Introducción: La bioimpedancia espectroscópica multifrecuencia (BIS) es un método preciso y objetivo para estimar la composición corporal y el estado de hidratación, que podría ser de utilidad en el tratamiento de la enfermedad renal crónica avanzada (ERCA). Objetivos: Los objetivos del presente estudio fueron determinar la prevalencia de las alteraciones del estado de hidratación estimadas por BIS y su relación con las características clínicas y bioquímicas, y establecer el valor de predicción del ángulo de fase a 50 kHz (AF) sobre la mortalidad. Pacientes y métodos: Se estudiaron 175 pacientes con ERCA. Para la medición de la BIS se utilizó un monitor BCM Fresenius. Los resultados del estado de hidratación (desviación con respecto a la teórica hidratación normal) fueron correlacionados con las principales características clínicas y parámetros bioquímicos. Tras un seguimiento prospectivo (mediana seguimiento = 481 días), se establecieron los mejores determinantes de la mortalidad mediante regresión de Cox, incluyendo entre otras covariables el AF. Resultados: El 85% de los pacientes tenían un estado de hidratación dentro de unos límites de ± 5% agua corporal total. El estado de hidratación se correlacionó con las características clínicas (edemas e hipertensión arterial severa), y con algunos parámetros bioquímicos, entre los que destacaba la asociación negativa con la albúmina plasmática, el índice de masa corporal y el cociente Na/K en orina. Durante el período de seguimiento fallecieron 16 pacientes (9%). Los mejores determinantes de la mortalidad fueron: índice de comorbilidad (HR = 4,304; p = 0,001), y el ángulo de fase (por cada grado; HR = 0,491; p = 0,026). Conclusiones: En conclusión, la estimación de la composición corporal y el estado de hidratación por BIS aportan información útil en la ERCA que podría ayudar a su tratamiento. El AF es un predictor independiente de mortalidad a corto plazo.
Introduction: Body composition assessment has the potential to improve the care of patients with chronic kidney disease (CKD). Whole-body multiple-frequency bioimpedance spectroscopy (BIS) appears to be a useful and appropriate technique for assessing hydration status and body composition in CKD patients. Objective: The aims of this study were to determine the hydration status by BIS in patients with advanced CKD, and to analyse the association of body fluid status with common clinical and biochemical characteristics. The prognostic value of the phase angle at 50KHz (PA) was also evaluated. Patients and methods: The study group consisted of 175 patients (66±14 year, 77 females) with eGFR<40ml/min not yet on dialysis. Body composition was assessed by BIS (BCM, Fresenius). Hydration status was expressed as a percentage of the total body water (TBW). Patients were prospectively followed-up for a median of 481 days, and the main determinants of mortality were estimated by Cox regression analysis. Results: The majority of patients (85%) showed a hydration status within ±5% TBW. Patients with oedemas or uncontrolled arterial hypertension showed mean estimate fluid overload significantly higher than that of the other study patients. Fluid overload was negatively associated with serum albumin levels, body mass index and urinary sodium/potassium ratio; and positively with male gender and diabetes. During the follow-up period, 16 patients died (9%). The main determinants of mortality adjusted for other potential covariates were: Davies comorbidity index (HR=4.304; P=.001), and PA (per each º; HR=0.491; P=.026). Conclusions: BIS may help identify changes in hydration status in CKD patients not fully appreciated by clinical or biochemical assessment. PA was a significant predictor of mortality in these patients.
INTRODUCTION
Knowing body composition is considered a valuable diagnostic tool in many medical specialities.1-3 It is becoming easier to diagnose the different changes in the status of nutrition and hydration as methods that estimate body composition are developed.1-3 These changes are relevant in nephrology, and especially in chronic kidney disease (CKD), where wasting-malnutrition4 and over-hydration5,6 are processes that significantly effect the vital prognosis of these patients. Furthermore, conventional diagnostic tests based on clinical and biochemical examinations are very inaccurate.
There are currently many methods to estimate body composition,2,7,8 with the following being the most important: isotopic dilution, density assessment (weight underwater or plethysmography), anthropometry, DEXA (dual energy X-ray absorptiometry), body imaging (magnetic resonance [MR] or computerised tomography [CT]), and bioimpedance (BIA).
BIA is the most widespread method for estimating body composition due to its safety, ease of use, low cost and portability.7-10
BIA is based on the principle of bioelectrical impedance (the vector sum of resistance and reactance).10 Although monofrequency BIA (50kHz) has been the most used method to date, multi-frequency BIA spectroscopy (BIS) has arisen as a method with more developed and complex theoretical bases. The aim of this second method is to estimate total body water (TBW) more accurately and also to measure the distribution of water between the intra- and extracellular water spaces.7,11,12
There is extensive experience with the use of BIA in patients undergoing haemodialysis and peritoneal dialysis.3,7,13-15 The parameters obtained with this procedure are very useful for monitoring lean mass, body fat and hydration status. However, these parameters have still not been used extensively in patients with advanced chronic kidney disease (ACKD) who are not undergoing dialysis. It is therefore unknown how useful they are.16-19
This study had the following objectives: 1) determine body composition using BIS in ACKD patients who are not undergoing dialysis; 2) analyse the prevalence of changes in hydration status and how they are linked with common signs and symptoms in ACKD such as oedemas, cramp or uncontrolled arterial hypertension (AHT), as well as studying other clinical and biochemical characteristics associated with hydration status, and 3) establish the prognostic value of a phase angle at 50kHz, which is a parameter that has been previously referred to as a determinant of a worse disease course in patients with CKD20,21 and patients with other chronic diseases.22,23
PATIENTS AND METHODS
Patients
A total of 175 patients were included in this study (mean age 66±14 years old, 77 women and 98 men). Patients were selected during the period from 1 September 2009 and 1 February 2010 in ACKD outpatient visits of Infanta Cristina University Hospital, Badajoz, Spain.
Inclusion criteria were as follows: patients over the age of 18 with chronic renal failure not secondary to failure of kidney transplantation with estimated glomerular filtration rate (eGFR) below 40ml/min/1.73m2 who were not on dialysis, absence of acute intercurrent condition that could potentially have an effect on the state of hydration or nutrition, absence of pacemakers or metallic intravascular devices, consent after being informed on the aims and safety of the test, patient mobile enough to be able to be examined in bed, and absence of amputations or other extreme alterations of the body composition (morbid obesity or emaciation).
All of the examinations were performed in the morning after blood was taken for the laboratory analyses and after having had a small breakfast. Room temperature oscillated between 22ºC and 24ºC when the test was performed.
The clinical data were obtained from the patient’s history and medical records. The presence of oedemas in the patients’ legs was confirmed by at least two observers before the results of the BIS were known. The final results were included in the study as presence/absence of oedemas, without determining how serious they were.
The information on the presence or absence of cramps was obtained by looking at the patient’s medical records. This was classed as positive if the symptoms were common (more than three times per week) in the arms or legs during the day or at night.
Uncontrolled AHT was defined as systolic blood pressure (SBP) or diastolic blood pressure (DBP) over 150/190mm Hg in over half of the outpatient or home measurements in patients who had been prescribed at least three antihypertensive drugs.
Blood pressure at rest was measured in all the patients with an Omron M3 automatic device and the results were included in the study.
Each patient included in the study was weighed with a calibrated digital scale and measured. The body mass index (BMI) was calculated using the conventional Quételet formula (kg/m2). No other anthropometric measurements were carried out.
Laboratory analysis
Blood was taken from all the patients for the haematological (blood count) and biochemical study after overnight fasting and on the same day as the BIS examination. This included the following parameters, among others: urea, creatinine, albumin (bromocresol purple method), sodium, potassium, chlorine, calcium and phosphorus. The biochemical measurements were made with an auto-analyser (Advia Chemistry, Siemens Healthcare Diagnostics). The level of bicarbonate in venous blood was also measured (ABL 800 FLEX blood gas analyser, Radiometer Ibérica).
The following biochemical parameters were analysed in 24-hour urine collection before the analytical study: urea, creatinine, proteinuria, sodium and potassium.
The eGFR was estimated using the MDRD-4 formula.24
Multi-frequency bioimpedance spectroscopy
A body composition monitor was used for this test (Body Composition Monitor, Fresenius) and the Fluid Management Tool version 3.1.11 software programme was used to calculate the different parameters. With an emission frequency range between 5 and 1000kHz, the intracellular and extracellular space was measured using the Cole-Cole model25 and the body composition spectroscopy equations.12
The patients removed their shoes and socks and any metallic elements and laid down on the bed on a non-conductive surface. The patients made sure that their limbs were sufficiently far apart so that there was no contact between them. If the limbs could not be completely separated due to the patient’s anatomical characteristics, a draw-sheet was placed between the legs. The electrodes were stuck to the skin on the back of the hand and wrist, and on the back of the foot and ankle.
The following parameters were collected for the study: hydration status or deviation from normal hydration (± in litres), total body water (l), intracellular water, extracellular water, total lean mass (kg), total body fat (kg), total cell mass (kg) and phase angle at 50kHz (º).
The hydration status was standardised to total body water and extracellular water and was expressed as percentages of these parameters.
The phase angle was calculated from resistance and reactance26 according to the formula:
Phase angle = arc-tangent reactance/resistance x180º/π
At a frequency of 50kHz, body impedance always has a resistance and reactance component with small coefficients of variance. The variability of these parameters can be a lot greater if a current of higher frequency is used. Thus, 50kHz frequency was chosen to measure reactance and resistance in order to calculate the phase angle.
Study design and statistical methods
The study has two parts. The first one is a transversal descriptive section which shows the parameters of hydration and their association with the clinical and biochemical characteristics. After a prospective follow-up, the determinants of mortality were analysed, including the 50kHz phase angle at the start of the follow-up, among other parameters.
The patients were followed up after the initial assessment with regular medical visits every 1-3 months while they continued in ACKD consultation, or by communicating any change in their evolution after starting dialysis. Patients were removed from the study if they died for any reason or at the end of the study period (1 February 2011).
The median follow-up until death or removal from the follow-up was 481 days (interquartile range: 435-505 days).
Student’s t-test was used to compare the means of the continuous variables with normal distribution for related and independent samples. Non-parametric tests were used to compare the continuous variables without normal distribution, such as the Mann-Whitney test for independent samples.
Univariate and multivariate linear regression models were used to measure how far the continuous variables were associated. Automatic backward stepwise regression analyses were used to select the best predictor variables.
Cox multivariate proportional hazard models were used to establish if there was an independent association between the phase angle and survival of the patients included in the study. The hazard ratios and their 95% confidence intervals were also calculated. The phase angle parameter was also analysed as a continuous variable in the Cox regression models, and as a discrete variable (above or below the median) in the univariate survival analysis with Kaplan-Meier curves. The covariables included in the analysis that were potentially related with mortality were: age, sex, comorbidity index (Davies index27), BMI, haemoglobin, serum albumin, serum phosphorus, proteinuria, baseline glomerular filtration rate, diagnosis of diabetes, SBP and DBP.
There was less than 0.5% missing values in all the variables. The quantitative variables lost were replaced with the value of the arithmetic mean of the rest of the data present.
The results were expressed as mean or median and standard deviation or ranges, respectively. A value of P<.05 was considered to be statistically significant. SPSS version 15 was used for the statistical analysis and to create the graphs.
RESULTS
Hydration status and clinical associations
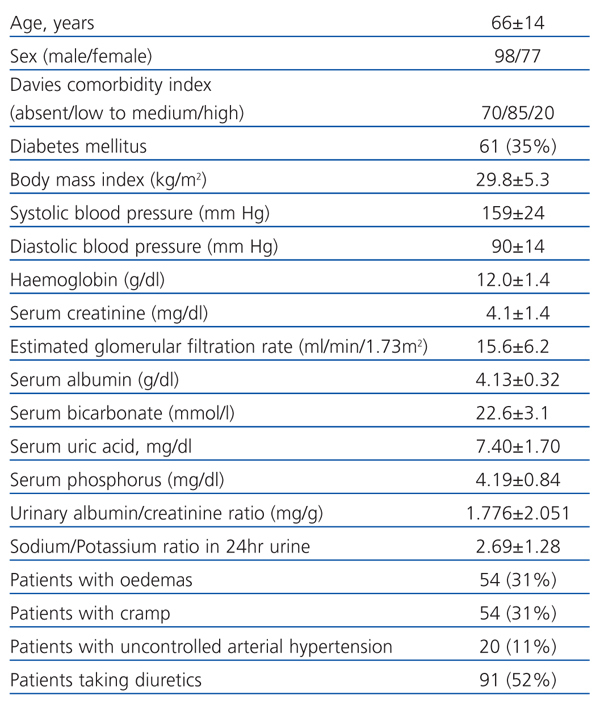
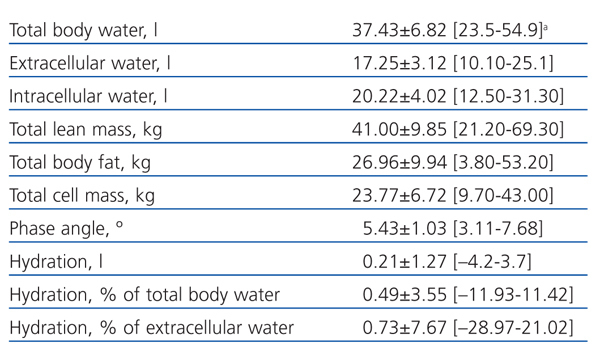
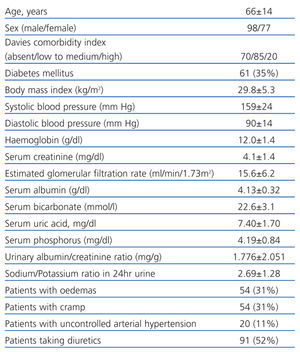
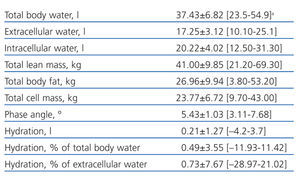
Table 1 shows the clinical and biochemical characteristics of the patients included in the study. Table 2 shows the parameters obtained using BIS.
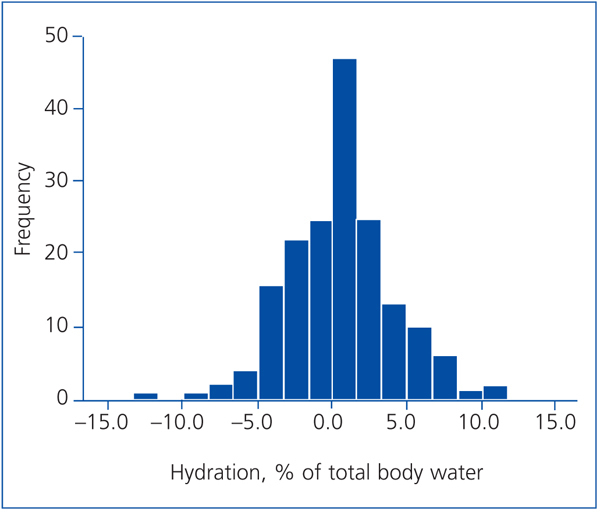
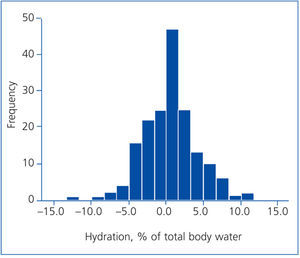
The frequency histogram (Figure 1) of the variation in hydration status (%) with regard to total body water shows that most of the patients’ hydration status was within normal ranges. Very few of them had overhydration or dehydration over 5% of total body water (19 and 8 patients, respectively).
Patients with oedemas in the physical examination had a significantly higher hydration status by BIS than those who did not have oedemas (1.51±3.78 compared to 0.04±3.36% TBW; P=.001 Man-Whitney test). However, there were no significant differences in the hydration status between patients who did and did not have cramps (0.53±3.54 compared to 0.48±3.57% TBW).
The 20 patients with uncontrolled AHT, according to the criteria used in this study, had a significantly higher hydration status than those that had their blood pressure under control (2.36±4.01 compared to 0.25±3.42% TBW; P=.003 Mann-Whitney test), although, as can be seen in Figure 2, poorly controlled blood pressure was also compatible with some theoretical dehydration.
The patients that were being treated with diuretics (52%) did not have any significant differences in their hydration status compared to those not being treated (0.85±3.68 compared to 0.11±3.38% TBW).
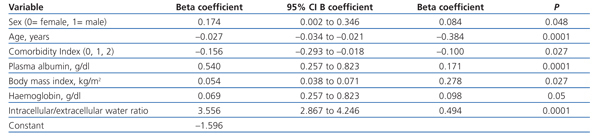
Using multiple linear regression (Table 3), the variables that were best associated with hydration status, expressed as percentages of TBW, were male gender and diabetes (greater overhydration), while BMI, urinary sodium/potassium ratio, plasma albumin and haemoglobin showed a negative association with overhydration.
Phase angle and mortality
The mean phase angle in the study group was 5.43±1.03º and the median was 5.30º.
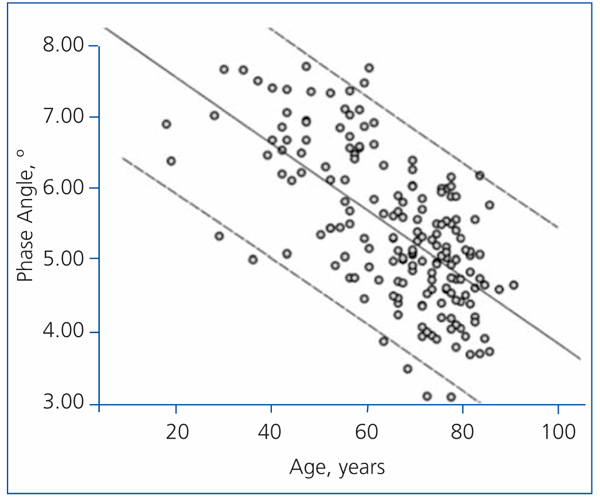
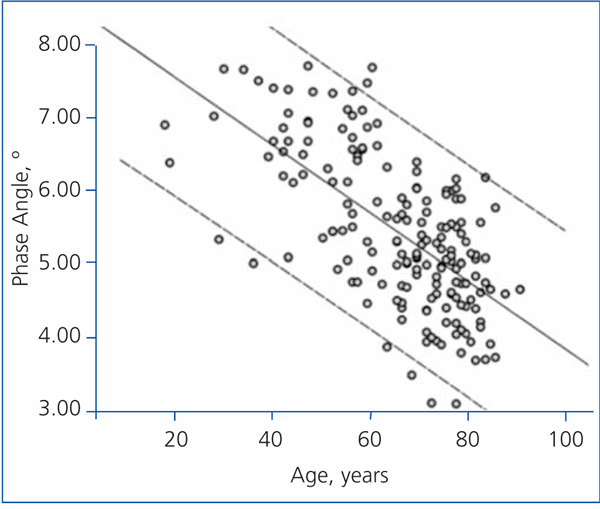
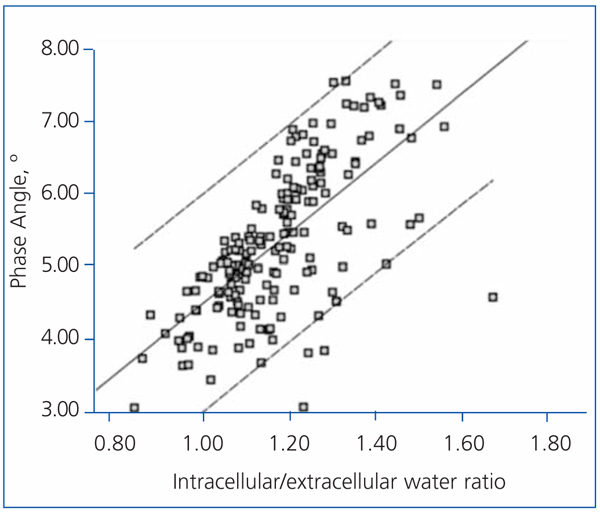
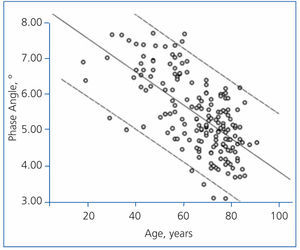
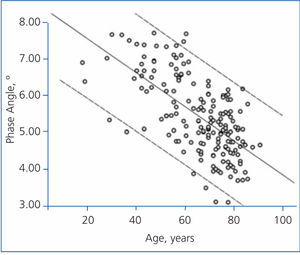
The best determinants of the phase angle using multiple linear regression are shown in Table 4. As you can see, the phase angle was associated with some well-known determinants of mortality in patients with chronic renal failure. Age was negatively associated with the phase angle (Figure 3), while serum albumin and the intracellular/extracellular water ratio (intracellular water divided by extracellular water in litres) had a strong positive association (Figure 4).
Sixteen patients (9%) died during the follow-up period. Two patients died suddenly in their homes, 6 due to heart problems, 1 for infectious pathology, 6 for cancer and 1 patient for liver failure. Two patients needed to start treatment with dialysis and no patients were lost to follow up.
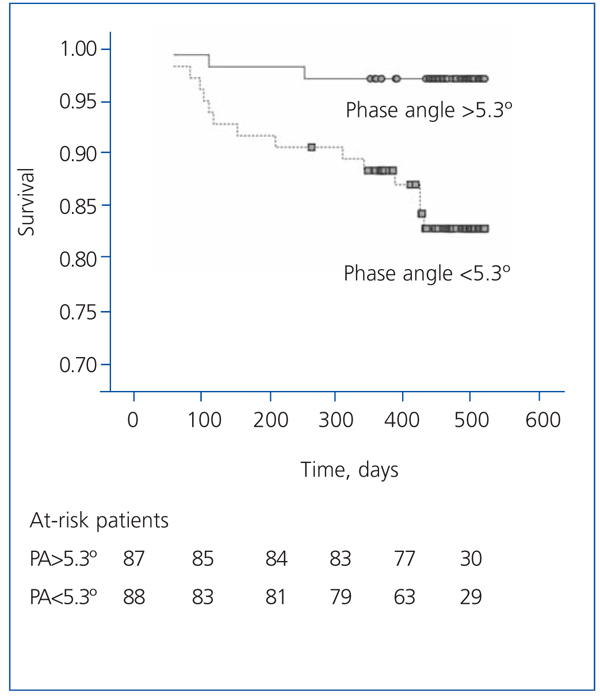
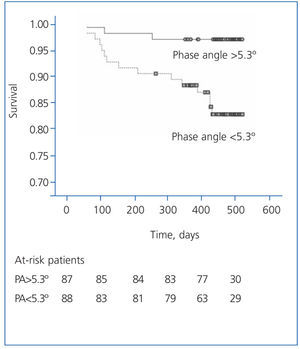
The patients with a phase angle above the median value (5.3º) had a higher survival than the other patients (Figure 5).
According to the Cox regression analysis, the best determinants of mortality were: comorbidity index (HR=4.304; 95% confidence interval [CI], 1.824-10 155; P=.001) and phase angle (per each º; HR=0.491; 95% CI, 0.263-0.917; P=.026). It is important to mention that age, as a covariable, was not part of the best predictive equation for mortality as usually happens, whereas the phase angle was.
DISCUSSION
The results show that most ACKD patients in this study had a hydration status with variations within ±5% of total body water. In spite of the small changes in the hydration status, this was correlated with the clinical characteristics (oedemas and severe AHT) as well the biochemical parameters. It is worth mentioning the negative association with plasma albumin, body mass index and urinary sodium/potassium ratio. Diabetic patients also tended to be more overhydrated.
Although BIA is a very widespread method for studying body composition in patients undergoing dialysis,3,7,13-15 it has been used a lot less in ACKD patients.16-19
A common finding in these predialysis assessments was the large proportion of patients that were overhydrated (>60%),18,19 in addition to changes in nutrition parameters, especially in lean mass16,17 and the decrease in the phase angle.16-19
Although the number of patients with uncontrolled AHT was low (20 patients), according to the criteria used in this study; the level of overhydration in this subgroup was significant compared to the other patients. These results suggest that determining the hydration status using BIS could be useful in making therapeutic decisions in cases with refractory arterial hypertension.28,29
The negative relation between hydration status and plasma albumin could be explained by haemodilution (e.g. greater extracellular volume, greater plasma volume, dilution and lower albumin concentration). In the same way, this situation could explain, at least partially, the inverse relation between hydration and the haemoglobin concentration.
In addition to haemodilution, hypoalbuminaemia secondary to nephrotic syndrome stimulates the positive balance between water and salt and expands the extracellular volume,30 although this does not always guarantee that intravascular volume will expand adequately.31 The association between overhydration and urinary sodium/potassium ratio (the lower this ratio is, the greater the level of overhydration) would support that hyperaldosteronism plays a role in the development of extracellular volume overload in these patients.
The negative association between BMI and hydration cannot be explained by biological and pathologic reasons. This may be related with interferences due to the large variations in body composition when estimating the extracellular/intracellular water ratio, which is the basis for calculating excess fluids.32
The phase angle is a parameter that is based on resistance and reactance.26 The biological significance that it has is still uncertain, but it could be an indicator of cell membrane integrity and distribution of intracellular and extracellular water.33,34 It may also show total cell mass and, therefore, play an important role in assessing the nutrition state,22,23,33,34 In accordance with these hypotheses, 50kHz phase angle values of the patients included in this study were correlated with age, distribution of body water, comorbidity index, BMI, serum albumin and haemoglobin. Therefore, the association was always the same as the prognostic significance that each of these variables had.
The phase angle value was related with vital prognosis not only in patients on dialysis20,21,35 but also in patients with other chronic pathological conditions.22,23,33 In accordance with these observations, the phase angle along with the comorbidity index were the most important determinants of short-term mortality of the patients included in this study.
The predictive strength of the phase angle on mortality was greater than that of age. This surprising finding could be explained by the high mean age of the group and the short follow-up period, circumstances that meant that factors related with the severity of comorbidity or with biological age took on a greater role in prediction, as might be seen in the phase angle value.
This study has certain limitations. The study group might not represent the characteristics of all patients referred to an ACKD medical visit, as some patients with metallic endovascular devices, pacemakers or extreme alterations to their body composition were excluded. The selection bias could have influenced the prospective study results.
The follow-up period was very short (around one year) and this might have had an effect on the determinants of mortality, as has been reported previously. Furthermore, as the parameters obtained by BIS are subject to changes over time, its relationship may be more faithful in the short term than in the long term.
Although the main covariables that are potentially related with mortality in CKD were included in this study, we did not analyse any inflammation markers.
To conclude, estimating body composition and hydration status by BIS provides useful information for treating patients with ACKD. The 50kHz phase angle represents an overview of many prognostic factors, but it is an independent determinant of short-term mortality, even in elderly patient and patients with associated diseases.
Table 1. Clinical and biochemical characteristics of patients included in the study
Table 2. Parameters obtained using multi-frequency bioimpedance spectroscopy in patients included in the study
Table 3. Covariables associated with state of hydration (percentage of total body fluid) by multiple linear regression
Table 4. Covariables associated with phase angle by multiple linear regression
Figure 1. Frequency histogram showing the distribution of the hydration status expressed as a percentage of total body water
Figure 2. Box plot showing hydration status expressed as the percentage of total body water in patients with blood pressure suitably under control (n=155) and those that did not have blood pressure under control (n=20)
Figure 3. Linear regression (phase angle and age)
Figure 4. Linear regression correlating the phase angle and intracellular/extracellular water ratio (litres of intracellular water divided by litres of extracellular water).
Figure 5. Actuarial survival graph (Kaplan-Meier) for patients with a phase angle greater or lower than 5.3º (study group median)