Introduction: Post-transplant lymphoproliferative disease (PTLD) represents a heterogeneous group of diseases characterised by a proliferation of lymphocytes occurring after solid organ transplantation. Most cases of PTLD are B-cell and their development has been closely associated with the Epstein-Barr virus (EBV), whose proliferation is encouraged by the inhibition of the cytotoxic function of T lymphocytes due to immunosuppressive drug treatment for transplant recipients. Several risk factors have been described for the development of this disorder, such as the seronegative state of the EBV receptor, the degree of overall net immunosuppression, especially with the use of monoclonal and polyclonal antibodies, acute rejection and cytomegalovirus (CMV) disease. Material and method: We studied the incidence of PTLD and its relationship with EBV as well as its evolution and possible risk factors in 1176 adult recipients of cadaveric renal transplantation performed in our hospital between 1988 and 2009, with a follow-up of 1-255 months. The presence of EBV in the lymphoproliferative tissue was determined using in situ hybridisation. We analysed the incidence of PTLD over two time periods, 1988-1998 and 1999-2009 with 472 and 704 patients respectively. Results: A total of 28 recipients (2.38%), 22 men and 6 women with a mean age of 46.5 (15.36) years (18-70 years) with a mean post-transplant evolution of 72.9 (56.3) months (1-180 months), developed PTLD. Thirteen (46.4%) did not show any of the classic risk factors described. The presence of EBV in lymphoproliferative tissue was detected in 18 out of 26 patients studied (69.2%). In terms of histology, 25 out of 28 were type B (89.2%). Ten out of 28 patients diagnosed (35.7%) received treatment with rituximab, six died during the follow-up, five as a direct result of their illness. The incidence for the two time periods was very similar for both groups, with 0.003922 cases/year-patient in the 1988-1998 period and 0.003995 cases/year-patient in the 1999-2009 period. Overall post-transplant survival for patients with PTLD was 73.6% at 5 years and 36.9% at 10 years, versus 87.8% and 75.9% for disease-free recipients (P<.0001). We calculated a graft survival of 62.6% at 5 years and 27.3% at 10 years versus 72.4% and 53.9% for grafts in disease-free recipients (P<.0001). In our study, patient survival one year after presenting the disease was 30.9% and 23.2% at year two. For the graft, survival was 15.5% and 7.7%, respectively. Conclusions: We conclude that PTLD is a disorder that is generally type B; it is significantly associated with EBV. Its incidence has not changed over time and half of all PTLD cases had no identifiable risk factors, which led to a poor prognosis despite the development of new treatments.
Introducción: La enfermedad linfoproliferativa postrasplante (ELP) representa un grupo heterogéneo de enfermedades que se caracterizan por una proliferación de linfocitos que se presenta después del trasplante de órganos sólidos. La mayoría de los casos de ELP son de estirpe B y su desarrollo se ha asociado estrechamente con el virus de Epstein-Barr (VEB), cuya proliferación se vería favorecida por la inhibición de la función citotóxica de los linfocitos T debido a la inmunosupresión farmacológica a la que se somete a los receptores de trasplante. Se han descrito varios factores de riesgo para el desarrollo de esta entidad, como son la seronegatividad del receptor para VEB, el grado de inmunosupresión neta global, sobre todo con el uso de anticuerpos monoclonales o policlonales, el rechazo agudo y la enfermedad por citomegalovirus (CMV). Material y métodos: Hemos estudiado la incidencia de ELP y su relación con el VEB, así como su evolución y los posibles factores de riesgo en su desarrollo, en 1.176 receptores adultos de trasplante renal de cadáver realizados en nuestro hospital, entre 1988 y 2009, con un seguimiento de uno a 255 meses. Se determinó la presencia de VEB en el tejido linfoproliferativo mediante hibridación. Analizamos la incidencia de ELP en dos períodos de tiempo, 1988-1998 y 1999-2009 con 472 y 704 pacientes, respectivamente. Resultados: Un total de 28 receptores (2,38 %), 22 hombres y 6 mujeres, con una edad media de 46,5 ± 15,36 años (18-70 años) y con una evolución media postrasplante de 72,9 ± 56,3 meses (1-180 meses), desarrollaron ELP. Trece de ellos (46,4%) no presentaban ninguno de los factores de riesgo clásicos descritos. Se detectó la presencia de VEB en el tejido linfoproliferativo de 18 de los 26 pacientes estudiados (69,2%). Respecto a su estirpe histológica 25 de los 28 eran tipo B (89,2%). Diez de los 28 pacientes diagnosticados (35,7%) recibieron tratamiento con rituximab, seis de ellos fallecieron durante el seguimiento, cinco como consecuencia directa de su enfermedad. Calculada la densidad de incidencia en los dos períodos, ésta fue muy similar en ambos grupos, de 0,003922 casos/años-paciente en el período 1988-1998 y de 0,003995 casos/años-paciente en el período 1999-2009. La supervivencia global postrasplante del paciente que presentó ELP fue del 73,6% a los 5 años y del 36,9 % a los 10 años frente al 87,8% y al 75,9% del receptor libre de enfermedad (p <0,0001). Evidenciamos una supervivencia del injerto del 62,6% a los 5 años y del 27,3% a los 10 años frente al 72,4% y al 53,9% de los injertos de los receptores libres de enfermedad (p <0,0001). En nuestra serie, la supervivencia del paciente al año de presentar la enfermedad fue del 30,9%, y del 23,2% al segundo año, y para el injerto del 15,5% del 7,7%, respectivamente. Conclusiones: Concluimos que la ELP es una entidad en su mayoría de estirpe B, asociada de forma significativa con el VEB, cuya incidencia no ha variado en el tiempo y en la que en la mitad de los casos no se identifican factores de riesgo, condicionando muy mal pronóstico a pesar de los nuevos tratamientos desarrollados.
INTRODUCTION
Post-transplant lymphoproliferative disease (PTLD) represents a heterogeneous group of diseases characterised by the proliferation of lymphocytes occurring after solid organ transplantation.1
Most cases of PTLD cases are B-cell2 and their development has been closely associated with the Epstein-Barr virus (EBV1,3,4) whose proliferation is encouraged by the inhibition of the cytotoxic function of the T-lymphocytes due to the overall net immunosuppressive drug treatment that transplant recipients must undergo.5
Several common risk factors have been described for the development of this disorder, such as the seronegative state of the EBV receptor,6,7 the degree of overall net immunosuppression, especially with the use of monoclonal and polyclonal antibodies,6-8 acute rejection,7 and cytomegalovirus (CMV) disease.9,10 Recently, Opelz described the mismatch at the DR locus as a risk factor for developing the disease, but could not conclude whether this factor reflects the need for further immunosuppression or an increased incidence of immunogenicity and acute rejection.11
Using two decades of experience, we have studied the incidence of PTLD and its possible variation over time, the relationship between EBV and PTLD, the prognosis of the condition, possible risk factors for its development and the influence of new strategies in its treatment.
MATERIAL AND METHOD
Patients
This is a descriptive study of the incidence of PTLD in 1176 adult recipients who received cadaver donor kidney transplants over a period of 21 years, from July 1988 to December 2009. Post-transplant follow-up time was from one to 255 months. A total of 472 patients underwent transplantation in the 1988-1998 period and 705 in the 1999-2009 period. The initial immunosuppression regimen included cyclosporine, azathioprine and prednisone, substituting azathioprine with mycophenolate from 1998 on, when tacrolimus started to be used as anti-calcineurin in many patients. High immunological risk recipients received induction therapy with OKT3 until 2000 and thymoglobulin thereafter.
Acute rejection was diagnosed through biopsy and treated initially with three IV boluses of 6-methyl-prednisolone (500mg). In case of corticoid resistance or Banff grade II or III, patients received monoclonal or polyclonal antibodies.
The presence of common risk factors for developing the disease was evaluated by examining medical records. These risk factors included EBV seronegativity, use of monoclonal or polyclonal antibodies, CMV infection and treated acute rejection.
Method
Diagnosis
Histology
Histological material and/or cellularity were obtained from all patients with the disease, and necropsy was requested for those recipients who died. The histological assessment was carried out according to morphological and immunohistochemical studies. The morphology study was performed, according to the classification of haematopoietic diseases by the World Health Organization,12 on sections stained with haematoxylin and eosin. Giemsa and PAS results were obtained from material fixed in neutral buffered formalin and embedded in paraffin. Immunohistochemical studies were performed on tissue fixed and embedded in paraffin using the streptavidin biotin peroxidase method, with antigen retrieval in a pressure cooker for 15 minutes. The following antibodies were used: CD 45 (pan-leukocyte), B lymphocytes markers (CD 20, CD 79, CD 45), T lymphocytes markers (CD 43, CD 3, CD 45RO=UCL1), cell proliferation marker Ki67, light chains, CD 30 (anaplastic), CD 15, and the bcl2 and p53 proteins.
EBV Studies
EBV serology was determined using EBV-VCA IgG and IgM before transplantation in all recipients by ELISA. The presence of EBV in the lymphoproliferative tissue was determined using in situ hybridisation with EBER PNA probes (Dako).
Other studies
Imaging tests were performed that included thoracic-abdominal CT in patients diagnosed with PTLD in life.
Statistical analysis
Descriptions of patient baseline characteristics are expressed as percentages for qualitative variables. For quantitative variables with a normal distribution, the mean was determined with standard deviation. For quantitative variables that did not follow a normal distribution, the median with its interquartile range was used.
Independent qualitative variables were analysed using contingency tables with the associated chi-squared statistics. Comparisons between quantitative variables were analysed using the Student's t-test.
We calculated the incidence rate for the two periods. It is expressed as cases/years-patient.
The curves for graft and recipient survival were calculated using Kaplan-Maier and the different comparisons between them with the log-rank test.
A comparison was considered significant if P<.05. The SPSS statistical software was used.
RESULTS
Out of the total population of 1176 patients, 28 were diagnosed with PTLD (2.38%), 22 of them were men (78.5%) and 6 women (21.5%), with a mean age of 46.5 (15.36) years (18-70 years). Post-transplant follow-up was 1-255 months and the mean time between transplant and diagnosis of the disease was 72.9 (56.32) months (1-180). A total of five patients (17.8%) developed the disease during the first year after transplantation and 23 (82.2%) developed it later (Table 1). 78.6% were EBV seropositive before transplantation versus 21.4% who were seronegative (Table 1). Twenty-three of the 28 patients diagnosed with PTLD (82.1%) had received cyclosporine as anti-calcineurin and 16 out of the 28 patients received azathioprine (57.1%). Five patients (17.8%) received tacrolimus and 11 (39.2%) received mycophenolate in combination with cyclosporine or tacrolimus. Only one patient received sirolimus as part of their immunosuppression treatment. A total of four patients (14.2%) received treatment with monoclonal and polyclonal antibodies (Table 1).
Thirteen out of the 28 patients (46.4%) showed no common risk factors. Five (17.8%) out of the 15 that did show some risk factor, had more than one (Table 1).
PTLD was diagnosed post mortem in six cases (21.4%), while the rest was diagnosed based on histological material or cellularity in vivo (Table 1). The lymphocyte strain detected was B in 25 out of the 28 patients (89.2%), (Table 2). The presence of EBV in the lymphoproliferative tissue was studied in 26 of the 28 patients (92.8%) and the virus was detected in 18 of them (69.2%), (Table 2).
Although the majority of cases belonged to the B cell strain, only 10 of the 28 patients diagnosed (35.7%) received treatment with rituximab (anti-CD20) since its use did not start until 2003 and not all cases had the CD20 antigen. Of these, six died during the follow-up and five as a direct result of their disease.
The incidence rate was similar in patients undergoing transplantation during the 1988-1998 period (0.003922) to that of recipients during the 1999-2009 period (0.003995).
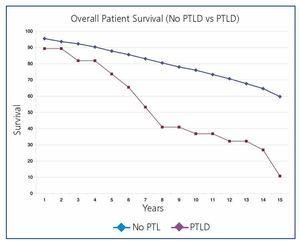
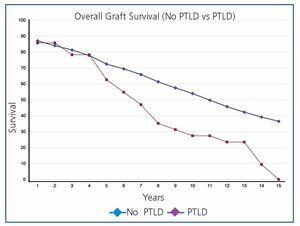
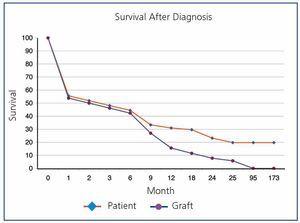
Overall post-transplant survival for patients with PTLD was 73.6% at 5 years and 36.9% at 10 years versus 87.8% and 75.9% for disease-free recipients (P<.0001) (Figure 1). The graft had a survival rate of 62.6% at 5 years and 27.3% at 10 years versus 72.4% and 53.9% for grafts in disease-free recipients (P<.0001), (Figure 2). In our study, patient survival at one year of having the disease was 30.9%, and 23.2% at two years (Figure 3). Survival for the graft was 15.5% and 7.7%, respectively (Figure 3).
DISCUSSION
According to our experience, the relationship between EBV and the development of the disease is close, as evidenced by the fact that most recipients who developed PTLD in our study had EBV in the proliferating tissue (Table 2). Seven of our patients were seronegative for this virus, i.e. a risk factor, and therefore, they were likely to develop a primary infection, which is the main risk factor identified in the large series.6,7,9 It is striking that in only half of the cases in our study we were able to identify a common risk factor (Table 1), a fact that could be explained by the existence of factors or combinations of factors still unknown that are involved in the disease.
The influence of different immunosuppressive agents in the development of the disease has been extensively studied in the literature. In general, immunosuppression is a key factor in its appearance, as shown by the fact that patients undergoing transplantation who restart dialysis significantly lower the risk of developing the disease.6,13 Therefore, the use of monoclonal or polyclonal antibodies has been established as a first-order risk factor.6-8 In our study, only four out of the 28 patients received this immunosuppressive agent (Table 1), indicating once again the existence of other factors involved in the development of this condition. The use of anti-CD25 antibodies in the induction does not add an increased risk of occurrence of the disease.7,8 As for other immunosuppressants, we should note the higher incidence of PTLD in kidney transplant recipients who have received FK instead of CsA.7,8 In our study, only five patients received this immunosuppressant (Table 1), which prevents us from reaching any conclusion. The antiproliferative azathioprine and mycophenolate are associated with a lower risk of developing the disease.7
It should be noted that the net overall immunosuppression used should be considered as the risk factor, although the importance of the use of monoclonal or polyclonal antibodies is significant. The identification of acute rejection as a common risk factor7,8 could be an indication of the importance of overall net immunosuppression in the development of the disease.
Given the possible influence that the change in immunosuppression during the last decade could have on the incidence of PTLD, we analysed incidence rate for the past two decades without finding a significant difference. These results are supported by those reported by Opelz, which provide evidence that the incidence of this condition has remained stable over the three time periods studied.8 This information is not reassuring since the follow-up time in the second period is shorter and it is expected that late cases of the disease have not yet presented.
There is controversy about whether there are two distinct conditions within PTLD; one with an early onset, which is closely related to EBV infection and prone to remission after reduction of immunosuppression, and one with a later onset, which is barely related to EBV and that has a poor clinical outcome with the reduction of immunosuppression.1,2,6 The data from Opelz goes counter to this hypothesis since it states a similar prognosis for PTLD whether early or late.8 In our study, most cases were of late onset, and therefore their poor prognosis could be related to the poor outcome ascribed to late onset by authors such as Leblond.2 Furthermore, most of our patients had EBV in the proliferating tissue, a rare finding under conditions of late development.2,4
As for time of onset after transplantation, the greatest risk seems to appear during the first year.14 Smith has reported a greater incidence of PTLD in the first year after transplantation, which decreases in subsequent years, although their data are of limited value due to the censoring of the series for non-medical reasons at the three year follow-up.13 Van Leeuwen, using the ANZDATA data, reports a lower incidence during the two to five year period than during the first two years.6 Opelz also refers to a higher incidence during the first year that remains stable during the following 10 years.8 Other series, however, report similar numbers of early and late cases4 and some, like our series, flip the ratio to favour a late incidence of the disease.15 Only five of our patients (17.8%) developed the disease within the first year after transplantation but in all of these cases we were able to demonstrate the presence of EBV in the proliferating tissue, confirming the relationship between a precocious development of the condition and the presence of EBV.2 The mean post-transplant time in which the disease was diagnosed in our series was 77.8 months allowing us to label our patients as suffering from late PTLD, which was similar to the experience of Trappe et al. who reported a mean post-transplant time of 88 months.15 In a meta-analysis by Pascual, the time to onset was much later, around 117 months,16 while other series report mean times that are much earlier.7,17
Most PTLD is of the B2 strain, a fact confirmed in our series where 89.2% of patients developed B-cell proliferations (Table 2). Treatment for these cases, provided they are CD20 positive, is established with anti-CD20 and conversion to mTOR inhibitors, having reported good outcomes in other series.15,16 In our limited experience, six out of the 10 treated patients died, five of them as a direct result of the disease, which indicates that despite recent advances this condition continues to have a poor prognosis. It must be stressed that the strategy mentioned has only been applied to the last few patients diagnosed, which means that the prognosis for the disease could change in the future.
The overall incidence of PTLD in our series is high, up to 2.38%, higher than other published series.6,7,17 The presence of risk factors in our patients does not explain this high incidence since only half of them showed some of the common risk factors described. It should be noted that 20% of our cases were diagnosed by performing autopsies (Table 1), a result of the departmental policy performing post-mortem studies on all patients who lack a definite cause of death, thus increasing the number of cases diagnosed.
According to our experience, the prognosis for patients with PTLD is poor, with patient survival at one year and at two years of 30.9% and 23.2%, respectively, which is significantly lower than that of patients who do not develop the disease. The development of PTLD therefore significantly determined patient and graft survival. Other authors confirm these data, although with higher survival rates7, with 40% mortality at one year in the Opelz series.8
Comparing our current results with those published 8 years ago,18 which reported data from patients undergoing transplantation up to 2001, we do not observe significant changes in patient age at the onset of the disease, time of onset after transplantation, percentage of patients with risk factors and prognosis of the disease. We must emphasise, however, that our incidence has almost doubled due to a much longer patient follow-up, with a significant incidence of late-developing disease after transplantation. This is confirmed by the data from Opelz, which show the existence of a year-after-year cumulative risk of developing the disease from the moment of transplantation.8
We conclude that PTLD, mostly of B cells, is a condition whose incidence has not changed over time. It is significantly associated with EBV, no risk factors are identified in half of all cases and it has a poor prognosis despite the new treatments developed.
Figure 1. Overall Patient Survival (no PTLD Versus PTLD)
Figure 2. Overall Survival of Graft (no PTLD Versus PTLD)
Figure 3. Patient Survival After Diagnosis
10361_108_10770_en_w4777105831410361_t1_en.doc
Table 1. Post-Transplant Time, Diagnostic Method, Immunosuppression and Risk Factors in Recipients who Developed the Disease
10361_108_10771_en_w4777105831510361_tabla_2_en.doc
Table 2. Histological Classification According to WHO12, Treatment with Rituximab and the Presence of EBV in Tissue of Recipients with Lymphoproliferative Disease