The number of patients starting renal replacement therapy increases every year, with hemodialysis (HD) being the therapy with the highest incidence and second in prevalence, only behind renal transplantation.1 Over the years, the HD population has become progressively older and more comorbid, with a high mortality rate of around 13–14%, much higher than that of other forms of renal replacement therapy.1
Historically, the assessment of "adequate dialysis" has been based on the clearance of small molecular weight solutes. However, the 2019 KDIGO guidelines on dialysis initiation, the choice of dialysis modality, access and prescription of dialysis remind us to pursue patient-centered treatment goals having in mind the patient preferences, being required the use of multiple measures to assess dialysis adequacy.2
Aware of all these challenges, and with the aim of promoting collaboration between different centers and professionals to achieve true individualization in the prescription of HD, the In-center Hemodialysis Group of the Spanish Society of Nephrology has been formed recently. The aim of this article is to highlight the main aspects that form part of the individualization of prescription and the challenges of in-center HD for the coming years.
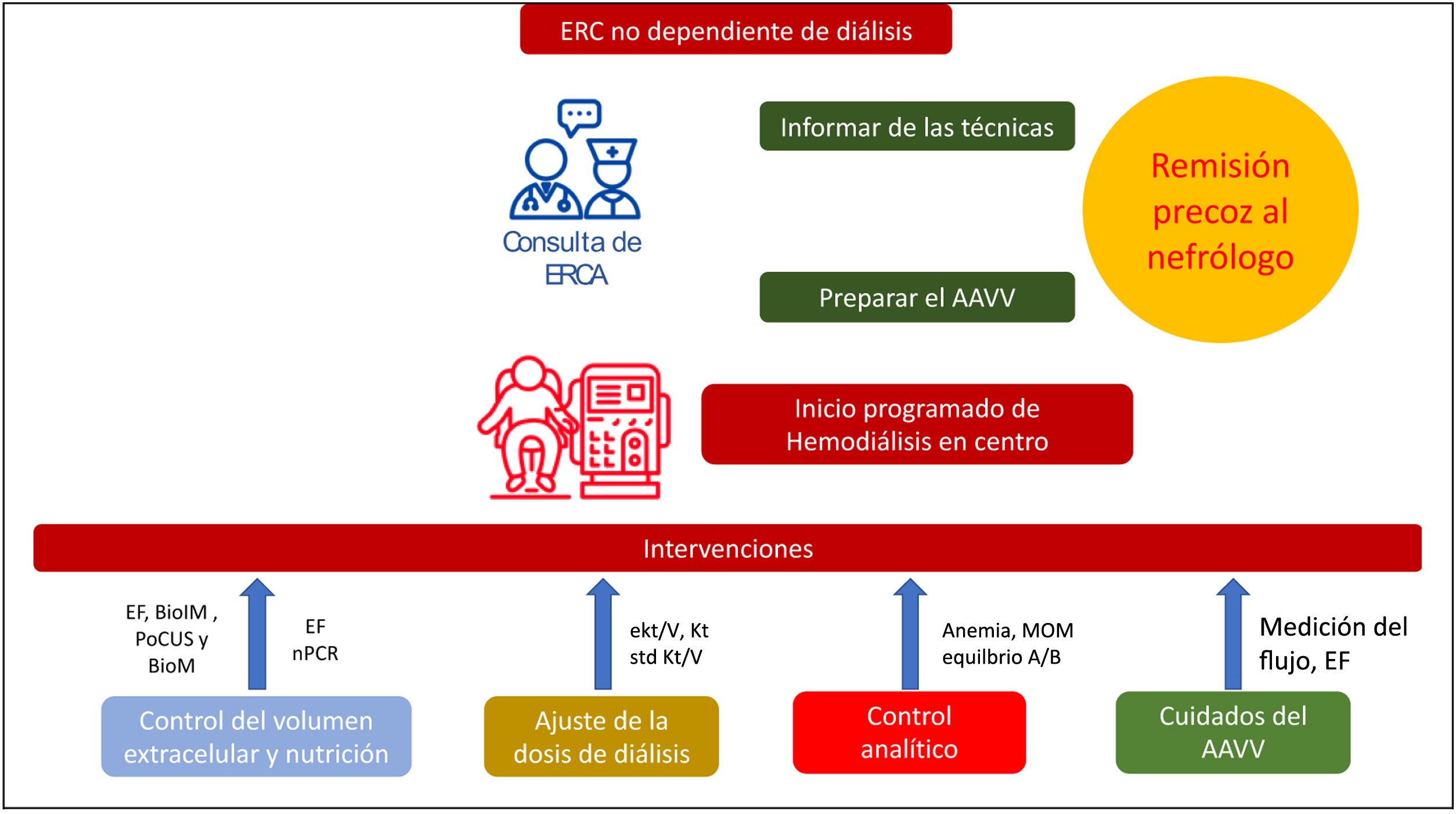
The path to carry out this individualization begins in the clinics of advanced chronic kidney disease, which have among other objectives the appropriate information on the different modalities of renal replacement therapy. For those who choose the modality of HD in a center, it continues to be a challenge to do so in a programmed manner with permanent and mature vascular access. Fig. 1 shows a schematic representation of the main care of the dialysis patient in which some of the aspects that form part of this individualization can be perceived.
Schematic representation of the main care of the hemodialysis patient.
VA: vascular access; A/B: acid-base; BioIM: bioimpedance; BioM: biomarkers of congestion; PE: physical examination; eKt/V: equilibrated Kt/V urea; CKD: chronic kidney disease; ACKD: advanced chronic kidney disease; Kt: sitting clearance; BMM: bone-mineral metabolism; nPCR: normalized protein catabolism rate; PoCUS: Point-of- care ultrasonography; std Kt/V: standard weekly Kt/V.
Despite the important technological evolution of HD, cardiovascular diseases are the main cause of mortality in this group of patients.3 In addition to the traditional risk factors, factors related to uremia and dialysis technique, such as arteriovenous fistulas, especially high flow, electrolyte gradients and interaction with membranes are additional mechanisms that facilitate the development of morphological and functional alterations in the heart,4 increasing the risk of cardiovascular pathologies such as heart failure, which appears in 20–40% of HD patients.5,6
Recently, congestion has become of special interest in HD patients as it is associated with high mortality7 and morphological alterations of the heart such as left ventricular hypertrophy, diastolic dysfunction, tricuspid regurgitation or right ventricular dysfunction.8 One of the greatest challenges when dealing with congestion in the HD patient is its difficult quantification by clinical parameters such as dry weight9 or even by bioimpedance.10 These, assessed in isolation, have a low sensitivity and their interpretation is complex, so the concept of multiparametric assessment of congestion emerges.11 This new vision includes the use of new tools such as point-of-care ultrasonography (PoCUS12 and the use of novel biomarkers of congestion such as carbohydrate antigen 125.13 The integration of clinical, ultrasound, biochemical
parameters and bioimpedance will make it possible in the future to personalize congestion and establish individualized decongestion strategies with the aim of achieving adequate ultrafiltration.
The art of ultrafiltrationIn recent years we have learned that depletion states due to high ultrafiltrations (>13 ml/kg/h) are also associated with increased mortality due to tissue ischemia and development of inflammatory states,14,15 which underscores the need for individualized volemia monitoring and ultrafiltration. HD monitors are equipped with biosensors and biocontrols based on ultrafiltration adjusted to intravascular volume, modification of conductivity, blood pressure, vascular filling rate, temperature modification, or oscillations in oxygen saturation. An adequate knowledge of the tools will be useful to improve the tolerance to ultrafiltration.
Therefore, in the "art of ultrafiltration" we must take into account several issues: it is necessary to objectively estimate the dry weight without forgetting to individualize it through clinical assessment, as well as recalculate it when the patient's situation changes. In addition, we must use all the tools provided by the monitors to carry out adequate ultrafiltration, avoiding excessive losses and ensuring adequate tolerance. Our objective in the next decade must be to continue incorporating all these biosensors into our routine practice.
Search for the ideal frequencyThe optimal number of weekly sessions that a patient should receive is currently unknown and is likely to vary depending on the patient's clinical situation.
Most patients receive 3 weekly sessions based arbitrary reasons and on the management of the dialysis unit. This frequency with a duration of 3−5 h per session is considered by the KDOQI/2015 Guidelines16 as "conventional HD program". However, this approach is questionable as it does not differentiate between incident or prevalent patients, with or without residual renal function (RRF).
In addition, in prevalent patients a higher number of sessions would be more similar to native renal clearance. Thus, programs with 5–7 weekly sessions are defined as frequent HD.16 However, this approach has obtained inconclusive results in several trials.17–20 Moreover, frequent HD is associated with a deleterious effect on vascular access and an accelerated loss of RRF.16
On the contrary, in incident patients it can be implemented an incremental HD program, starting with one or two weekly sessions depending on their renal urea clearance and quantifying periodically their RRF. The published data from the first trial with incremental HD are very promising,21 and there are other studies underway.22 We should also consider alternate-day HD programs (every 48 h), which avoid the long period and its high mortality23 or palliative or decremental dialysis, which shortens the sessions prioritizing the patient's well-being and quality of life. Thus, the optimal frequency should be dynamic, patient-centered, with 1–2 sessions at the beginning (if the patient maintains RRF), moving to conventional (3–4 sessions) or frequent (5–7) according to the need of the patient and decreasing their number in final stages to improve their quality of life.
Individualizing dialysis fluidDialysis fluid (DF) plays a crucial role in each HD session because depending on its composition, it will produce changes in the patient's blood that can have clinical consequences.
The composition of the DF is key to obtain the purification efficacy being pursued, without forgetting that it must be performed under optimal safety conditions.24 When prescribing the session, we must bear in mind what is our objective, according to the characteristics of each patient. We know that we can modify certain parameters in the DF, which allows us to improve the patient's tolerance to the session, such as sodium or potassium,25 or improve certain clinical parameters such as the control of bone- mineral metabolism, by means of changes in the concentration of calcium26 or magnesium.27
One of the key points in the individualization of the LOD is conductivity, since small changes can have relevant clinical consequences in the patient. Two of the chemical components essential for maintaining correct conductivity of the DF, and therefore the conductivity with which we dialyze the patient, are sodium3 and bicarbonate.28 Another element that will directly influence the conductivity of the LOD is temperature; in general, the specific conductivity increases by 2% of its value for each one-degree increase in temperature.
At present, it is possible to quickly, easily and effectively individualize the DF in each HD session for each patient. This possibility is a powerful tool available to us in our daily clinical practice, allowing us to improve tolerance to HD sessions without losing efficacy.
Choice of membranes - dialyzerThe evolution in the development of dialysis membranes has been really very productive and always in line with the technological evolution of dialysis monitors and modalities. Continuous improvement in these membranes, with better production systems, such as nanotechnology, have provided new generations of dialyzers with improved efficiency. Most high-flux dialyzers may be used in high-flux HD and hemodiafiltration modalities. Other membranes have the property of higher adsorption capacity, such as PMMA,29 being one of the membranes with the best survival results in HD modality in Japanese studies.30 As an alternative to synthetic dialyzers are the latest generation cellulosic dialyzers, such as cellulose triacetate, with similar efficacy and biocompatibility to synthetics. Even the latest generation can be used in hemodiafiltration techniques with high convective volume.31 At present we have an unsolved problem with synthetic membranes which is that in a small percentage (3%) they present moderate or severe adverse reactions, which disappear when changing to a cellulose membrane. It is important to identify the cause and triggering factors to avoid this complication.32
Recently, a new advance in membranes has been made by increasing the pore size, half-cut-off pores, which allow higher permeability without the need for convection.33 In fact, these dialyzers should only be used in HD, or as it has been called, extended HD.34
There is last step in which further progress could be made which is that of adding a second membrane (activated carbon or hydrophobic resins) to the diffusive and convective membranes to enhance adsorption. We refer to double filter dialysis with regeneration of the ultrafiltrate from the patient after passing through an adsorbing cartridge,35 or experiences coming mainly from China that are working with an adsorbing cartridge that they add to the conventional HD treatment.36
The use of adsorption for the removal of protein-bound uremic toxins, such as p-cresol sulfate and indoxyl sulfate, could be a useful tool to decrease morbidity and mortality in dialysis patients, since the elevation of their plasma levels has been associated with increased mortality and cardiovascular events in patients with chronic kidney disease.37
An aspect that is becoming increasingly relevant is the exposure of HD patients to bisphenol A, an environmental toxicant that forms part of the polycarbonate present in the casings or in the membrane of some dialysis filters, as is the case of polysulfone or polyester-polymer alloy.38 Several studies, among which we highlight a Spanish one, have shown that the use of polysulfone versus polynephron (without bisphenol A) was associated in these patients with an increase in blood levels of bisphenol A and, secondarily, in the levels of intracellular free radicals and circulating inflammatory markers (IL-6, TNF-α, C-reactive protein).39
Reversing fragilityDespite the progressive aging of the population, a better management of cardiovascular complications and an improved tolerance to the treatment thanks to technological advances have allowed greater flexibility in the inclusion in HD programs HD of increasingly older patients with greater comorbidity. These patients have an increased prevalence of frailty, considered a predictor of disability, hospitalization, falls, loss of mobility, cardiovascular disease and death.40 People with CKD are more predisposed to develop frailty (15–21%), especially those on HD (up to 73% depending on the tool used).41 Frailty is a biological syndrome of decreased reserve and resistance to stressors and may be associated with malnutrition, sarcopenia, dynapenia and other complications of CKD.42
This situation poses a change of scenario in HD patient care, being essential the systematic introduction of frailty screening, in order to identify those frail and pre-frail people, more vulnerable to the occurrence of adverse events so that they can benefit from preventive or therapeutic measures to reverse all or part of this state.
Despite the clinical implications of frailty, screening is currently not routinely performed in many HD units, perhaps because there is no consensus on which tool to use.43
Current frailty assessment tools can be divided into those based on physical frailty such as Fried's frailty phenotype,44 which focuses on functional assessment and is the most accepted definition at present, or multidimensional, the most widely used being the Frailty Index,45 derived from this, the Clinical Frailty Scale,46the Edmonton Frailty Scale47 or the FRAIL scale,48 validated for the dialysis population. The implementation of the use of these scales in daily clinical practice is not easy because some are time- consuming and require auxiliary instruments.
Vascular access and nutritional statusIt is well known that starting dialysis with a permanent access is associated with better patient outcomes, however, starting dialysis with a mature and functional access remains a challenge.
Although the benefits of fistulas are widely accepted,49 the 2019 KDIGO guidelines2 remind us that "fistula first" is not appropriate for all patients, the established paradigms for vascular access need to be reconsidered within the structure of the individual "life plan" of the patient with chronic kidney disease, taking into account, not only their vascular characteristics, but also their goals and preferences.
The prevalence of protein-energy wasting is much more frequent in dialysis (up to 20–50 %) than in pre-dialysis phases because the dialysis procedure induces a net protein catabolic state, influenced by the dialysis technique and a systemic inflammatory response in relation to the biocompatibility of the system.50 Given that nutritional status is one of the main treatable factors affecting the prognosis and evolution of patients with chronic kidney disease, the only way to prevent and treat these patients early is to perform an assessment of the nutritional status of patients with chronic kidney disease, if possible as early as the pre-dialysis stage. The simplest way is to carry out a 3-day dietary record (including dialysis, non-dialysis and holidays), but there are different validated tests in the dialysis population such as the Malnutrition Inflammation Score (MIS) or the Subjective Global Assessment, as well as new practical computer tools (www.nutrendial.cat)50 that allow us to easily calculate the prevalence of protein energy wasting (PEW) and compare it with these scores (MIS and SGA).
ConclusionIn-center HD has undergone important changes both from the technological point of view and in its concept of appropriateness, as well as in the profile of the patients it serves, making it essential to truly individualize dialysis prescription based on patient- centered treatment objectives.