbetta, C. Soto; Fresenius Medical Care, Granollers: J. Mora, A. Juan, O. Ibrik; Diaverum Baix Llobregat, Hospitalet: A. Foraster, J. Carreras; Fresenius Medical Care, Hospitalet: F. Moreso, M. Hueso, J. Nin, A. Fernández; Fresenius Medical Care, Reus: J. Soler, M. Arruche, C. Sánchez, J. Vidiella; Fresenius Medical Care Diagonal, Barcelona: F. Barbosa, M. Chiné, S. Hurtado; CETIRSA, Terrassa: J. Llibre, A. Ruiz, M. Serra, M. Salvó, T. Poyuelo; Hospital Clínic, Barcelona: F. Maduell, M. Carrera, N. Fontseré, M. Arias, Josep M Campistol,; Fresenius Medical Care Julio Verne, Barcelona: A. Merín, L. Ribera; Fundació Althaia, Manresa: JM. Galceran, J. Mòdol, E. Moliner, A. Ramirez; Hospital Santa Tecla, Tarragona: J. Aguilera, M. Alvarez; Diaverum Bonanova, Barcelona: B. de la Torre, M. Molera; Diaverum IHB, Barcelona: J. Casellas, G. Martín; Fundació Puigvert, Barcelona: E. Andres, E. Coll; Hospital Josep Trueta, Girona: M. Valles, C. Martínez; Hospital General, Vic: E. Castellote; Diaverum, Mataró: JM. Casals, J. Gabàs, M. Romero; Hospital Universitari Bellvitge, Hospitalet: A. Martinez-Castelao, X. Fulladosa; Hospital de Terrassa: M. Ramirez-Arellano, M Fulquet; Diaverum Verge de Montserrat, Santa Coloma: A. Pelegrí, M. el Manouari, N. Ramos; Centre Secretari Coloma, Barcelona: J. Bartolomé; Hospital de Figueres, R. Sans; Hospital Arnau de Vilanova, Lleida: E. Fernández, F. Sarró; Hospital Santa Creu, Tortosa: T. Compte; Diaverum Nephros, Barcelona: F. Marco, R. Mauri; Clínica Girona: J. Bronsoms.
Clinical Trials Unit: JA. Arnaiz, H. Beleta, A. Pejenaute (UASP Farmacología Clínica, Hospital Clínic Barcelona).
Statistical analysis: F. Torres, J. Ríos and J. Lara (Biostatistics Unit, School of Medicine, Universitat Autònoma de Barcelona; Biostatistics and Data Management Platform, IDIBAPS, Hospital Clinic; Barcelona).
The institutions and investigators in the study group are listed in the appendix.
Please cite this article as: Maduell F, Moreso F, Mora-Macià J, Pons M, Ramos R, Carreras J, et al. Reanálisis del estudio ESHOL: mortalidad por todas las causas considerando riesgos de competición y tiempo-dependientes para trasplante renal. 2016;36:156–163.


![Sensitivity analyses for the main outcome showing hazard ratios [95% CI] for the intervention based on relevant variables that were found to be independent predictors for all-cause mortality. Multivariate I: age, gender, diabetes and vascular access. Multivariate II: age, gender, diabetes, vascular access and the Charlson comorbidity index. Multivariate III: age, gender, diabetes, vascular access, Charlson comorbidity index and censoring for transplantation. 1T, 2T and 3T: first, second and third tertiles. Sensitivity analyses for the main outcome showing hazard ratios [95% CI] for the intervention based on relevant variables that were found to be independent predictors for all-cause mortality. Multivariate I: age, gender, diabetes and vascular access. Multivariate II: age, gender, diabetes, vascular access and the Charlson comorbidity index. Multivariate III: age, gender, diabetes, vascular access, Charlson comorbidity index and censoring for transplantation. 1T, 2T and 3T: first, second and third tertiles.](https://static.elsevier.es/multimedia/20132514/0000003600000002/v4_201703310238/S2013251416000298/v4_201703310238/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w94GCRvdQBB6xyQjMrWMzrts=)