Chronic kidney disease-associated pruritus (CKD-aP) is one of the most common and disabling comorbidities in patients with advanced CKD. In addition, it is associated with an increased risk of mortality, poorer quality of life, sleep disorders, mental health disorders, and increased use of health care resources. The clinical presentation of CKD-aP is very heterogeneous, making it difficult to diagnose and treat. Currently, there are no national guidelines on the management of CKD-aP.
The aim of this document is to provide national consensus recommendations for the diagnostic and therapeutic management of CKD-aP.
The document was prepared in three phases: a diagnostic and therapeutic management algorithm was proposed by a small group of nephrology specialists; the proposal was validated by a larger group of nephrologists; and a second validation by a multidisciplinary group that also included dermatology specialists.
The diagnostic and therapeutic management algorithm attempts to cover the current need of a lack of specific guidelines for the adequate management of CKD-aP. At the same time, it introduces the use of difelikefalin, the first and only drug specifically approved for CKD-aP, with a good safety and efficacy profile.
El prurito asociado a la enfermedad renal crónica (Pa-ERC), es una de las comorbilidades más comunes e incapacitantes en los pacientes con ERC avanzada. Además, está asociado a un mayor riesgo de mortalidad, peor calidad de vida, aparición de trastornos del sueño, alteraciones de la salud mental y mayor uso de recursos sanitarios. La presentación clínica del Pa-ERC es muy heterogénea, lo que dificulta su diagnóstico y su tratamiento. Actualmente, no existen guías a nivel nacional sobre el manejo del Pa-ERC.
El objetivo de este documento es proporcionar unas recomendaciones de consenso a nivel nacional para el manejo diagnóstico y terapéutico del Pa-ERC.
La elaboración del documento se realizó en tres fases: la realización de una propuesta de algoritmo de manejo diagnóstico y terapéutico por parte de un grupo reducido de especialistas en nefrología; la validación de la propuesta por un grupo más amplio de nefrólogos y, finalmente, una segunda validación por un grupo multidisciplinar en el que se incluyeron, además, especialistas en dermatología.
El algoritmo de manejo diagnóstico y terapéutico trata de cubrir la actual necesidad por una falta de directrices específicas para el control adecuado del Pa-ERC en España. A la vez, introduce el uso de difelicefalina, el primer y único fármaco aprobado específicamente para el Pa-ERC de pacientes en hemodiálisis, con un buen perfil de seguridad y eficacia.
Pruritus associated with chronic kidney disease (Pa-CKD) is one of the most common and disabling comorbidities in patients with advanced CKD.1–3 In Spain, between 51% and 59% of hemodialysis patients suffer from Pa-CKD.4–6 In addition, it is associated with a higher risk of mortality, worse quality of life, sleep disorders, mental health disorders and greater use of health resources.5,7 The clinical presentation of Pa-CKD is very heterogeneous, making it difficult to diagnose and treat.1,8
Currently, there are no specific guidelines for the management of Pa-CKD. At the European level, there is only the "European S2K Guideline on chronic pruritus" developed by the European Dermatology Forum (EDF) and the European Academy of Dermatology and Venereology (EADV).9 This guideline provides summarized evidence on therapeutic options for the different types of chronic pruritus (including Pa- CKD), and for each of the therapeutic options; an evidence-based expert recommendation is provided. Currently, there is no consensus on the most appropriate diagnostic and treatment algorithm for Pa- CKD.9 In Spain, Pa-CKD is mainly treated by nephrology specialists; however, the clinical practice guidelines for CKD published by the different Spanish nephrology societies do not include the management of Pa- CKD.
The lack of specific therapeutic options for Pa-CKD promotes the lack of consensus guidelines for diagnosis and treatment, which, in turn, leads to under-diagnosis of Pa-CKD and a suboptimal therapeutic approach.8
In April 2022, the European Medicines Agency (EMA) authorized the first drug indicated for Pa-CKD, difelicefalin, which has been shown to be effective in the treatment of Pa-CKD in two Phase 3 clinical trials (KALM-1 and KALM-2) (12-week duration) and in the additional 52-week open-label extension study in hemodialysis patients.10 The reduction in pruritus intensity translates into substantial long-term improvements in patients' health-related quality of life (HRQoL), as demonstrated in KALM-1 and its open-label extension (OLE) phase. In both studies (KALM-1 and KALM-2), a significantly higher proportion of patients treated with difelicefalin achieved an improvement of ≥3 points on the WI-NRS scale over placebo. Patients in the difelicefalin group during the double-blind phase also experienced sustained improvement in pruritus and in their HRQoL (5-D Itch Scale and Skindex-10). In addition, patients who received placebo in the double-blind phase and difelicefalin in the KALM-1 OLE had a mean change of 6.9 (95% CI −7.7 to −6.2) points on the 5-D Itch Scale from baseline to week 52 of the OLE phase.11
Difelicefalin is administered 3 times a week by rapid intravenous injection into the venous line of the dialysis circuit at the end of the hemodialysis treatment, during or after pumping the blood back to the patient. Thanks to its form of administration, at the end of the hemodialysis session, difelicefalin does not place an additional burden of visits on the patient, so it has a high potential for compliance. Moreover, it does not require additional monitoring, which simplifies its use. The recommended dose of difelicefalin is 0.5 µg/kg dry body weight (i.e., target weight after dialysis). The total dose volume (ml) required from the vial should be calculated as follows: 0.01 × dry body weight (kg), rounded to the nearest one-tenth (0.1 ml) (see data sheet).12
Thanks to the development of a specific drug for Pa-CKD with robust evidence on its efficacy and safety, the doors have been opened to greater knowledge and awareness of this pathology.
The aim of this consensus document is to provide national recommendations for the updated diagnostic and therapeutic management of Pa-CKD for hemodialysis patients.
MethodologyPhysicians specialized in nephrology and dermatology have participated in the development of the algorithm. To this end, several virtual coordination, discussion and consensus meetings have been held.
Based on an initial working outline, the experts divided the recommendations to be made into three sections: differential diagnosis, assessment of the severity of Pa-CKD and treatment of pruritus, which were subsequently reviewed and discussed by the entire group.
Each proposed recommendation was finalized after a literature search and, to a large extent, supported by the experience and opinions of experts. Once the final version of the algorithm had been validated by the entire Spanish Pruritus Group and dermatology specialists, the final document was drafted.
Following approval of the algorithm, the document has been submitted for public review by members of the Spanish Society of Nephrology (SENEFRO).
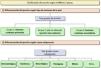
ResultsThe diagnostic and treatment algorithm for Pa-CKD is presented in Fig. 1. The recommendations developed by the group of experts are shown in Table 1.
Recommendations for the diagnosis and treatment of Pa-CKD.
| Phase | Recommendations | Level of evidence |
|---|---|---|
| Screening | 1. It is recommended that the initial screening be performed by the nephrologist or nursing professional and use only question 20 of the KDQoL-36: "In the last 4 weeks, to what extent have you felt discomfort caused by itching". | Expert opinion |
| Differential diagnosis | 2. It is recommended to perform a skin examination of the patient, at the clinic, to verify the presence of lesions on the patient's skin and to rule out those not attributable to Pa-CKD. | Expert opinion |
| 3. In case of the presence of primary skin lesions not attributable to Pa-CKD, it is recommended referral to dermatology. | Expert opinion | |
| 4. If no lesions are visualized or if they are secondary, it is also recommended to rule out other potential causes of active pruritus in the patient and if there are any, refer to the corresponding specialist. | Expert opinion | |
| 5. If there are no primary lesions or other causes associated with pruritus, it is recommended to consider the diagnosis of Pa-CKD. | Expert opinion | |
| 6. If Pa-CKD is diagnosed, it is recommended an assessment of the intensity and severity of pruritus using the WI-NRS and SADS questionnaires. | Expert opinion | |
| Registration | 7. It is recommended that the healthcare professional register the patient diagnosed with Pa-CKD in the computer system, using the coding of the pathology, with the tools currently available (ICD-9, ICD-10) until ICD-11 is available. | Expert opinion |
| Treatment and follow-up | 8. For the treatment of mild Pa-CKD, general measures such as skin hydration, maintenance of barrier function and other skin care techniques are recommended. In addition, adequate dialysis efficacy and control of calcium-phosphorus metabolism should be ensured. | Expert opinion |
| 9. For the treatment of moderate to severe Pa-CKD, it is recommended to use specific therapy for itching, being difelicefalin the only drug with specific indication approved for Pa-CKD in hemodialysis patients. | Based on clinical trials | |
| 10. For the treatment of moderate to severe Pa-CKD, the off-label use of gabapentinoids, mirtazapine and/or montelukast could be considered. | Expert opinion | |
| 11. Once treatment for Pa-CKD has been initiated, it is recommended that the intensity of pruritus be monitored (using the WI-NRS and SADS questionnaires) at least at 3 months. | Expert opinion | |
| 12. If after 3 months of treatment, the patient suffers severe side effects or no improvement, it is recommended to consider re-evaluating the treatment. | Expert opinion |
The questionnaires used for the diagnosis and assessment of the severity of pruritus (KDQoL-36 and WI-NRS and SADS, respectively) have been selected because they are simple to understand and easy to answer or being fulfilled by the patient.13–16
With regard to the diagnosis of chronic pruritus, it was decided to use only question 20 of the KDQoL-36 for the initial screening, since the answer obtained provides relevant and sufficient information for the screening: "In the last 4 weeks, to what extent have you felt discomfort caused by itching" (and as answers: none, some, quite a lot, a lot or very much). In this way, the presence and intensity of itching can be screened quickly and easily. This initial screening should be performed by the nephrology or nursing professional. If the patient responds "somewhat, quite a lot, a lot or very much" a differential diagnosis will be made by the nephrologist to rule out other possible causes. This screening will be performed every 3 months in patients in whom Pa-CKD has not been diagnosed.
Differential diagnosisThe following explains how to perform the differential diagnosis. First of all, it is necessary to verify whether or not skin lesions are present. These could be primary or secondary (Fig. 2). Although primary lesions are not attributable to Pa-CKD, secondary lesions may be related. To rule out the presence of such lesions not attributable to Pa-CKD, it is necessary to perform a skin examination of the patient, ideally in the consultation room in order to maintain the patient's privacy. It is also recommended to consult with the dermatologist. For more information on the type of lesions, see Appendix Annex 2. In case of presence of skin lesions not attributable to Pa-CKD, these should be treated by the dermatologist. In case no lesions are visualized or these are secondary, other potential causes of active pruritus in the patient should also be ruled out (e.g., hematological disease, liver/bile disease, neurological disease, psychiatric disease, neoplasia, drugs). In the case of other possible causes, the patient will be referred to the corresponding specialist to receive the most appropriate treatment. Finally, the diagnosis of Pa-CKD should be considered if there are no primary lesions or other causes associated with pruritus. It should be noted that, in the case of xerosis, despite being a primary lesion, it could coexist with Pa-CKD.
Classification of pruritus according to the IFSI in two steps.9
Once a potential patient has been identified, an assessment of the intensity and severity of pruritus is made using the WI-NRS and SADS questionnaires (Fig. 3). Patients should complete the WI-NRS questionnaire daily for one week, or at least 3 days in one week. For the evaluation of the results, the average of the evaluations of all days will be taken. Patients who obtain a mean score ≥4 points on the WI-NRS and categories B or C on the SADS will be categorized as patients with moderate-severe pruritus (Table 2).
Suggested assessment scales for measuring the severity of itching.14,15
If the patient is diagnosed with Pa-CKD, the healthcare professional involved should register it as a health problem or diagnosis in a structured and systematized manner. To do this, the detailed medical history of any patient with chronic pruritus should be collected first. This should include the general characteristics of the pruritus (e.g., duration, time course, location, intensity and quality), knowledge of the patient's medical history, including accurate information on medication changes in the last year and family history, as well as the performance of a complete dermatological examination.
Pathology coding is used to record the pathology in the computer system. To do this, it is necessary to classify and assign a specific code to this pathology.
Currently, in the international disease classification systems 9 and 10 (ICD-9 and ICD-10) there are no specific codes for Pa-CKD, but the disease classification system 11 (ICD-11), which will be implemented in hospitals in the coming years, does incorporate specific codes for Pa-CKD (EC90.1). Until ICD-11 is available, it is recommended that Pa-CKD be coded with the tools currently available (ICD-9, ICD-10), as shown in Table 3, indicating in the clinical history the association of pruritus with CKD. It is also possible to perform a direct mapping (Table 3), so when implemented in the hospitals of the National Health System it will improve the system of registration and diagnosis of patients with Pa-CKD.3,17,18
Diagnostic coding.
| Search coding by ICD | ||||||||
|---|---|---|---|---|---|---|---|---|
| CIE | Search | Tabular list | Select | |||||
| ICD-9-CM | 698.9 | Tab.D | Ch.12 | 690−698 | 698.9 | Unspecified pruritic disorders | ||
| ICD-10-CM | L29.9 | Tab.D | Ch.12 | L20-L30 | L29.9 | Itching, unspecified | ||
| Direct mapping | ||||||||
|---|---|---|---|---|---|---|---|---|
| Code | Classification origin | Destination classification | Select | |||||
| L29.9 | ICD-10-EN Diag. | ICD-9-CM | 698.9 Unspecified pruritic diseases | |||||
In the SNOMED CT terminology there is also a specific code for Pa-CKD: 403733004 - "Hemodialysis-associated pruritus",3 which could also be used in hospitals where it is in effect.
Treatment and follow-upRegarding the treatment of pruritus, patients with Pa-CKD will receive one or another therapy depending on the severity. For mild Pa-CKD, general measures will be used, such as skin hydration and maintenance of the barrier function (Table 4) and other skin care techniques, as well as other methods to reduce pruritus (Table 5). In addition, adequate dialysis efficacy and control of calcium-phosphorus metabolism should be ensured. In case the patient has moderate to severe pruritus (WI-NRS ≥4 or SADS B or C), specific itch therapy is recommended, with difelicefalin being the only drug with specific indication approved for Pa-CKD in hemodialysis patients. Difelicefalin is not yet marketed in Spain1. Since it is currently only available intravenously and in clinical trials it has been administered at the end of the hemodialysis session, it can only be applied to hemodialysis patients. Other off-label options to consider include gabapentinoids, mirtazapine and/or montelukasts. In a systematic review, 6 of the 7 included studies reported favorable results in decreasing Pa-CKD after gabapentin administration. However, it should be noted that the number of patients included in these studies is limited and most of them are under 65 years of age.19,20 In addition, important side effects have been reported, so the healthcare professional should consider the risk/benefit profile before using them (Table 6). Likewise, it should be taken into account that the use of gabapentinoids requires dose adjustments and monitoring; furthermore, there are safety alerts and problems of dependence, abuse and withdrawal syndrome in several countries (United States, United Kingdom, France and Spain).21–24 Another drug such as montelukast, a leukotriene receptor antagonist used in the treatment of asthma, allergic rhinitis, atopic dermatitis and idiopathic urticaria has shown favorable results in the reduction of Pa-CKD in 2 randomized placebo-controlled studies.25,26 However, as in the gabapentin studies, the number of patients in the 2 montelukast studies is limited and the mean age of patients is below 65 years, while the mean age of hemodialysis patients is mostly above 65.20 Mirtazapine is a serotonergic antagonist with antihistamine action that demonstrated efficacy in reducing the intensity of pruritus in a clinical trial with 77 patients treated initially with gabapentin and subsequently with mirtazapine.27 Finally, for refractory pruritus, phototherapy is recommended. However, it should be noted that there is currently no clinical evidence on the combination of treatments.
Emollients and moisturizers for pruritus associated with CKD.
| Pa-CKD Patient Skin Care Guide | - Moisturize skin daily or twice daily, especially after showers/baths. |
|---|---|
| - Apply emollients, especially at night: | |
| ◦ Creams | |
| ◦ Lotions | |
| ◦ Gels | |
| Considerations to take into account | - The term "moisturizer" includes emollients, humectants and occlusives: |
| - Emollients: improve skin barrier function, membrane fluidity and cell signaling, resulting in an overall improvement in skin texture and appearance. | |
| - Humectants: attract water to the stratum corneum. | |
| - Occlusives: form a layer on the skin surface to physically block evaporation of water from the skin. | |
| - Moisturizer and emollient are often used interchangeably; however, moisturizers often contain humectants to hydrate the stratum corneum of the skin. |
General advice for the management of pruritus associated with chronic kidney disease.
| Skin care |
| - Wear permeable and soft clothing, e.g., cotton; dress in several layers to avoid sweating. |
| - Keep room temperature low overnight |
| - Use mild, non-alkaline, fragrance-free soaps and bath oils. |
| - Moisturize skin daily, especially after showering/bathing. |
| - Apply emollients, especially at night. |
| - Creams/lotions/gels |
| - Apply refreshing wet wraps |
| - Limit baths to 20 min |
| - Use lukewarm water ± oatmeal or potassium permanganate; pat the skin dry. |
| Avoid |
| - Factors that may contribute to skin dryness |
| - Excitement, tension, negative stress |
| - Very hot and spicy foods, large quantities of hot beverages and alcohol |
| - Contact with allergenic and irritating substances |
| Others |
| - Relaxation techniques: Autogenic training, relaxation therapy, psychosocial education. |
| - Education: Coping with the vicious circle of |
Very frequent and frequent adverse reactions according to the technical data sheet of the drugs used to treat Pa-CKD.
| Gabapentin35 | Mirtazapine36 | Montelukast37 | Difelicefalin38 | |
|---|---|---|---|---|
| Infections and infestations | Viral infection, pneumonia, respiratory infection, urinary tract infection, infection, otitis media | |||
| Blood disorders | Leukopenia | |||
| Immune system disorders | Allergic reactions (e.g., urticaria) | |||
| Metabolic and nutritional disorders | Anorexia, increased appetite | Increased appetite, weight gain | ||
| Psychiatric disorders | Hostility, emotional turmoil and instability, depression, anxiety, nervousness, abnormal thinking | Abnormal dreams, confusion, anxiety, insomnia | ||
| Nervous system disorders | Drowsiness, dizziness, ataxia, seizures, hyperkinesia, dysarthria, amnesia, tremor, insomnia, headache, sensations such as paresthesia, hyposthesia, abnormal coordination, nystagmus, increased/decreased/absent reflexes | drowsiness, sedation, headache, lethargy, dizziness, tremor, amnesia | Headache | Drowsiness, paresthesia |
| Ocular disorders | Amblyopia, diplopia | |||
| Ear and labyrinth disorders | Vertigo | |||
| Vascular disorders | Hypertension, vasodilatation | Orthostatic hypotension | ||
| Respiratory, thoracic and mediastinal disorders | Dyspnea, bronchitis, pharyngitis, cough, rhinitis | |||
| Gastrointestinal disorders | Vomiting, nausea, dental abnormalities, gingivitis, diarrhea, abdominal pain, dyspepsia, constipation, dry mouth or dry throat, flatulence | Dry mouth, nausea, nausea, diarrhea, vomiting, constipation | Abdominal pain | |
| Skin and subcutaneous tissue disorders | Facial edema, purpura most often described as contusions resulting from physical trauma, rash, pruritus, acne | Exanthema | ||
| Musculoskeletal and connective tissue disorders | Arthralgia, myalgia, back pain, spasms, spasms | Arthralgia, myalgia, back pain | ||
| Renal and urinary disorders | Impotence | |||
| General disorders | Fatigue, fever, peripheral edema, abnormal gait, asthenia, pain, malaise, flu-like syndrome | Peripheral edema, fatigue |
Once treatment for Pa-CKD has been initiated, it is necessary to follow up the intensity of pruritus (by passing the WI-NRS and SADS questionnaires) at least at 3 months. If there are severe side effects or no improvement after 3 months, re-evaluation of treatment at this time could be considered. It should be considered that with some drugs the effect may not be immediate, and the results are maximized after the first 2 weeks, so it would not be recommended to abandon treatment before such an effect can be observed. However, dermatology specialists recommend taking the WI-NRS questionnaire daily/periodically, as far as possible, in order to observe the effect of the treatment continuously.
DiscussionTo date, diagnostic guidelines for Pa-CKD are scarce. There is no globally accepted method or diagnostic guideline for measuring Pa-CKD. Furthermore there is not a specific diagnostic code for Pa-CKD in ICD-10.28 This generates a situation of underdiagnosis and underreporting, resulting in global prevalence data of Pa-CKD between very wide ranges.8 It is necessary to promote the specific coding of Pa-CKD in order to have accurate data on these parameters in Spain.
There are no specific guidelines for Pa-CKD in Spain, and the CKD treatment guidelines do not include the management of Pa-CKD. This makes the therapeutic approach dissimilar and not optimized, highlighting a clear unmet need for this pathology.7,29,30 Internationally, the German S2K guidelines for the diagnosis and treatment of chronic pruritus recommend difelicefalin as the first treatment option for moderate or severe Pa-CKD in hemodialysis patients.31
The perception of most Spanish nephrologists (91%) is that presently the Pa-CKD is an unsolved problem due mainly to the low efficacy of the therapeutic options available to date; this was reflected in a survey conducted through the SEN in 2021.20 In this analysis a16% of nephrologists stated that the prevalence of pruritus in their center was greater than 20%, while 84% considered the current prevalence to be 10–20%.20 However, in the survey conducted by the SEN in the framework of Pruritus Week,it was reported a prevalence of Pa-CKD of 50.5% of hemodialysis patients, being moderate to severe in 26.7%.6
In Spain, there is still a long way to go before ICD-11 is implemented, since most hospitals have the previous versions ICD-9 and ICD-10, which prevents proper coding of the Pa-CKD and, therefore, its recording is incorrect.
In the nationwide survey conducted by Alcer (Association for the Fight Against Kidney Diseases), 26% of hemodialysis patients with Pa-CKD reported that never felt an improvement in their quality of life with the treatment received for pruritus. A 35% of hemodialysis patients with Pa-CKD claimed not to have expressed their itching symptoms to any health professional, of which 26% explained that the reason was the belief that they would not give it importance, 23% because they thought it had no solution and 20% because they did not know that it could be related to CKD.7
The introduction of new specific treatments for Pa-CKD opens a new horizon for improving the quality of life of these patients. In the nation wide survey, it was observed that 44% of hemodialysis patients with Pa-CKD claimed to feel irritability more than once a week due to pruritus; 32% frustration; 21% anguish and 11% depression.7
An article by Agarwal et al. published in August 2022, explains how understanding of the pathophysiology of a particularly bothersome symptom of hemodialysis (Pa-CKD) led to the design, development, and regulatory approval of a treatment for that symptom. It highlights the importance to patients of improving itching symptoms and quality of life, and proposes an algorithm for the screening, diagnosis, assessment and treatment of Pa-CKD among hemodialysis patients, taking into account the introduction of the only drug approved for this pathology, difelicephalin.32
The diagnostic and treatment algorithm developed by the authors of this manuscript, which is largely based on the one proposed above, is intended to address the current lack of specific guidelines for the differential diagnosis and management of Pa-CKD in Spain.33
Conflict of interestAll authors have received honoraria for report or consultancies from CSL-VIFOR.