Chronic kidney disease (CKD) is a prevalent condition that requires reliable tools to predict its progression to end-stage renal disease (ESRD). The KFRE equation, internationally validated, allows for estimating the risk of progression at 2 and 5 years. However, it has not been validated in the Spanish population. This study aims to evaluate the predictive capacity of the KFRE in a cohort of Spanish patients.
MethodsThis is a multicenter, observational, and retrospective study conducted in patients with an estimated glomerular filtration rate (eGFR)<30ml/min who were followed in nephrology clinics between January 2016 and 2021. A total of 602 patients with clinical and demographic data were included. The predictive capacity of the KFRE, in its 4-variable and 8-variable versions, was evaluated using Cox regression analysis and ROC curves.
ResultsOf the 602 patients included, 60% were male. Diabetes was the main etiology. Among the patients who started renal replacement therapy (RRT), 37% did so within two years, and 70% began with hemodialysis. Patients who initiated RRT (50.6%) were younger, had a lower eGFR, and exhibited higher baseline albuminuria. The 4-variable KFRE equation showed an AUC of 0.7639 (95% CI: 0.71−0.81) and demonstrated superior accuracy compared to the 8-variable model.
ConclusionsThe KFRE equation, particularly in its 4-variable version, proves to be useful in predicting the progression to ESRD in the Spanish population. However, recalibration is necessary to improve its accuracy in this context.
La enfermedad renal crónica (ERC) es una condición prevalente que requiere herramientas fiables para predecir la progresión a enfermedad renal crónica terminal (ERCT). La ecuación KFRE, validada internacionalmente, permite estimar el riesgo de progresión a 2 y 5 años. Sin embargo, no ha sido validada en población española. Este estudio tiene como objetivo evaluar la capacidad predictiva de la KFRE en una cohorte de pacientes españoles.
MétodosEstudio multicéntrico, observacional y retrospectivo, en pacientes con GFRe<30ml/min seguidos en consultas de nefrología entre enero de 2016 y 2021. Se incluyeron 602 pacientes con datos clínicos y demográficos. La capacidad predictiva de la KFRE, en sus versiones de 4 y 8 variables, se evaluó mediante análisis de regresión de Cox y curvas ROC.
ResultadosDe los 602 pacientes incluidos, el 60% eran varones. La diabetes fue la principal etiología. El 37% de los pacientes que iniciaron TRS lo hicieron a los dos años y el 70% mediante hemodiálisis. Los pacientes que iniciaron TRS (50,6%) eran más jóvenes, con peor GFRe y mayor albuminuria basal. La ecuación KFRE de 4 variables mostró un AUC de 0,7639 (IC 95%: 0,71–0,81) y precisión superior al modelo de 8 variables.
ConclusionesLa ecuación KFRE, especialmente en su versión de 4 variables, demuestra ser útil para predecir la progresión a ERCT en población española. No obstante, es necesaria su recalibración para mejorar la precisión en este contexto.
Chronic kidney disease (CKD) represents an important global public health problem, with a prevalence of 15% in Spain.1,2 This condition is associated with an increase of 23%, in the initiation of renal replacement therapy (RRT) with significantly higher rates in the Canary Islands and the Valencian Community than other autonomous communities, and more frequent in men and people over 75 years of age.2
The progression of CKD to end-stage CKD has a substantial impact on survival and healthcare costs associated with the initiation of RRT. Therefore, early identification of patients at risk is crucial to optimize clinical management and adequately plan the initiation of RRT.3,4
In response to these needs, investigators developed risk prediction models, such as the Kidney Failure Risk Equation (KFRE), which estimates the risk of progression to end-stage renal disease (ESRD) over 2 and 5 years using demographic and basic analytical variables. International guidelines such as KDIGO 2024 highlight the use of these tools to improve risk stratification in patients with advanced CKD, allowing faster and more efficient interventions.3
However, referral protocols to nephrology vary widely among international guidelines.3,5–7 A premature referral may result in unnecessary interventions in low-risk patients, while a late referral may delay the appropriate initiation of RRT in those at high risk of progression.8 Although parameters such as estimated glomerular filtration rate (eGFR) and albuminuria are useful for classifying CKD, their ability to predict progression to ESRD in isolation is limited.9
To address these limitations, clinicians have incorporated predictive models such as the KFRE, developed by Tangri et al., into clinical guidelines such as ERBP and KDIGO to estimate the risk of progression to ESRD over 2- and 5-year periods.3,10,11 There are different versions of the KFRE, with different numbers of variables, applicable to different populations.
There are 2 main versions of the KFRE:
- 1
4-variable KFRE: this is the most widely used version and the simplest for implementation in clinical practice. It includes the variables age, sex, GFR and urine albumin/creatinine ratio (ACR). Its simplicity allows integration into risk stratification tools without the need for additional laboratory tests.
- 2
8-variable KFRE: in addition to the above parameters, it incorporates serum values of calcium, phosphate, bicarbonate and albumin. Although it offers greater predictive accuracy in some contexts, its use is less common in daily practice due to the need for more specific biochemical analyses.
In terms of its development and validation, KFRE has 2 main approaches:
- •
Original KFRE: derived and validated in Canadian cohorts, with regression coefficients adjusted to the population. It has shown excellent discriminative ability in different studies, but its calibration may change when applied to populations with different clinical and demographic characteristics.11
- •
Pooled KFRE: developed through a meta-analysis of individual data from multiple international cohorts to improve the generalization of the model to different populations. Although it maintains the structure of the original equation, its coefficients reflect the baseline risk for different regions. Previous studies have shown that the pooled version improves calibration in other than North American cohorts, reducing the risk overestimation in the original equation.12
The KFRE equation has been validated in North American, European and Asian cohorts, showing good predictive capacity.12–14
However, the KFRE has not been validated in the Spanish population. Given that demographic, clinical, and social factors can influence its performance, it is essential to evaluate and, if necessary, recalibrate this tool to ensure its applicability to Spain. This multicenter study aims to validate the predictive capacity of the KFRE in Spanish patients with advanced CKD.
Material and methodsObjectiveThe aim of the study is to validate the applicability of the 4- and 8-variable KFRE equations in the Spanish population by assessing the area under the curve (AUC) resulting from the classification generated by the KFRE (high or low risk of renal failure) and the real clinical situation of the patient.
Study designMulticenter retrospective observational multicenter study that consecutively included all adult patients under follow-up in nephrology consultations of participating hospitals, with eGFR<30ml/min/1.73m2 (CKD stage 4) as measured by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula. We followed patients from January 1, 2016, until completion of 5 years of follow-up or initiation of RRT, renal transplantation, or renal failure-related death.
The sample size calculated to estimate an AUC greater than 0.9 (excellent classification), with a 95% confidence interval and an error of 0.05, was 628 patients, distributed into 314 patients who initiated RRT before the end of the 5-year follow-up and 314 patients who completed follow-up without requiring initiation of RRT.
PatientsInclusion criteria:
- •
Age>18 years.
- •
eGFR<30ml/min/1.73m2 at the start of follow-up in the ERCA consultation and who, despite being candidates for RRT, have not begun such therapy through the follow-up period (5 years).
- •
eGFR<30ml/min/1.73m2 at the start of follow-up in ACKD patients who have started RRT or died of renal failure before the end of follow-up (5 years).
Exclusion criteria:
- •
eGFR<10ml/min/1.73m2, previous renal transplantation or previous requirement of RRT at the start of follow-up.
- •
Unavailability of at least 2 measurements with a 3-month interval of baseline eGFR or albuminuria.
- •
Loss to follow-up before study completion.
- •
Pregnancy.
- •
Death before the end of the 5-year follow-up due to non-renal causes.
- •
Patients who are not candidates for RRT.
All patients required at least 2 measurements of eGFR and albuminuria measured by albumin/creatinine ratio (ACR) at an interval of 3 months.
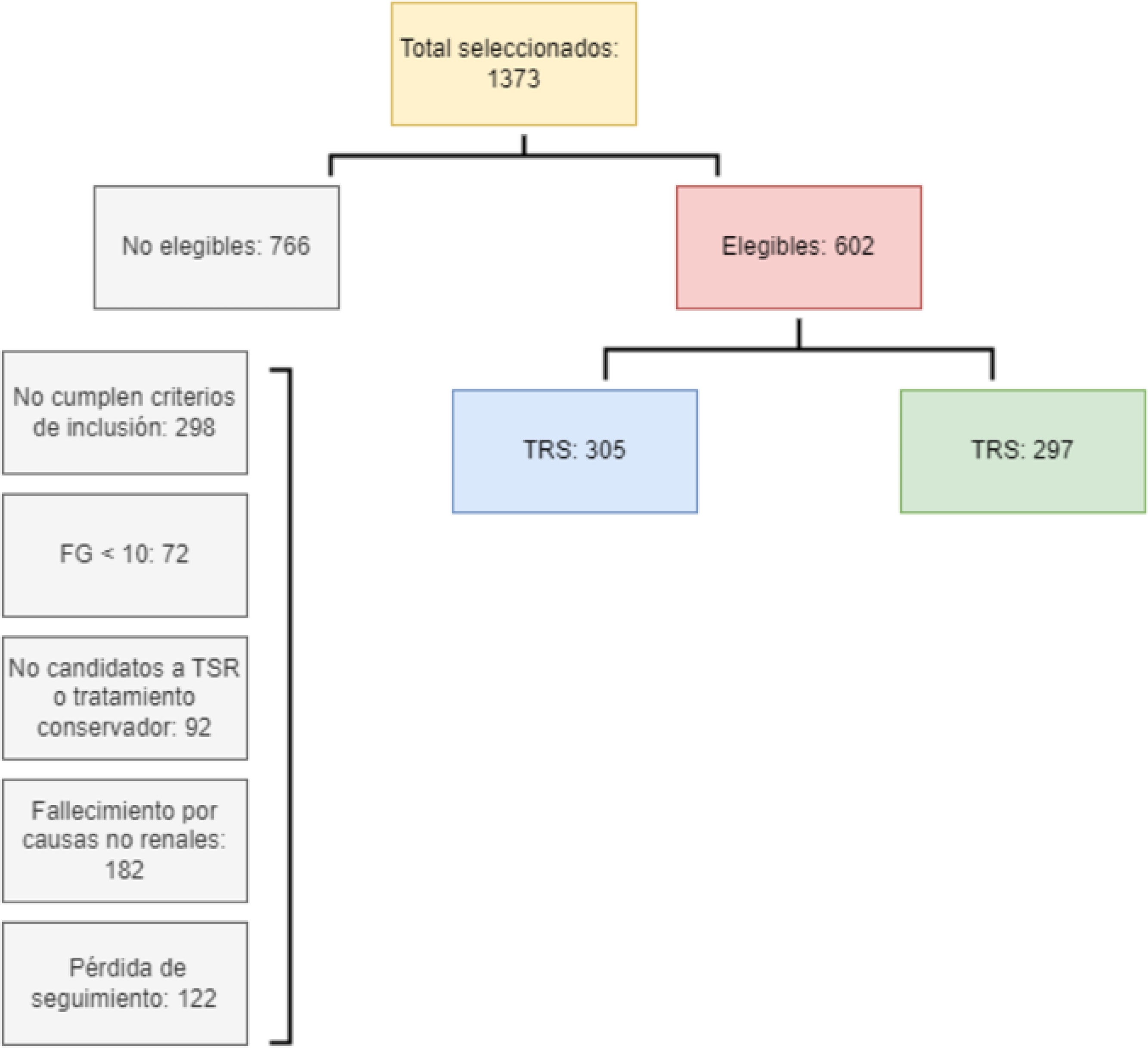
Fig. 1 shows the patient selection process in the study. Of the initial 1373 patients, 766 were excluded for various criteria. Finally, 602 patients were considered eligible, of whom 305 received RRT and 297 did not.
VariablesDemographic variables and laboratory results were collected: age, sex and ethnicity. We estimated GFR using the 2009 CKD-EPI creatinine equation. Albuminuria was represented as a logarithmically transformed ACR. We transformed other measures of urinary protein excretion to ACR using previously developed equations.15 Baseline serum albumin, phosphorus, calcium and bicarbonate values were obtained for each patient. We excluded from the analysis participants whose baseline data were not available.
The primary endpoint was the KFRE calculation of 4 and 8 variables at baseline, 2 and 5 years of follow-up. The secondary variables were baseline renal disease, the modality of RRT initiated and whether the initiation was urgent or programmed, as well as the cause of initiation and, if applicable, renal failure related death.
Eligibility for RRT in this study was based on the clinical decision of the physician in consensus with his patient, without unified criteria due to the retrospective nature of the study. This reflects actual practice, where the indication for RRTdoes not depend only on biological parameters, but also on shared decision making.
Definition of renal failure-related death: death with ESRD without initiation of hemodialysis, with no other cause of death, or when dialysis treatment was suspended or not initiated due to therapeutic uselessness or refusal by the patient or family.
Statistical analysisCategorical variables were described as total number and percentage for each category. Continuous variables were expressed as mean±standard deviation. Comparisons between continuous variables were performed with Student's t-test and categorical variables with the Chi-square test.
Recalibration of the KFRE equation for the Spanish population was performed using a Cox proportional hazards model, maintaining the same variables of the original model (age, sex, eGFR and ACR). To adapt the equation to the Spanish context, three risk calibration approaches were implemented at 2 and 5 years (Appendix B, Supplementary Material 1):
- 1
Original KFRE: predictive model developed in Canadian cohorts, based on clinical and laboratory variables, with high discriminative capacity to estimate the risk of progression to end-stage renal failure in 2 and 5 years.
- 2
Calibrated KFRE (Regional Calibrated-non-North America): variant of the original equation adjusted by a regional calibration factor, correcting for the overestimation of risk observed in some cohorts outside North America, thus optimizing its predictive accuracy in specific populations.
- 3
Pooled KFRE: model derived from a meta-analysis of multiple international cohorts, in which the regression coefficients are estimated using random-effects methods, allowing greater generalization and global applicability of the model.
To compare the performance of each model, we calculated the Brier Score, the bias and the accuracy in risk prediction at 2 and 5 years. The equation with the lowest Brier Score was considered the most accurate. Likewise, the discriminatory capacity of each model was evaluated by calculating the AUC using ROC curves.
In addition, stratified analyses were performed according to baseline eGFR level, differentiating between patients with eGFR≤20ml/min and eGFR>20ml/min to assess the validity of the model in different subgroups.
All calculations were performed using the SAS statistical package version 9.4 (SAS Institute).
Ethical approvalThis study was approved by the clinical trials committee of the Canarias University Hospital -approval number (CHUC_2021_67 [KFRESPAÑA]).
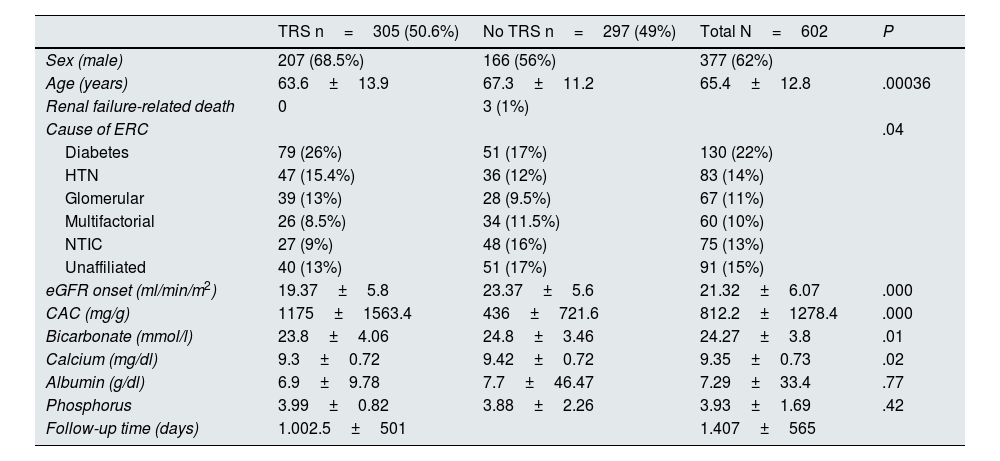
ResultsBaseline characteristicsA total of 602 patients were included in the study, of whom approximately 60% were male in both cohorts. Table 1 shows the baseline characteristics of the patients at 2 and 5 years.
- •
Patients who started RRT were younger (63.6±13.9 years, P<.0003) and had worse GFR (19.37±5.8ml/min, P<.000) and higher baseline albuminuria (1175±1563mg/g, P<.000).
- •
The main cause of kidney disease in both groups was diabetes, followed by hypertension.
- •
The overall mean follow-up was 1407±565 days.
Baseline characteristics.
| TRS n=305 (50.6%) | No TRS n=297 (49%) | Total N=602 | P | |
|---|---|---|---|---|
| Sex (male) | 207 (68.5%) | 166 (56%) | 377 (62%) | |
| Age (years) | 63.6±13.9 | 67.3±11.2 | 65.4±12.8 | .00036 |
| Renal failure-related death | 0 | 3 (1%) | ||
| Cause of ERC | .04 | |||
| Diabetes | 79 (26%) | 51 (17%) | 130 (22%) | |
| HTN | 47 (15.4%) | 36 (12%) | 83 (14%) | |
| Glomerular | 39 (13%) | 28 (9.5%) | 67 (11%) | |
| Multifactorial | 26 (8.5%) | 34 (11.5%) | 60 (10%) | |
| NTIC | 27 (9%) | 48 (16%) | 75 (13%) | |
| Unaffiliated | 40 (13%) | 51 (17%) | 91 (15%) | |
| eGFR onset (ml/min/m2) | 19.37±5.8 | 23.37±5.6 | 21.32±6.07 | .000 |
| CAC (mg/g) | 1175±1563.4 | 436±721.6 | 812.2±1278.4 | .000 |
| Bicarbonate (mmol/l) | 23.8±4.06 | 24.8±3.46 | 24.27±3.8 | .01 |
| Calcium (mg/dl) | 9.3±0.72 | 9.42±0.72 | 9.35±0.73 | .02 |
| Albumin (g/dl) | 6.9±9.78 | 7.7±46.47 | 7.29±33.4 | .77 |
| Phosphorus | 3.99±0.82 | 3.88±2.26 | 3.93±1.69 | .42 |
| Follow-up time (days) | 1.002.5±501 | 1.407±565 |
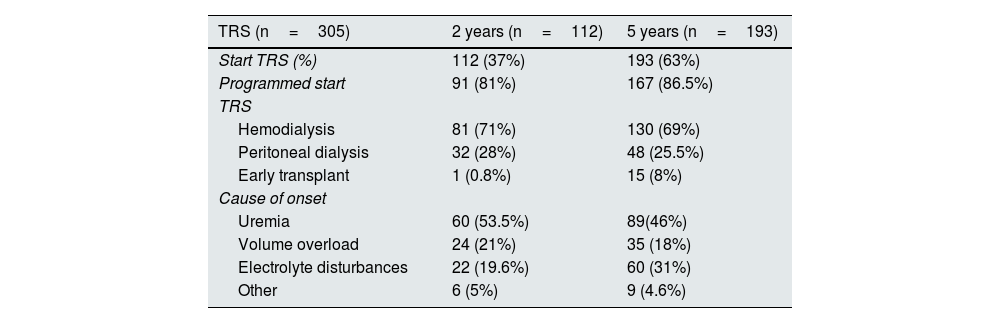
Of the 305 patients who started RRT, 112 (37%) did so at 2 years and 193 (63%) at 5 years (Table 2). Hemodialysis was the main starting procedure in both groups (70%), with programmed initiation in approximately 80% of patients. The main cause of initiation was the presence of uremic symptoms (50%), followed by volume overload (20%).
Baseline characteristics of the renal replacement therapy group.
| TRS (n=305) | 2 years (n=112) | 5 years (n=193) |
|---|---|---|
| Start TRS (%) | 112 (37%) | 193 (63%) |
| Programmed start | 91 (81%) | 167 (86.5%) |
| TRS | ||
| Hemodialysis | 81 (71%) | 130 (69%) |
| Peritoneal dialysis | 32 (28%) | 48 (25.5%) |
| Early transplant | 1 (0.8%) | 15 (8%) |
| Cause of onset | ||
| Uremia | 60 (53.5%) | 89(46%) |
| Volume overload | 24 (21%) | 35 (18%) |
| Electrolyte disturbances | 22 (19.6%) | 60 (31%) |
| Other | 6 (5%) | 9 (4.6%) |
To assess the risk of RRT and compare it with the actual event rate, the models with the lowest bias were analyzed, as described in Appendix B Supplementary Material 2.
- •
Event at 2 years: the 4-variable weighted KFRE equation showed better predictive ability with an AUC of 0.766, Brier Score of 0.196, bias of 0.29 and precision of 0.84, compared to the original KFRE. The weighted 8-variable KFRE equation also showed lower bias (0.35), higher precision (0.89) and an AUC of 0.68 versus the original KFRE (Appendix B Table 1 of Supplementary Material).
- •
Event at 5 years: the weighted KFRE equation showed lower bias in both the 4-variable version (bias of 0.01, precision of 0.64) and the 8-variable version (bias of 0.07, precision of 0.72) (Appendix B Table 1 of Supplementary Material 2).
To identify the model with the best discriminative ability, we compared the 4- and 8-variable weighted KFRE models at 2 and 5 years (Appendix B Table 2 of Supplementary Material 2):
- •
At 2 years: the 4-variable weighted KFRE had a higher AUC (0.763; 95% CI 0.71−0.81) as compared to the 8-variable weighted KFRE (AUC 0.682; 95% CI 0.62−0.73) (P<.0001).
- •
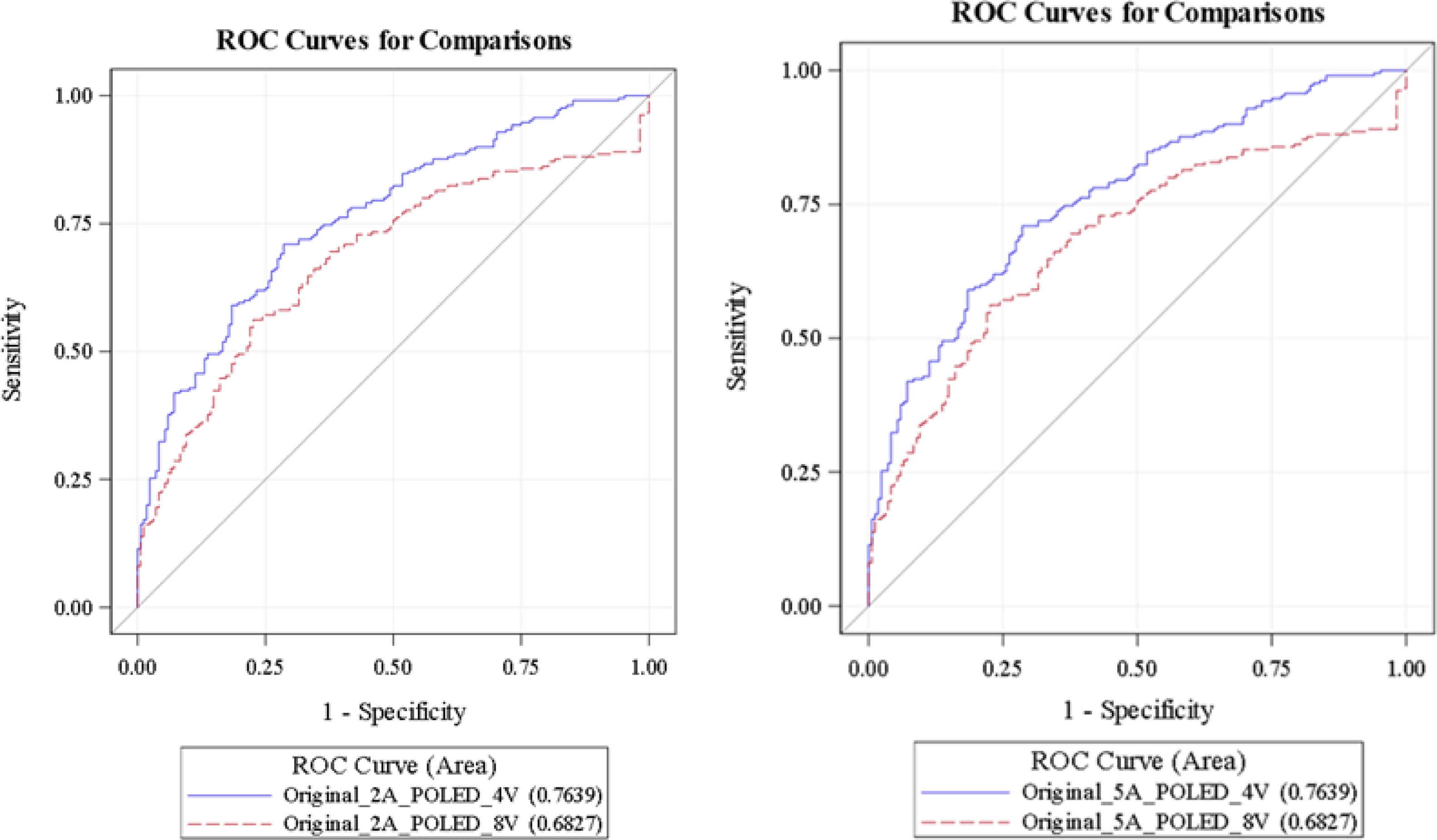
At 5 years: similarly, the 4-variable weighted KFRE showed a higher AUC (0.7639; 95% CI: 0.71−0.81) as compared to the 8-variable weighted KFRE (0.682; 95% CI: 0.62−0.73) (P<.001) (Fig. 2).
GFR<=20ml/min:
- •
Event at 2 years: the 4-variable weighted KFRE showed better predictive ability (AUC 0.733, Brier Score 0.202, bias 0.29 and precision 0.84) compared to the original KFRE. The 8-variable weighted KFRE also showed lower bias (0.35), higher precision (0.89) and an AUC of 0.656 versus the original KFRE.
- •
Event at 5 years: the weighted KFRE showed lower bias in the 4-variable version (bias 0.01, precision 0.64) and in the 8-variable version (bias 0.07, precision 0.72). The weighted 4-variable KFRE had a higher AUC (0.752, 95% CI 0.682−0.823) compared to the weighted 8-variable KFRE (AUC 0.656, 95% CI 0.578−0.736) (P=.0017).
GFR>20ml/min:
- •
Event at 2 years: the 4-variable weighted KFRE showed better predictive ability (AUC 0.673, Brier Score 0.277, bias 0.29 and precision 0.84 compared to the original KFRE. The 8-variable weighted KFRE also showed lower bias (0.35), higher precision (0.89) and an AUC of 0.662 versus the original KFRE.
- •
Event at 5 years: The weighted KFRE showed lower bias in both the 4-variable version (bias of 0.01, precision of 0.64) and the 8-variable version (bias of 0.07, precision of 0.72). There were no significant differences in terms of the AUC of both models for events at 2 and 5 years (P=1.000).
Analysis of the ROC curves showed a superior predictive capacity of the 4-variable KFRE model compared to the 8-variable model, both at 2 and 5 years. At 2 years the area under the curve (AUC) was higher in the 4-variable model (0.7639; 95% CI: 0.71−0.81) versus the 8-variable model (0.682; 95% CI: 0.62−0.73), suggesting that the 4-variable model discriminates better among patients who will progress to ESRD in this period. This pattern endured at 5 years, with a consistent difference in AUC (0.7639 vs. 0.682).
DiscussionThis study validates the predictive capacity of the 4-variable KFRE formula in Spanish patients with ACKD, demonstrating better performance as compared to the 8-variable model. The ROC curve showed an AUC of 0.7639, both at 2 and 5 years, which reinforces the utility of this simplified model as a practical and efficient tool for risk stratification of progression to RRT. This finding is consistent with previous studies that evaluated the performance of the KFRE model in other populations, such as the UK analysis, where an AUC above 0.9 was observed, but with a need for recalibration to adjust for overestimation of risk in low-risk patients.14 Similarly, in the Portuguese cohort the 4-variable model had a high AUC (0.903 at 2 years), although we identified challenges in terms of specificity in certain groups.16
In our Spanish population, the overall predictive capacity of the KFRE was limited, with an AUC of less than 0.8, which underscores the importance of recalibrating the formula to adjust it to the characteristics of each population.17,18 Furthermore, in patients with ACKD (GFR<20ml/min/1.73m2), although useful, the KFRE formula does not fully capture clinical, social and emotional factors that influence the decision to initiate RRT, such as the presence of uremic symptoms or volume overload.19 This is consistent with previous observations that the KFRE tends to overestimate risk in elderly patients due to the competing risk of death before reaching renal failure.20,21
Shared decision making (SDM) is an essential approach, integrating not only biomedical factors, but also patient preferences, quality of life and personal goals. Clinicians could complement tools such as the KFRE with personalized measures, such as the Bansal index, to guide individualized decisions, especially in frail elderly patients where quality of life may prevail over prolongation of life.19 A Japanese study by Okada et al. suggested that including dynamic biomarkers, such as annual changes in GFR and proteinuria, significantly improves prediction, indicating a direction for future research.17 In addition, artificial intelligence (AI)-based approaches, such as those proposed by Bai et al., have shown comparable or superior performance to KFRE, allowing integration of multiple clinical variables and dynamic biomarkers, which could address current limitations of sensitivity and specificity in specific subgroups.22
Involvement and planning for initiation of renal replacement therapyA positive aspect of this study is that more than 80% of patients started RRT in a planned manner, which highlights the effectiveness of ACKD consultations in treatment planning. This finding is crucial, since planned initiation, especially in patients starting hemodialysis, is associated with better clinical outcomes, such as less use of temporary catheters and greater preparation of permanent vascular access. This result is consistent with the review by Smart et al. who showed that early referral to nephrology significantly reduces initiation with temporary vascular access (RR 0.47, 95% CI 0.45−0.50).23
The proportion of scheduled starts in our cohort exceeds international standards, where unscheduled start rates range from 24% to 49%.24,25 This underscores the importance of implementing predictive tools such as the KFRE, along with proactive strategies to optimize patient preparedness and clinical outcomes. However, there is also a clear need to incorporate additional variables, such as psychosocial or contextual factors, to improve its accuracy. Prospective studies will be essential to validate these adjustments in the Spanish population and to explore advanced approaches, such as machine learning models, that address the complexity of clinical management in ACKD.
Strengths and limitations of the studyStrengths:
- •
Validation in the Spanish population: this is one of the first studies to validate the KFRE in a multicenter cohort of patients with ACKD in Spain, which provides specific and relevant data for this population.
- •
Clinically relevant approach: the selection of patients who are candidates for RRT reflects standard practice in nephrology, ensuring that the findings are applicable in the real clinical context.
- •
Comparative evaluation: we analyzed different versions of the KFRE equation (4 and 8 variables) and performed subgroup analyses according to the eGFR, which allows more detailed evaluation of its predictive performance.
- •
Use of robust measures of predictive performance: we included metrics such as Brier Score, bias and precision, in addition to the AUC of the ROC curve, which reinforces the validity of the results.
Limitations:
- •
Retrospective nature of the study: the inclusion of patients based on clinical registries may have introduced selection biases and limitations in the standardization of criteria for the indication of RRT.
- •
Exclusion of patients who died before follow-up: this may have generated a survival bias, underestimating the impact of mortality on renal disease progression.
- •
Absence of objective criteria for the indication of RRT: the clinical decision to initiate RRT was by the treating physician in consensus with the patient, without a unified protocol, which could affect the comparison with previous studies.
- •
Need for recalibration of the model: although the KFRE equation showed good performance, the AUC values in this population were lower than those reported in international studies, indicating the need for further adjustments to improve its accuracy in Spanish patients.
- •
Lack of integration of competing events: the main analyses did not consider mortality as an alternative outcome, which could have limited the complete risk assessment in this population.
The 4-variable KFRE formula proved to be a useful and practical tool for risk stratification of progression to SRT. However, its implementation in clinical practice requires adjustments to maximize its applicability in the Spanish context. This study highlights both the strengths and limitations of the model, stressing the need to combine it with comprehensive clinical strategies in ACKD consultations.
CRediT authorship contribution statementBeatriz Escamilla and Marco Montomoli share lead authorship of this study.
FinancingThe study was funded in part by grants from the Instituto de Salud Carlos III, Madrid, Spain (ICI21/00042 and Red RICORS RD21/0005/0012), FONDOS FEDER and FIISC (PFIISC23/12).
The authors declare that they have no conflicts of interest.