Allogeneic hematopoietic progenitor cell transplantation is a widely used procedure, and graft-versus-host disease (GVHD) is a common complication. Glomerular involvement due to GVHD is exceptional. Objective: to describe the clinical and histopathological presentation of patients with glomerular disease secondary to GVHD. Materials and methods: a retrospective review of the Uruguayan registry of glomerulopathies was conducted to identify renal biopsies from patients with confirmed GVHD. Results: seven patients were identified, four male, with a median age of 41 years (range 8–58). The most common clinical and analytical presentation was nephrotic syndrome. Histopathological findings included membranous glomerulonephritis (4), segmental and focal sclerohyalinosis (1), membranoproliferative glomerulonephritis (1), and thrombotic microangiopathy (1). All patients were treated with corticosteroids in combination with immunosuppressors, most frequently mycophenolate mofetil and rituximab. Five patients achieved complete remission, one had partial remission, and one did not respond. The combination of corticosteroids with rituximab showed a good response rate in those presenting with podocytopathy in the renal biopsy.
El trasplante alogénico de progenitores hematopoyéticos es un procedimiento ampliamente utilizado y el desarrollo de enfermedad injerto versus huésped (EIVH) una complicación frecuente. El compromiso glomerular por EIVH es excepcional. Objetivo: describir la presentación clínica e histopatológica de pacientes con enfermedad glomerular secundaria a EIVH. Material y métodos: se revisó retrospectivamente el registro uruguayo de glomerulopatías en busca de biopsias renales de pacientes con EIVH demostrada. Resultados: se identificaron 7 pacientes, 4 de sexo masculino, edad 41 (8–58) años. La presentación clínica-analítica más frecuente fue el sindrome nefrótico. Los hallazgos histopatológicos fueron glomerulonefritis membranosa (4), esclerohialinosis segmentaria y focal (1), glomerulonefritis membranoproliferativa (1) y microangiopatía trombótica (1). La totalidad de pacientes se trataron con corticosteroides asociados con inmunosupresores, más frecuentemente micofenolato mofetilo y rituximab. Presentaron remisión completa 5 pacientes, remisión parcial 1 y no remisión 1. La combinación de corticoides con rituximab mostró una buena tasa de respuesta en quienes se presentaron con podocitopatía en la biopsia renal.
Allogeneic hematopoietic stem cell transplantation (ASCT) is a widely used procedure for the treatment of multiple hematological diseases.1 A complication of ASCT is graft-versus-host disease (GVHD), which can develop acutely during the first 100 days after ASCT (a-GVHD) or be a chronic complication (c-GVHD) when it occurs after this time period.2 c-GVHD is the most common late complication in patients with ASCT, present in 40–80% of transplant recipients, and is responsible for high morbidity and mortality3. The most common manifestations of c-GVHD are cutaneous and mucosal (lichen planus, scleroderma-like lesions, mucosal erosions with ulcerated lesions), gastrointestinal, and hepatic.1
The kidney may be affected during the course of ASCT as a consequence of drug toxicity linked to pre-transplant conditioning, sepsis, or toxicity from calcineurin inhibitors used to prevent GVHD.4 This involvement may be clinically expressed as acute kidney failure with an incidence of 50–75%,5 or as chronic kidney disease with reported incidence that ranges between 7% and 66% depending on the series.6 Glomerular involvement secondary to c-GVHD is a rare complication, with a prevalence of less than 2% in published series. The most frequent histopathological patterns are membranous nephropathy and minimal change disease, followed by much less frequent lesions such as focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, IgA nephropathy, crescentic glomerulonephritis, and lupus nephritis-like glomerular lesions.7 The objective of this study is to describe the clinical and analytical characteristics of patients with c-GVHD who underwent renal biopsy due to manifestations of glomerular injury.
MethodsPatientsThe Uruguayan Registry of Glomerulopathies (RUG) was retrospectively reviewed searching for renal biopsies from patients receiving ASCT with a confirmed diagnosis of c-GVHD. Patients aged ≥14 years with biopsies obtained between 2000 and 2024 were included. The RUG was launched in 1970 at the Unidad Académica de Nefrología de la Facultad de Medicina, Universidad de la República, and gradually included data on patients with biopsy-confirmed glomerular disease. Since 2000, reporting of renal biopsies has been mandatory, and the RUG therefore collects information on all renal biopsies performed in the country. The medical records of the included patients were reviewed, obtaining epidemiological data, including the disease that led to the indication for ASCT, medications received during the pretransplant conditioning process, nephrological clinical presentation, and laboratory data before and after the renal biopsy.
To evaluate the renal response to established treatments, complete remission was defined as a reduction in proteinuria <0.3 g/day, a reduction in creatinine levels to baseline values prior to the development of renal impairment, and serum albumin levels ≥3.5 g/dL; partial remission was defined as a reduction in proteinuria <3.5 g/day and >0.3 m g/day, and stabilization at creatinine levels no greater than 25% above the baseline values prior to the development of renal impairment; and non-remission was defined as a failure to meet the above definitions.
Ethical aspectsThe study protocol was approved by the Research Ethics Committee of the Hospital de Clínicas, Facultad de Medicina (No. 55-24). In Uruguay it is mandatory to report the Glomerular diseases, and an informed consent is obtained by which Patients agree that the data obtained from the renal biopsy, as well as the clinical data collected, may be used for research purposes.
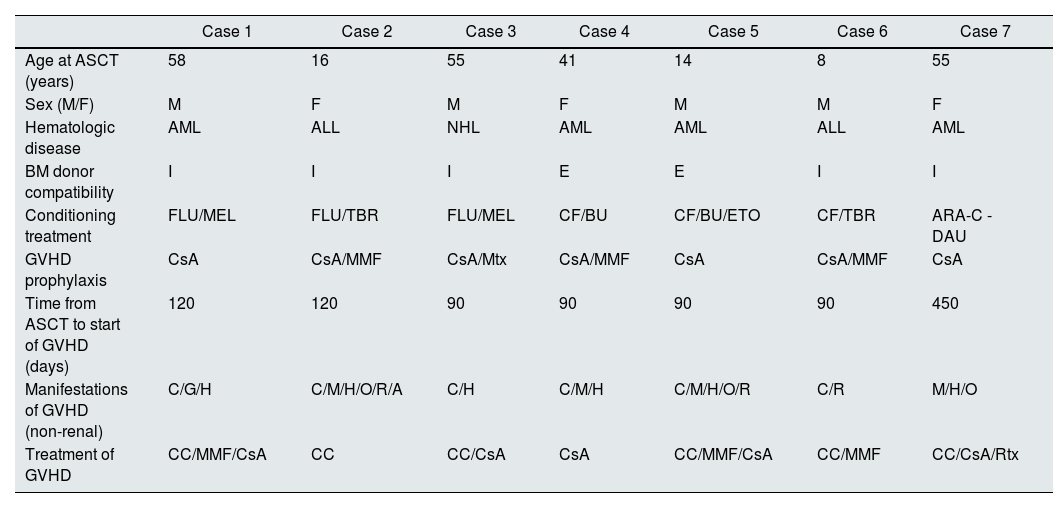
ResultsPopulationSeven patients biopsied during the study period were included. Four were male, and the age was 41 (8–58) years. Table 1 shows patient characteristics and data related to hematologic disease, ASCT, and the development of GVHD.
Population characteristics, ASCT, GVHD—nonrenal—and treatment received.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age at ASCT (years) | 58 | 16 | 55 | 41 | 14 | 8 | 55 |
| Sex (M/F) | M | F | M | F | M | M | F |
| Hematologic disease | AML | ALL | NHL | AML | AML | ALL | AML |
| BM donor compatibility | I | I | I | E | E | I | I |
| Conditioning treatment | FLU/MEL | FLU/TBR | FLU/MEL | CF/BU | CF/BU/ETO | CF/TBR | ARA-C -DAU |
| GVHD prophylaxis | CsA | CsA/MMF | CsA/Mtx | CsA/MMF | CsA | CsA/MMF | CsA |
| Time from ASCT to start of GVHD (days) | 120 | 120 | 90 | 90 | 90 | 90 | 450 |
| Manifestations of GVHD (non-renal) | C/G/H | C/M/H/O/R/A | C/H | C/M/H | C/M/H/O/R | C/R | M/H/O |
| Treatment of GVHD | CC/MMF/CsA | CC | CC/CsA | CsA | CC/MMF/CsA | CC/MMF | CC/CsA/Rtx |
Manifestations of GVHD: cutaneous (C), mucosal (M), gastrointestinal (G), hepatic (H), ocular (O), respiratory (R), osteoarticular (A).
ARA-C: cytosine arabinoside; BU: busulfan; CC: corticosteroids; CF: cyclophosphamide; CsA: cyclosporine A; DAU: daunorubicin; E: related donor; GVHD: graft-versus-host disease; ETO: etoposide; I: identical donor; F: female; FLU: fludarabine; AML: acute myeloblastic leukemia; ALL: acute lymphoblastic leukemia; NHL: non-Hodgkin lymphoma; M: male; MEL: melphalan; MMF: mycophenolate mofetil; Mtx: methotrexate; TBR: total body irradiation; Rtx: rituximab; ASCT: allogeneic hematopoietic stem cell transplant.
Four of the seven patients presented extrarenal manifestations of GVHD before the first 100 days after ASCT. The most frequent manifestations were cutaneous, presented as hyperpigmented lesions, lichen planus on the oral mucosa, and hepatic, primarily as elevated liver transaminases. GVHD prophylaxis was prescribed in all cases, with cyclosporine A being the most commonly used drug. None of the patients initially presented renal manifestations attributable to GVHD. Immunosuppressive treatment for the manifestations of GVHD included corticosteroids in all cases, with varying frequencies of cyclosporine A, mycophenolate mofetil, and rituximab (Table 1).
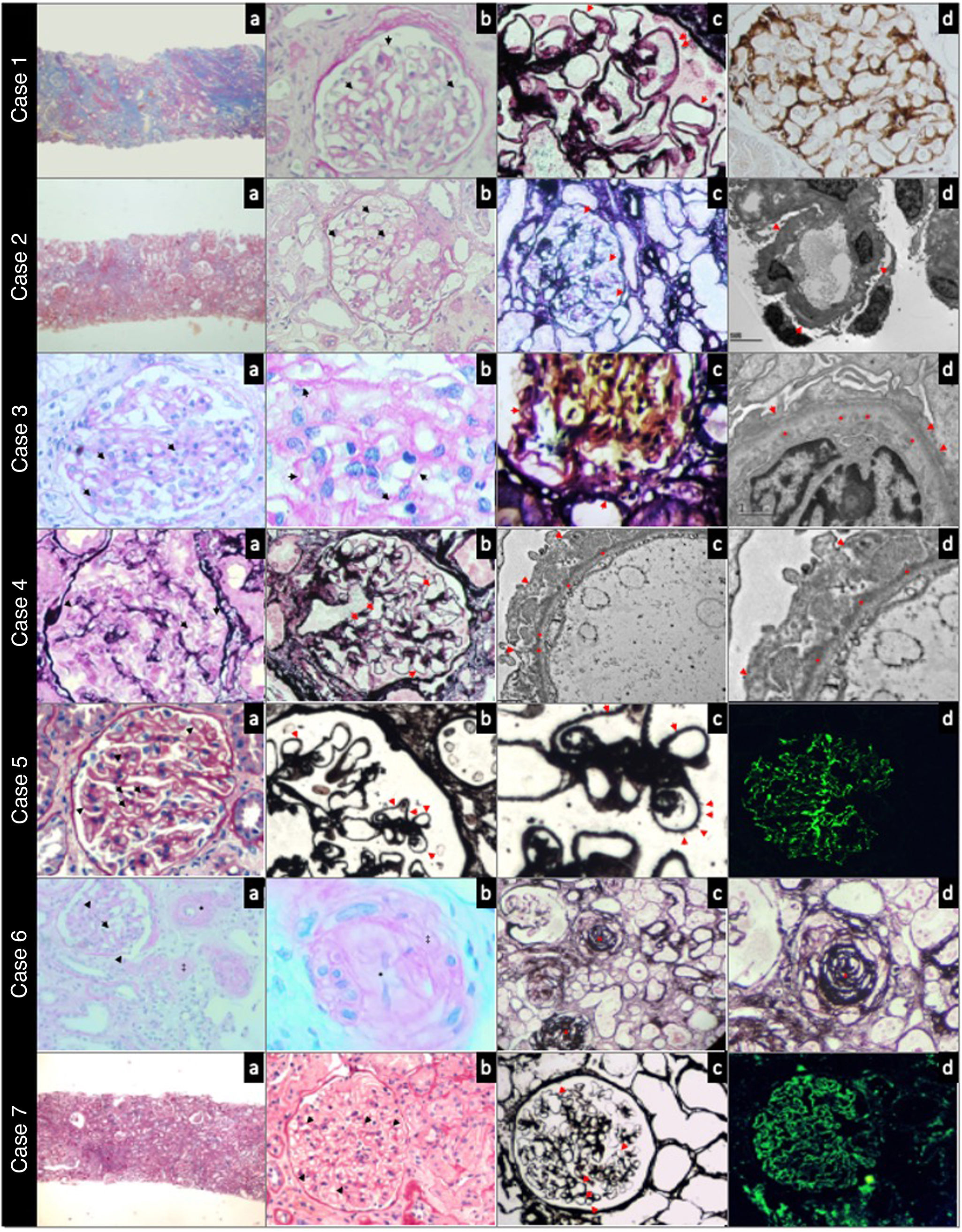
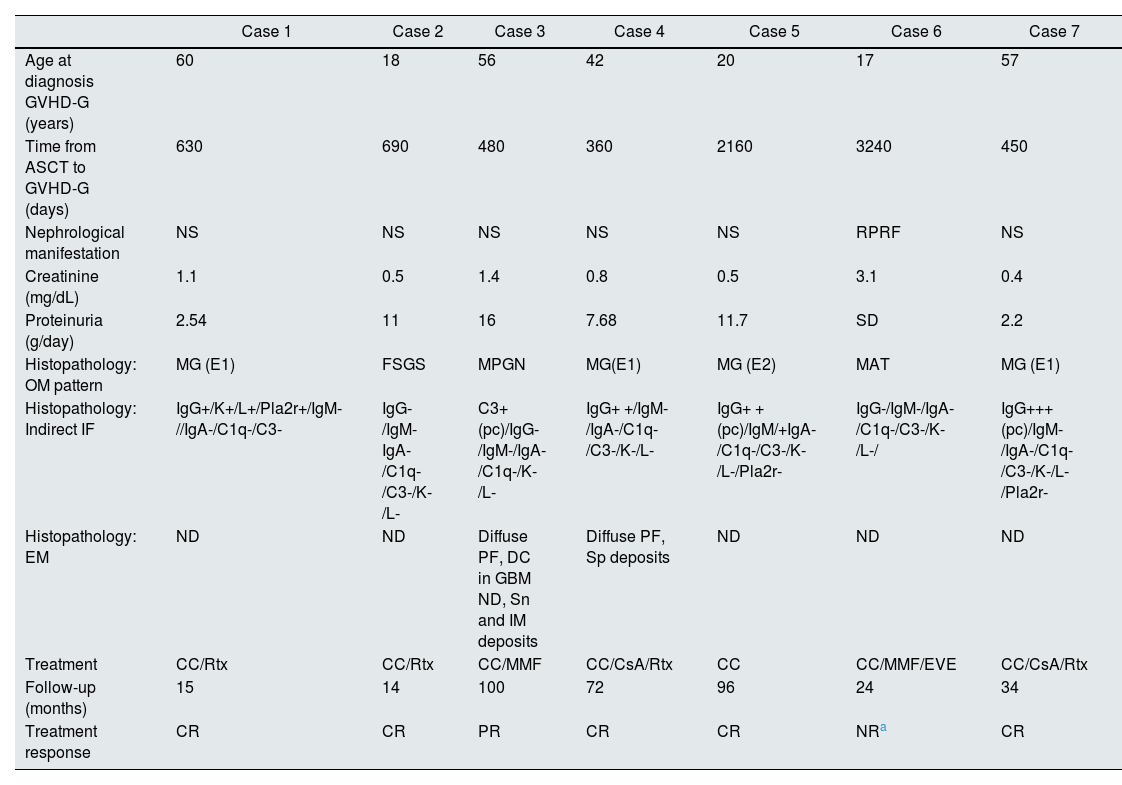
Glomerular disease associated with GVHDTable 2 shows the most relevant histopathological findings of patients of the present study. The age at the time of developing renal manifestations was 42 (17–60) years. The time between ASCT and the development of manifestations attributable to glomerular disease was 1144 (360–3240) days. The most frequent clinical presentation was nephrotic syndrome (6/7), with proteinuria 8.58 (2.2–16) g/day, creatinine 1.1 mg/dL, and a median creatinine level of 0.8 mg/dL. The most frequent histopathological pattern was membranous glomerulonephritis (MG) (4/7); less frequently, findings were compatible with focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis (MPGN), and thrombotic microangiopathy (TMA). One of the MG cases (case 1) was positive for Pla2r by immunofluorescence in the tissue sample, with negative plasma anti-Pla2r antibodies. Fig. 1 shows the most relevant histopathological findings in each case.
Clinical presentation, histopathology, treatment, and outcome of glomerular disease associated with GVHD (GVHD-G).
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis GVHD-G (years) | 60 | 18 | 56 | 42 | 20 | 17 | 57 |
| Time from ASCT to GVHD-G (days) | 630 | 690 | 480 | 360 | 2160 | 3240 | 450 |
| Nephrological manifestation | NS | NS | NS | NS | NS | RPRF | NS |
| Creatinine (mg/dL) | 1.1 | 0.5 | 1.4 | 0.8 | 0.5 | 3.1 | 0.4 |
| Proteinuria (g/day) | 2.54 | 11 | 16 | 7.68 | 11.7 | SD | 2.2 |
| Histopathology: OM pattern | MG (E1) | FSGS | MPGN | MG(E1) | MG (E2) | MAT | MG (E1) |
| Histopathology: Indirect IF | IgG+/K+/L+/Pla2r+/IgM-//IgA-/C1q-/C3- | IgG-/IgM-IgA-/C1q-/C3-/K-/L- | C3+(pc)/IgG-/IgM-/IgA-/C1q-/K-/L- | IgG+ +/IgM-/IgA-/C1q-/C3-/K-/L- | IgG+ +(pc)/IgM/+IgA-/C1q-/C3-/K-/L-/Pla2r- | IgG-/IgM-/IgA-/C1q-/C3-/K-/L-/ | IgG+++(pc)/IgM-/IgA-/C1q-/C3-/K-/L-/Pla2r- |
| Histopathology: EM | ND | ND | Diffuse PF, DC in GBM ND, Sn and IM deposits | Diffuse PF, Sp deposits | ND | ND | ND |
| Treatment | CC/Rtx | CC/Rtx | CC/MMF | CC/CsA/Rtx | CC | CC/MMF/EVE | CC/CsA/Rtx |
| Follow-up (months) | 15 | 14 | 100 | 72 | 96 | 24 | 34 |
| Treatment response | CR | CR | PR | CR | CR | NRa | CR |
C1q: complement fraction C1q; C3: complement fraction C3; CC: corticosteroids; CsA: cyclosporine A; DC: double contour; E1: stage 1; E2: stage 2; FSGS: focal segmental GS; GVHD-G: graft-versus-host disease (GVHD); EVE: everolimus; PF: pedicellar fusion; MG: membranous glomerulonephritis; MPGN: membranoproliferative glomerulonephritis; IF: indirect immunofluorescence pattern on histopathology; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; IM: intramembranous deposits; RPRF: rapidly progressive renal failure; K: kappa light chain; L: lambda light chain; TMA: thrombotic microangiopathy; GBM: glomerular basement membrane; EM: histopathological findings on electron microscopy; MMF: mofetil mycophenolate; BM: histopathological pattern on light microscopy; NR: non-remission; PR: partial remission; pc: pericapillary deposits; Pla2r: phospholipase A2 receptor; CR: complete remission; RT: rituximab; ND: no data; Sn: subendothelial deposits; Sp: subepithelial deposits; NS: nephrotic syndrome; ASCT: autologous hematopoietic progenitor cell transplant.
Case 1. Panoramic view of a cortical fragment —Masson’s trichrome— (a). Glomerulus showing rigid capillary loops (arrows) —periodic acid-Schiff— (b). Capillary loops with microspicules (arrows) in the external sector of the glomerular basement membrane —methenamine silver— (c). Immunohistochemistry with intense positivity for antibodies against phospholipase A2 in the glomerular basement membrane (d). Case 2. Panoramic view of a cortical fragment —Masson’s trichrome— (a). Glomerulus showing rigid capillary loops (arrows) —periodic acid-Schiff— (b). Thin basement membranes (arrows) without spicules or double contours —methenamine silver- (c). Electron microscopy showing a glomerular capillary loop without deposits and diffuse pedicellar fusion (arrows) (d). Case 3. Glomerulus with segmental intracapillary hypercellularity (arrows) —periodic acid-Schiff— (a). Capillary loops at higher magnification, with occasional double contour images (arrows) —periodic acid-Schiff— (b). Glomerulus with segmental double contour images in the glomerular basement membrane (arrows) —methenamine silver— (c). Electron microscopy showing diffuse subendothelial deposits (*) and pedicellar fusion (arrows) (d). Case 4. Glomerulus showing rigid capillary loops (arrows) —periodic acid-Schiff— (a). Rigid glomerular capillary loops (arrows) —methenamine silver— (b). Electron microscopy showing regular and diffuse subepithelial deposits (*) in the external sector of the glomerular basement membrane, and diffuse pedicellar fusion (arrows) (c and d). Case 5. Glomerulus showing thickened and rigid capillary loops (arrows) —periodic acid-Schiff staining— (a). Diffuse spicules on the outer slope of the glomerular basement membrane (arrows) —methenamine silver staining— (b and c). Direct immunofluorescence with IgG staining showing a fine granular pericapillary pattern (d). Case 6. Glomerulus with infolding of the basement membranes (arrows); interlobular artery with myointimal fibrosis (*) and arteriole with luminal thrombosis (‡) —periodic acid-Schiff staining— (a). Arteriole at higher magnification, with complete occlusion of the lumen (*) and collagenization of the arteriolar wall (‡) —periodic acid-Schiff staining— (b). Vessels of different calibers with complete occlusion of the vascular lumen (*) —methenamine silver staining— (c and d). Case 7. Panoramic view of a cortical fragment —periodic acid-Schiff— (a). Glomerulus showing regular thickening (arrows) of the glomerular basement membrane of the capillary loops —periodic acid-Schiff— (b). Glomerulus with rigid capillary loops (arrows) —methenamine silver— (c). Direct immunofluorescence staining for IgG with a fine granular pericapillary pattern (d).
Treatment included corticosteroids in all cases, with bolus of methylprednisolone followed by prednisone, combined with rituximab (3/7), mycophenolate mofetil (2/7), or cyclosporine (2/7).
The response to the treatment was satisfactory, with complete remission in 5/7 cases and partial remission in 1/7. There was no response in only one case (case 6). This patient presented with rapidly progressive renal failure, with histopathological findings consistent with thrombotic microangiopathy, which necessitated the initiation of chronic dialysis.
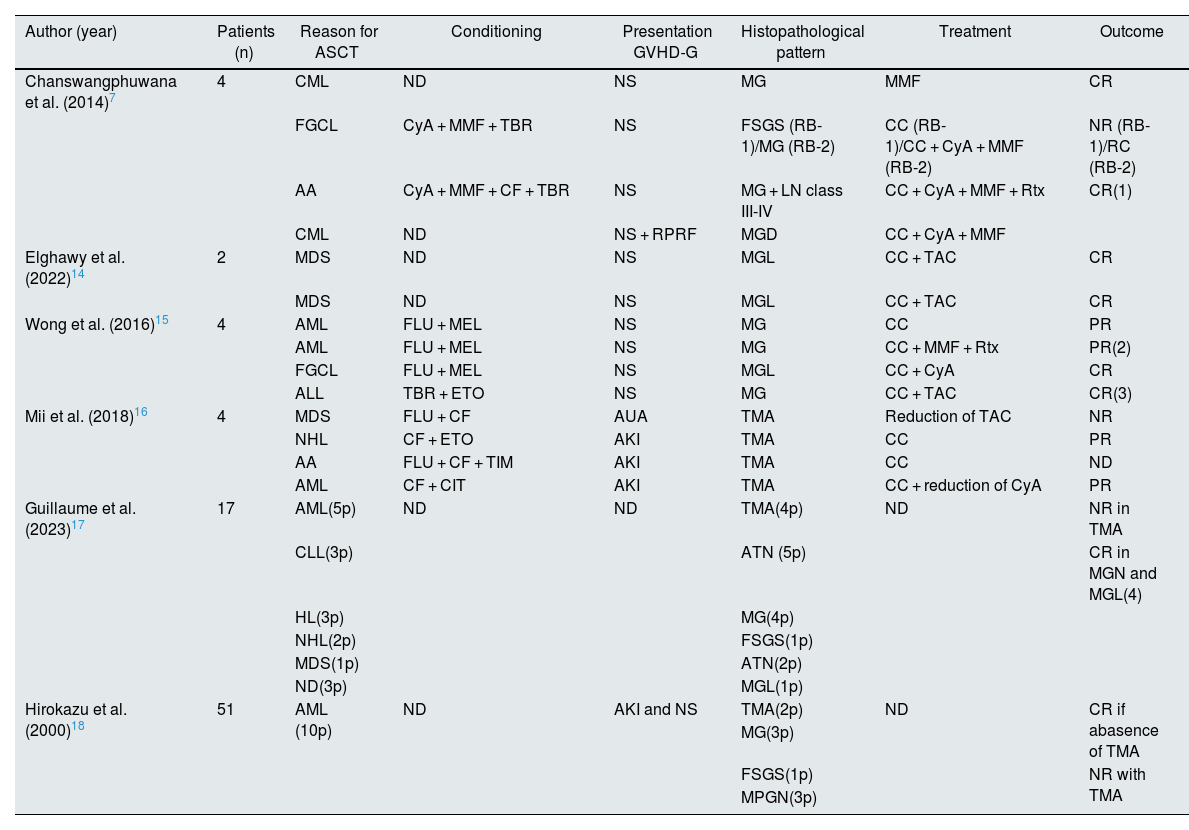
DiscussionWe present a group of patients with glomerular disease associated with chronic GVHD. In all cases, glomerular involvement occurred in patients with other manifestations of GVHD. The most frequent clinical presentation was NS in the context of MG and, less frequently, FSGS, consistent with other reports shown in Table 3. The finding of TMA, present in our group and also in other series (Table 3), may be a consequence of the immunological activity of GVHD or of treatment with calcineurin inhibitors used as prophylaxis. In our case (case 6), TMA appeared after the development of GVHD in a patient with multiple extrarenal manifestations of the disease, suggesting that it may be a direct consequence of the disease. In other series, the finding of TMA (Table 3) is primarily associated with the use of calcineurin inhibitors. One case of MG in our series (case 1) was tissue-positive for the phospholipase A2 receptor (immunohistochemistry) without detecting anti-phospholipase A2 receptor (Pla2r) antibodies in plasma. This situation, that has been described in isolated cases,8,9 may be explained in the context of GVHD by the production of donor-derived antibodies in response to allovariants of podocyte antigens (Plar2) from the recipient.10 Other podocyte antigens identified in MG from patients with GVHD were NELL-1 (neural epidermal growth factor-like protein 1),11 described in primary MG (without identified underlying disease), malignant diseases, drugs, autoimmune diseases, and solid organ and hematopoietic organ transplantation.12 In a recent report, Sethi et al. identified the procadherin FAT1 in 5 of 9 patients who developed MG in the context of GVHD.13 In most of our patients with MG, we did not study the antigen involved in basement membrane deposition. The increased use of laser microdissection and mass spectrophotometry may allow for greater understanding of the antigens involved in MG development in patients with GVHD. The treatments prescribed in our patients varied (Table 2), with a notable favorable response in those presenting with podocytopathies (MG/FSGS) compared to those with TMA or MPGN. In our series, three patients were treated with rituximab, achieving complete remission in a short period of time. In other series (Table 3), treatment with rituximab was associated with complete remission in a high number of cases, which may be explained by the involvement of B lymphocytes in the pathogenesis of GVHD, particularly in late stages (chronic GVHD).2,3 In our patients the prognosis of renal function was good; only one patient (case 6) with rapidly progressive renal failure and TMA lesions in the renal biopsy required chronic dialysis. The strength of this study is that it captures all patients with chronic GVHD biopsied in the country. Its weakness is the retrospective nature of the analysis, which can lead to the loss of relevant information.
Case series of glomerular disease associated with graft-versus-host disease in allogeneic hematopoietic stem cell transplant recipients published between 2014 and 2024.
| Author (year) | Patients (n) | Reason for ASCT | Conditioning | Presentation GVHD-G | Histopathological pattern | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Chanswangphuwana et al. (2014)7 | 4 | CML | ND | NS | MG | MMF | CR |
| FGCL | CyA + MMF + TBR | NS | FSGS (RB-1)/MG (RB-2) | CC (RB-1)/CC + CyA + MMF (RB-2) | NR (RB-1)/RC (RB-2) | ||
| AA | CyA + MMF + CF + TBR | NS | MG + LN class III-IV | CC + CyA + MMF + Rtx | CR(1) | ||
| CML | ND | NS + RPRF | MGD | CC + CyA + MMF | |||
| Elghawy et al. (2022)14 | 2 | MDS | ND | NS | MGL | CC + TAC | CR |
| MDS | ND | NS | MGL | CC + TAC | CR | ||
| Wong et al. (2016)15 | 4 | AML | FLU + MEL | NS | MG | CC | PR |
| AML | FLU + MEL | NS | MG | CC + MMF + Rtx | PR(2) | ||
| FGCL | FLU + MEL | NS | MGL | CC + CyA | CR | ||
| ALL | TBR + ETO | NS | MG | CC + TAC | CR(3) | ||
| Mii et al. (2018)16 | 4 | MDS | FLU + CF | AUA | TMA | Reduction of TAC | NR |
| NHL | CF + ETO | AKI | TMA | CC | PR | ||
| AA | FLU + CF + TIM | AKI | TMA | CC | ND | ||
| AML | CF + CIT | AKI | TMA | CC + reduction of CyA | PR | ||
| Guillaume et al. (2023)17 | 17 | AML(5p) | ND | ND | TMA(4p) | ND | NR in TMA |
| CLL(3p) | ATN (5p) | CR in MGN and MGL(4) | |||||
| HL(3p) | MG(4p) | ||||||
| NHL(2p) | FSGS(1p) | ||||||
| MDS(1p) | ATN(2p) | ||||||
| ND(3p) | MGL(1p) | ||||||
| Hirokazu et al. (2000)18 | 51 | AML (10p) | ND | AKI and NS | TMA(2p) | ND | CR if abasence of TMA |
| MG(3p) | |||||||
| FSGS(1p) | NR with TMA | ||||||
| MPGN(3p) |
- Initial complete response (CR) with CC + CyA; relapse treated with RTx + MMF with sustained CR.
- If no response (NR) with CC, RTx was added and partial response (PR) was achieved.
- If PR with CC, TAC was added and CR was achieved.
- They were treated with a combination of CC, CyA, and RTx.
AA: aplastic anemia; AUA: asymptomatic urinary abnormalities; RB: renal biopsy; CC: corticosteroids; CF: cyclophosphamide; CIT: cytarabine; CyA: cyclosporine A; FSGN: focal segmental sclerohyalinosis; G-GIVH: glomerular damage due graft-versus-host disease; ETO: etoposide; FLU: fludarabine; MG: membranous glomerulonephritis; MPGN: mesangial proliferative glomerulonephritis; AKI: acute kidney injury; RPRI: rapidly progressive renal failure; ALL: acute lymphoblastic leukemia; AML: acute myeloblastic leukemia; FGCL: follicular giant cell lymphoma; MGL: minimal glomerular lesion; HL: Hodgkin lymphoma; CML: chronic myeloid leukemia; NHL: non-Hodgkin lymphoma; CLL: chronic lymphoid leukemia; TMA: thrombotic microangiopathy; MEL: melphalan; MMF: mycophenolate mofetil; AIN: acute interstitial nephritis; LN: lupus nephritis; NR; no response; ATN: acute tubular necrosis; ITN: tubulointerstitial nephropathy; CR: complete response; TBR: total body irradiation; PR: partial response; RTx: rituximab; ND: no data; SIR: sirolimus; MDS: myelodysplastic syndrome; NS: nephrotic syndrome; TAC: tacrolimus; ASCT: allogeneic hematopoietic progenitor transplant; TIM: thymoglobulin.
Glomerular involvement secondary to chronic GVHD is rare. The most common histopathological pattern in renal biopsy is MG, followed by FSGS, MPGN, and TMA. Treatment based on corticosteroids combined with other immunosuppressants achieves adequate response rates. Rituximab appears to have a prominent place in the treatment of patients presenting with podocytopathy (MG, FSGS) in this context. The more extended use of mass spectrophotometry may allow identification of antigens associated with membrane deposits in those who develop MG in the context of GVHD secondary to ASCT.
FundingThis research has not received specific grants from public, commercial, or non-profit agencies.
The authors declare no conflict of interest.
To all the nephrologists reporting to the Glomerulopathies Prevention and Treatment Program of Uruguay.