Patients with type 2 diabetes mellitus (T2DM) have an increased risk of developing chronic kidney disease (CKD). CKD is defined by both a decline in glomerular filtration rate and the presence of albuminuria. Although the measurement of the urine albumin-to-creatinine ratio is recommended from the time of T2DM diagnosis and subsequently at least once a year, in clinical practice, this assessment is underutilized in many patients. The pathophysiology of diabetic kidney disease involves hemodynamic, metabolic, pro-inflammatory, and pro-fibrotic factors. Likewise, in cardio-reno-metabolic syndrome, excessive or dysfunctional adiposity plays a fundamental role, promoting the development of kidney disease, type 2 diabetes and cardiovascular disease. Therefore, its management requires a multifactorial approach that includes the use of renin-angiotensin-aldosterone system inhibitors (targeting hemodynamic factors), SGLT2 inhibitors (targeting both hemodynamic and metabolic factors), finerenone (with anti-inflammatory and anti-fibrotic effects), and GLP-1 receptor agonists with demonstrated renal benefits (targeting metabolic factors), with the aim of more effectively slowing CKD progression and reducing the risk of cardiovascular complications. In this review article, we propose strategies to facilitate the proper assessment and implementation of guideline-recommended treatment in patients with diabetes and albuminuria, presenting a ten-point framework to improve the comprehensive and collaborative management of these patients.
Los pacientes con diabetes mellitus tipo 2 (DM2) tienen un riesgo incrementado de desarrollar enfermedad renal crónica (ERC). La ERC se define tanto por el descenso del filtrado glomerular, como por la presencia de albuminuria. Aunque se recomienda la determinación del cociente albúmina-creatinina en orina desde el momento del diagnóstico de la DM 2 y posteriormente al menos una vez al año, en la práctica clínica dicha determinación se encuentra infrautilizada en muchos pacientes. En la etiopatogenia de la enfermedad renal diabética intervienen factores hemodinámicos, metabólicos, proinflamatorios y profibróticos. Asimismo, en el síndrome cardio-reno-metabólico, la adiposidad excesiva o disfuncional juega un papel fundamental, promoviendo el desarrollo de enfermedad renal, diabetes tipo 2 y enfermedad cardiovascular. Por ello, en su tratamiento es necesaria una estrategia multifactorial que incluya el uso de fármacos inhibidores del sistema renina- angiotensina-aldosterona (factores hemodinámicos), inhibidores de SGLT2 (factores hemodinámicos y metabólicos), finerenona (efectos antiinflamatorios y antifibróticos) y los agonistas del receptor de GLP-1 con beneficio renal demostrado (factores metabólicos) con el objetivo de reducir con mayor eficacia la progresión de la ERC y disminuir el riesgo de presentar complicaciones cardiovasculares. En este artículo de revisión proponemos estrategias para facilitar la adecuada evaluación e implementación del tratamiento recomendado por las guías de práctica clínica en el paciente con diabetes y albuminuria, presentando un decálogo para mejorar el manejo integral y colaborativo de estos pacientes.
Type 2 diabetes mellitus (T2DM) is a worldwide public health problem, creating a global epidemic. Thus, in the year 2024, the estimated prevalence was 589 million people with diabetes mellitus (DM) in the adult population between 20 and 79 years (11.1% of the total adult world population), with more than 90% accounting for people with T2DM.1 In Spain, the prevalence of the adult population with T2DM is 14%, and likely up to half of the actual cases are undiagnosed.2 Furthermore, there is an unequal distribution across the autonomous communities; it is higher in the Canary Islands and Murcia, followed by Andalusia, Valencia and the Balearic Islands.3 T2DM is associated with a high demand of healthcare and economic resources.4
The prevalence of chronic kidney disease (CKD) in people with DM is very high, affecting approximately 30–45% of people with DM, and conversely, in the population with CKD, concomitant T2DM is common. In fact, DM is the main cause of CKD, more common than other causes, such as hypertension.5–8 Moreover, investigators estimate that the prevalence and consequences associated with CKD will increase significantly in the coming years.9
The development of CKD in the DM population increases the risk of developing cardiovascular complications and progression to end-stage renal disease.5 Thus, the greater the deterioration of renal function, the greater the risk of cardiovascular and renal complications. Cardiovascular disease accounts for almost half of the causes of death in people with DM and CKD in its more advanced stages.10 It is important to remember that CKD may already be present at the time T2DM is diagnosed,11 so all patients with T2DM should be investigated for the presence of CKD, with urine albumin creatinine ratio (uACR) and estimated glomerular filtration rate (eGFR), with the aim of making the necessary interventions to prevent progression of CKD, as well as to avoid cardiovascular events.12–15
On the other hand, cardiovascular-reno-metabolic syndrome, due to excessive or dysfunctional adiposity, promotes the development of cardiovascular disease, CKD and T2DM. In this context, prevention and early treatment of this vicious cycle is essential to reduce the risk of complications.16
Importance of albuminuria in CKD patientsCKD is categorized according to eGFR (G1-G5) and albuminuria (A1-A3).5 Therefore, albuminuria is an essential element for the diagnosis of CKD, and prognostic, allowing clinicians to categorize CKD into various cardiovascular risk groups.11,12,17
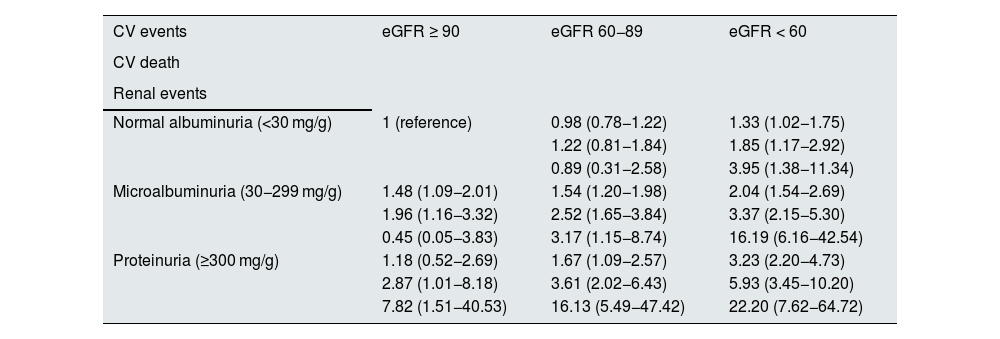
Albuminuria, in addition to being a non-invasive and highly cost-effective diagnostic method for cardiovascular risk assessment,18 is an independent risk factor for cardiovascular morbidity and mortality.6,7,13 In fact, both eGFR and albuminuria are independent predictors of cardiovascular and renal events, as well as of increased mortality, multiplying the risk when both are deranged concurrently.5,14 Thus, for example, in the ADVANCE study, where 10,640 patients with T2DM were followed for an average of 4.3 years, it was found that, as uACR increased or eGFR decreased, the risk of cardiovascular events increased. Furthermore, there was no evidence of interaction between the effects of higher uACR and lower eGFR (Table 1).19
Risk of cardiovascular and renal complications as a function of glomerular filtration rate and albumin-creatinine ratio in patients with type 2 diabetes.
| CV events | eGFR ≥ 90 | eGFR 60−89 | eGFR < 60 |
|---|---|---|---|
| CV death | |||
| Renal events | |||
| Normal albuminuria (<30 mg/g) | 1 (reference) | 0.98 (0.78−1.22) | 1.33 (1.02−1.75) |
| 1.22 (0.81−1.84) | 1.85 (1.17−2.92) | ||
| 0.89 (0.31−2.58) | 3.95 (1.38−11.34) | ||
| Microalbuminuria (30−299 mg/g) | 1.48 (1.09−2.01) | 1.54 (1.20−1.98) | 2.04 (1.54−2.69) |
| 1.96 (1.16−3.32) | 2.52 (1.65−3.84) | 3.37 (2.15−5.30) | |
| 0.45 (0.05−3.83) | 3.17 (1.15−8.74) | 16.19 (6.16−42.54) | |
| Proteinuria (≥300 mg/g) | 1.18 (0.52−2.69) | 1.67 (1.09−2.57) | 3.23 (2.20−4.73) |
| 2.87 (1.01−8.18) | 3.61 (2.02−6.43) | 5.93 (3.45−10.20) | |
| 7.82 (1.51−40.53) | 16.13 (5.49−47.42) | 22.20 (7.62−64.72) |
CV: cardiovascular; eGFR: estimated glomerular filtration rate (ml/min/1.73 m2).
In summary, persistent albuminuria alone is sufficient to diagnose CKD. In addition, it increases the risk of renal complications, cardiovascular complications and mortality.20–22
Albuminuria should be routinely evaluated in patients with DM, independently of the eGFR value, as it is an early indicator of renal damage. Delay in diagnosis is associated with increased risk of disease progression and cardiovascular complications. Therefore, identifying and treating patients with CKD early is crucial to prevent progression of CKD and to reduce cardiovascular events (Fig. 1).11,12 However, in clinical practice, detection of albuminuria is still insufficient.7 In this context, it is a priority to improve early detection strategies, including urine albumin-creatinine ratio tests.11 Most clinical practice guidelines recommend annual screening for CKD in the patient with DM. In patients with type 1 DM (T1DM) it should be started 5 years after diagnosis, and in T2DM, at the time of disease diagnosis, regardless of baseline treatment.5,11,12
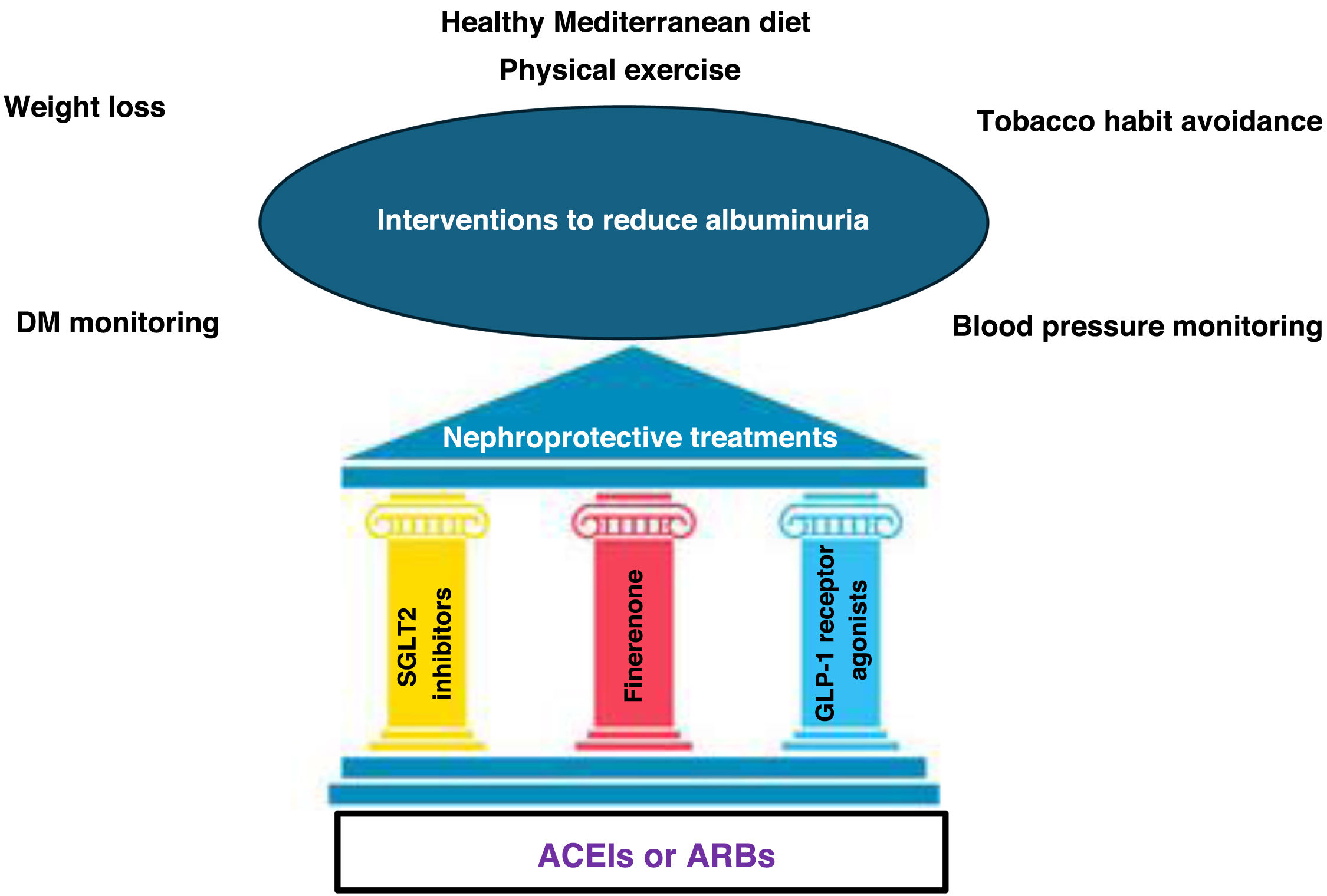
Global approach to reduce albuminuria.
ARBs: angiotensin II receptor antagonists; GLP-1 receptor agonists: glucagon-like peptide receptor type 1 agonists; DM: diabetes mellitus; ACEIs: angiotensin-converting enzyme inhibitors; SGLT2 inhibitors: sodium-glucose cotransporter 2 inhibitors.
Source: figure made with data from the American Diabetes Association Professional Practice Committee11 and from Montero et al.12
The management of patients with CKD and T2DM requires a global and comprehensive treatment that covers aspects associated with the progression of renal disease and the risk of developing cardiovascular complications. It involves, as a first step, lifestyle modifications, blood pressure and lipid control, together with the combination of different pharmacological treatments (Fig. 2).11,12
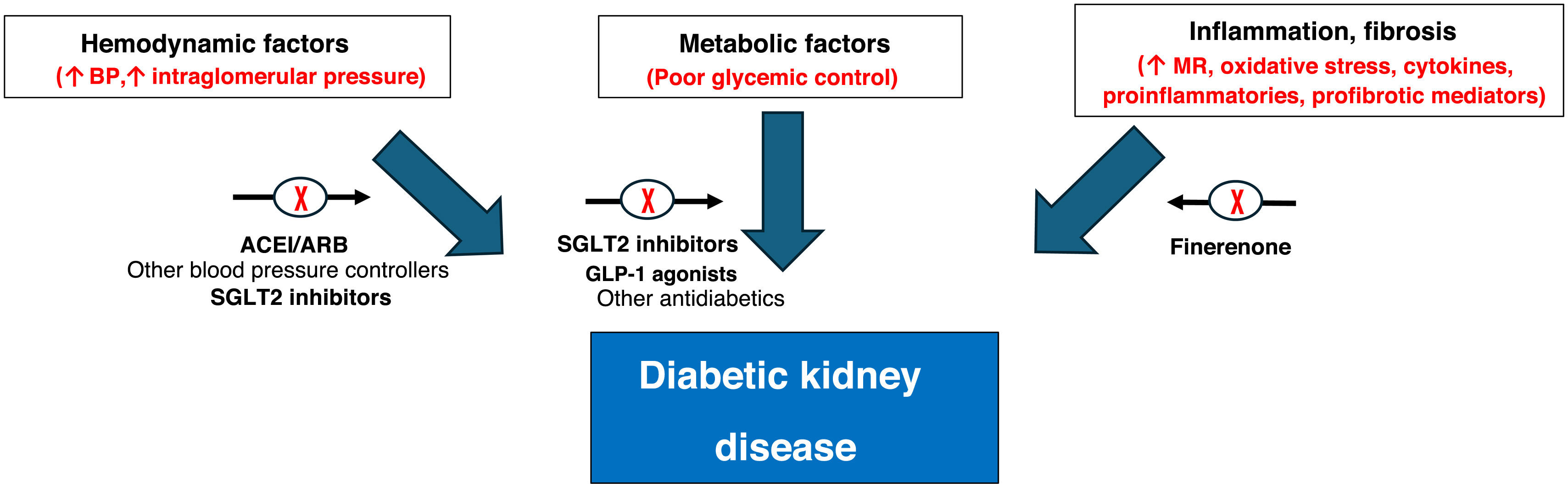
Etiopathogenesis of CKD in T2DM and therapeutic approach.
ARBs: angiotensin II receptor antagonists; GLP-1: glucagon-like peptide type 1; ACEI: angiotensin-converting enzyme inhibitors; SGLT2 inhibitors: sodium-glucose cotransporter 2 inhibitors; BP: blood pressure; MR: mineralocorticoid receptor.
Source: figure made with data from the American Diabetes Association Professional Practice Committee,11 de Montero et al.,12 Alicic et al.,43 Zelniker and Braunwald44 and Heerspink et al.45
Regarding lifestyle modifications, the acquisition of healthy dietary and lifestyle habits is recommended, including a diet with a predominance of vegetable proteins and fiber, avoiding ultra-processed foods. In addition, weight reduction is recommended in cases of obesity, to reduce the risk sarcopenia and dynapenia. Likewise, since patients are usually elderly, exercise is recommended, to avoid frailty.11,12
Within this holistic treatment, different drugs reduce the risk or delay the progression of CKD and reduce cardiovascular risk, with complementary and additive effects. This global approach would include the optimization of DM treatment, lipid control with statins, associated or not with other lipid-lowering treatments, which allow target LDL cholesterol to be reached, blood pressure control (<130/80 mmHg), with the baseline treatment being angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor antagonists (ARBs) for those with albuminuria and/or eGFR < 60 ml/min/1.73 m2 at the maximum tolerated dose. Similarly, for patients with T2DM and CKD, the use of a sodium-glucose cotransporter 2 inhibitors (SGLT2 inhibitors) is recommended with demonstrated benefit in reducing CKD progression and cardiovascular events in persons with eGFR ≥ 20 ml/min/1.73 m2. Another mainstay treatment to reduce cardiovascular events and CKD progression in people with T2DM, CKD, albuminuria and a GFR ≥ 25 ml/min/1.73 m2 is the use of finerenone, a non-steroidal mineralocorticoid receptor antagonist.11,12
This approach with different drugs makes it possible to act on the different factors that influence the development of CKD in the patient with T2DM. Thus, renin-angiotensin-aldosterone system inhibitors (RAASi) reduce the tone of the efferent arteriole, hyperfiltration and cardiac remodeling. Sodium-glucose cotransporter 2 inhibitors increase afferent arteriolar tone, have anti-inflammatory and antifibrotic effects, as well as reducing hyperfiltration, proteinuria and oxidative stress. Finerenone reduces inflammation, fibrosis, endothelial dysfunction, tissue remodeling and proteinuria.21,23 Finally, GLP-1 receptor agonists, in addition to reducing body weight, also reduces oxidative stress and endothelial dysfunction.11,12
Consequently, holistic treatment with all these drugs provides greater renal and cardiovascular protection in this high cardiovascular risk population.
Inhibition of the renin-angiotensin-aldosterone system (RAASi)Both ACEIs and ARBs constitute the treatment of choice in patients with T2DM, arterial hypertension and CKD, due to their ability to reduce the progression of renal disease and the risk of cardiovascular events.11,24–26 Thus, in the RENAAL study, the use of losartan reduced uACR by 35%,23 with ACEIs and ARBs offering similar results.27,28 Evidence on the benefit of ACEI or ARBs treatment in renal protection would be less robust for patients with albuminuria without arterial hypertension.29,30 Treatment with ACEI or ARBs can be associated with electrolyte abnormalities such a deranged potassium levels, so baseline blood biochemistry should be performed after initiation and/or titration of these drugs, with subsequent control analyses.11,12
It is important to highlight that, despite the benefits shown by ACEIs and ARBs in cases of albuminuria, in both the RENAAL study (with losartan) and the IDNT study (with irbesartan), progression of CKD was observed in 43.5% and 32.6% of patients, respectively,24,31 which indicates the need for additional treatments in this population due to the associated residual risk.21
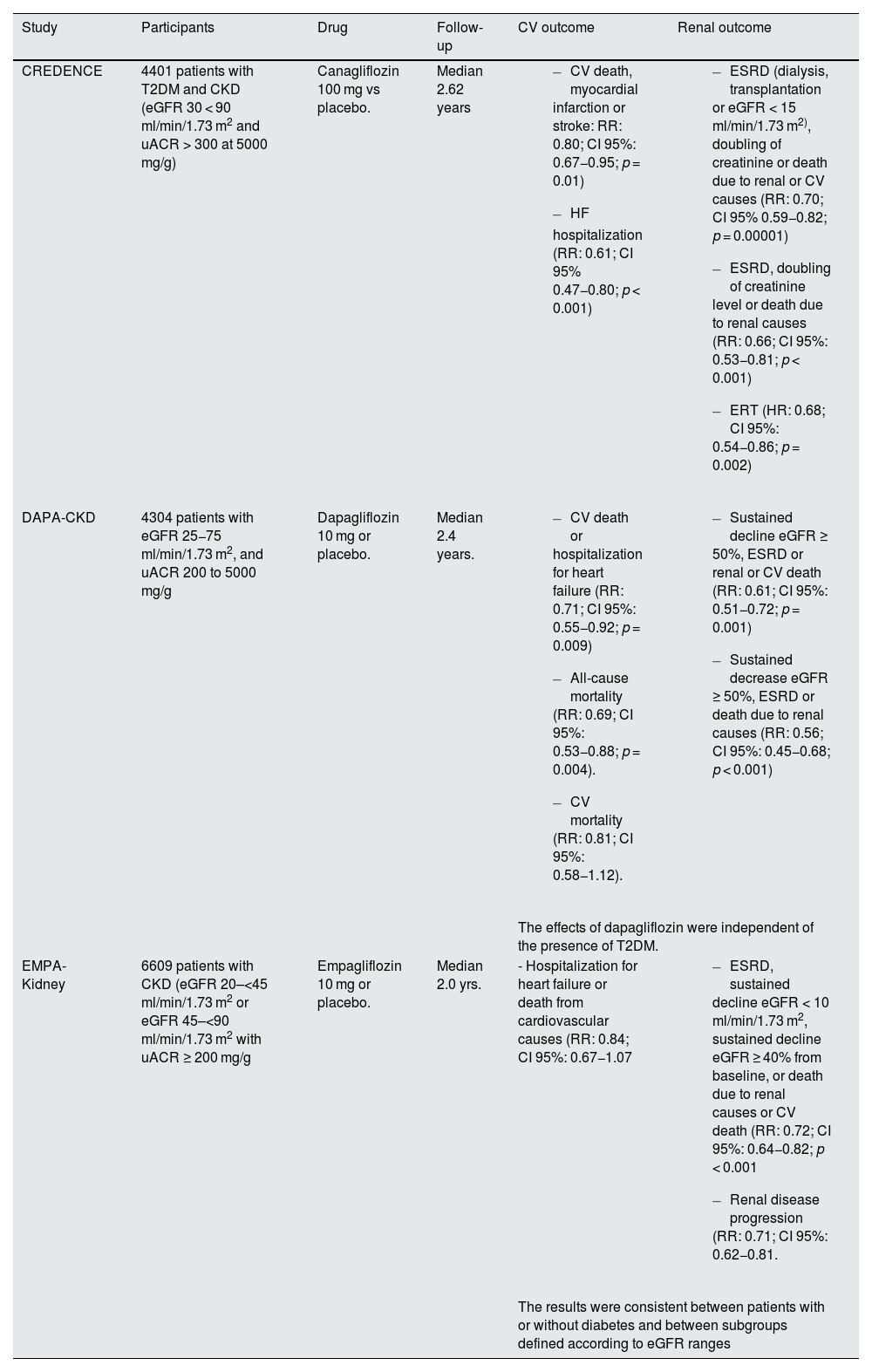
SGLT2 inhibitorsThe use of SGLT2 inhibitors is recommended in patients with eGFR ≥ 20 ml/min/1.73 m2 and T2DM, as they delay CKD progression and reduce the risk of heart failure independently of glycemic control.11,12 Canagliflozin (CREDENCE study), dapagliflozin (DAPA-CKD study) as well as empagliflozin (EMPA-KIDNEY study) have demonstrated the benefits of SGLT2 inhibitors in patients with CKD and albuminuria. This benefit, moreover, is independent of renal function, albuminuria levels and the presence of T2DM (Table 2).32–34 In these clinical trials, canagliflozin, dapagliflozin and empagliflozin reduced uACR by 31%, 29% and 19%, respectively.32–34 These drugs are very well tolerated, although they can cause a mild, transient, initial deterioration of renal function, as well as volume depletion and a drop in blood pressure; the patient's volume status, concomitant diuretic and antihypertensive use should be taken into consideration, especially in fragile patients, before initiating treatment with SGLT2 inhibitors. In addition, SGLT2 inhibitors treatment is also associated with an increased risk of genitourinary infections, as their mechanism of action results in glycosuria, an aspect which may justify antibiotic treatment.32–34
Main results of clinical trials with SGLT2 inhibitors in patients with CKD.
| Study | Participants | Drug | Follow-up | CV outcome | Renal outcome |
|---|---|---|---|---|---|
| CREDENCE | 4401 patients with T2DM and CKD (eGFR 30 < 90 ml/min/1.73 m2 and uACR > 300 at 5000 mg/g) | Canagliflozin 100 mg vs placebo. | Median 2.62 years |
|
|
| DAPA-CKD | 4304 patients with eGFR 25−75 ml/min/1.73 m2, and uACR 200 to 5000 mg/g | Dapagliflozin 10 mg or placebo. | Median 2.4 years. |
|
|
| The effects of dapagliflozin were independent of the presence of T2DM. | |||||
| EMPA-Kidney | 6609 patients with CKD (eGFR 20–<45 ml/min/1.73 m2 or eGFR 45–<90 ml/min/1.73 m2 with uACR ≥ 200 mg/g | Empagliflozin 10 mg or placebo. | Median 2.0 yrs. | - Hospitalization for heart failure or death from cardiovascular causes (RR: 0.84; CI 95%: 0.67−1.07 |
|
| The results were consistent between patients with or without diabetes and between subgroups defined according to eGFR ranges | |||||
uACR: albumin creatinine ratio; CV: cardiovascular; T2DM: Type 2 diabetes Mellitus; CKD: chronic kidney disease; ESRD: end-stage renal disease; eGFR: estimated glomerular filtration rate; 95% CI: 95% confidence interval; RR: relative risk; SGLT2: sodium-glucose cotransporter 2.
Several hemodynamic and metabolic mechanisms by which SGLT2 inhibitors slow the progression of renal disease have been described, beyond their effect on blood glucose control. SGLT2 inhibitors reduce the renal tubular reabsorption of glucose, hyperfiltration, oxidative stress and inflammation, in addition to increasing angiotensinogen and decreasing body weight, systemic blood pressure, intraglomerular pressure, volume overload and albuminuria. Thus, slowing the loss of renal function.35–38 Additional pleiotropic effects of SGLT2 inhibitors at the vascular, cardiac and renal levels have also been reported.39
Recent data show that SGLT2 inhibitors could also be beneficial after renal transplantation, since it has been shown that their use would be associated with a significant reduction in all-cause death, graft failure or doubling of serum creatinine.40 In contrast, in CKD patients on dialysis, although there is promising preliminary data, it is unclear whether SGLT2 inhibition will be effective in preventing clinically relevant outcomes and/or whether it is sufficiently safe in this setting.41
In any case, despite clear benefits on CKD progression, clinical trials have shown that there remains a residual risk of kidney disease progression despite treatment with RAASi and SGLT2 inhibitors, indicating that additional and complementary approaches are needed to reduce this risk.32–34
FinerenoneDespite treatment with ACEIs/ARBs and the use of SGLT2 inhibitors, there is still a significant residual risk of cardiovascular and renal complications in the patient with T2DM and CKD. For example, in the CREDENCE study, 11% of patients with CKD and T2DM presented progression of CKD or cardiovascular death at 36 months of follow-up.32 In fact, this progression of CKD is associated with increased cardiovascular morbidity and mortality, decreased life expectancy, poorer quality of life and increased healthcare costs.42
Not only hemodynamic and metabolic factors are involved in the etiopathogenesis of diabetic kidney disease: inflammation and fibrosis also play a fundamental role, where ACEIs/ARBs or SGLT2 inhibitors would not act specifically, which would therefore explain this residual risk despite treatment with these drugs (Fig. 2).43–45
Inflammation and fibrosis are mainly caused by mineralocorticoid hyperactivation, leading to increased oxidative stress, release of proinflammatory cytokines and profibrotic mediators. Therefore, it is important to differentiate systemic inflammation (due to atherosclerosis) from ligand-dependent inflammation, which occurs in patients with mineralocorticoid hyperactivity, especially in the T2DM population.43,45 The correction of this mineralocorticoid hyperactivity is a therapeutic target in these patients.11,12
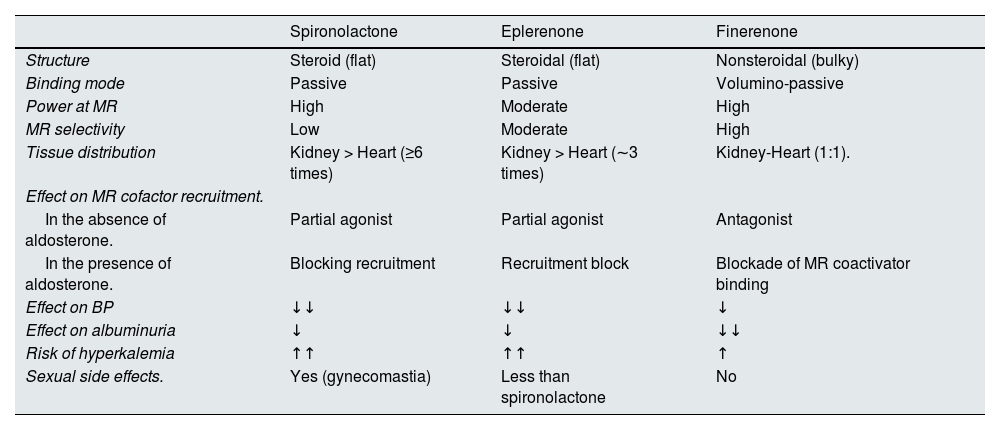
It is important to emphasize that not all mineralocorticoid receptor antagonists have the same physicochemical properties and, therefore, their effects on blood pressure, albuminuria and cardiovascular and renal events are different, as is the risk of side effects, mainly hyperkalemia (Table 3).21,46–51 In this regard, finerenone has unique properties, and its selective antagonism on the mineralocorticoid receptor induces a conformational change upon binding to the receptor, which is different from the change observed with steroidal mineralocorticoid receptor antagonists. Furthermore, this change results in cell type-specific cofactor recruitment. Finerenone blocks the transcription of proinflammatory and profibrotic genes in various cell types, which promotes its anti-inflammatory and antifibrotic action. Consequently, finerenone offers great advantages over spironolactone and eplerenone, even though these also reduce albuminuria to a lesser extent, which implies finerenone is the only mineralocorticoid receptor antagonist that has been shown to reduce the progression of renal disease and the risk of developing cardiovascular complications in patients with T2DM and CKD, plus a lower risk of hyperkalemia.21,46–51
Differential effects of different mineralocorticoid receptor antagonists.
| Spironolactone | Eplerenone | Finerenone | |
|---|---|---|---|
| Structure | Steroid (flat) | Steroidal (flat) | Nonsteroidal (bulky) |
| Binding mode | Passive | Passive | Volumino-passive |
| Power at MR | High | Moderate | High |
| MR selectivity | Low | Moderate | High |
| Tissue distribution | Kidney > Heart (≥6 times) | Kidney > Heart (∼3 times) | Kidney-Heart (1:1). |
| Effect on MR cofactor recruitment. | |||
| In the absence of aldosterone. | Partial agonist | Partial agonist | Antagonist |
| In the presence of aldosterone. | Blocking recruitment | Recruitment block | Blockade of MR coactivator binding |
| Effect on BP | ↓↓ | ↓↓ | ↓ |
| Effect on albuminuria | ↓ | ↓ | ↓↓ |
| Risk of hyperkalemia | ↑↑ | ↑↑ | ↑ |
| Sexual side effects. | Yes (gynecomastia) | Less than spironolactone | No |
BP: blood pressure; MR: mineralocorticoid receptor.
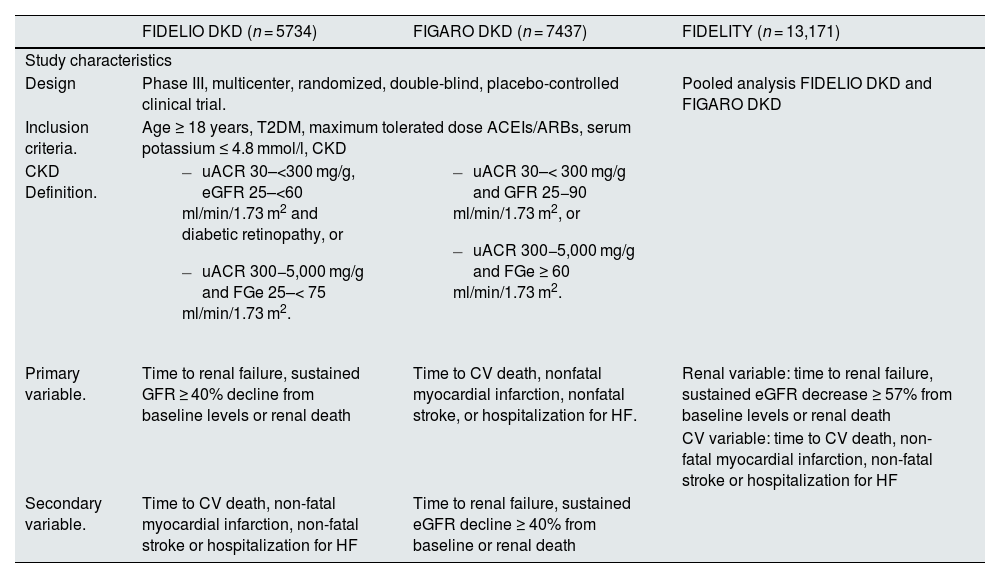
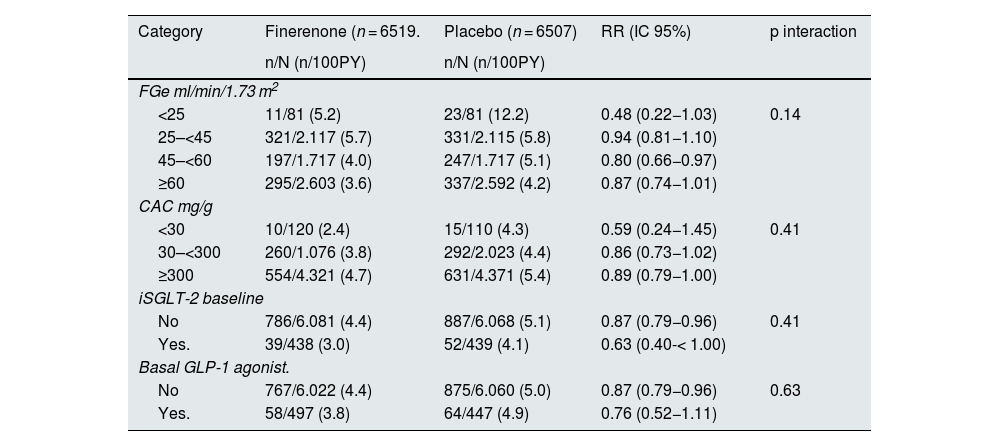
The phase III clinical trials that have demonstrated the cardiovascular and renal benefits of finerenone treatment in patients with T2DM and CKD with albuminuria have been the FIDELIO-DKD and FIGARO-DKD studies, along with the combined analysis of both, the FIDELITY study (Table 4).52–54 Together, these studies provide a comprehensive clinical program on patients with T2DM and CKD across the spectrum of CKD severity. Overall, finerenone significantly reduced albuminuria by 32% after only 4 months of treatment, with this reduction seen across studies. Likewise, finerenone reduced the risk of renal disease progression by 15–23%, depending on the endpoint, and by 14% the risk of cardiovascular events after 3 years of follow-up.54 The benefits of finerenone were consistent and it was a well-tolerated treatment, regardless of baseline eGFR, uACR and the use of SGLT2 inhibitors or GLP-1 receptor agonists (Table 5).52–55 Thus, in the FIDELITY study, adverse events related to hyperkalemia occurred more frequently with finerenone (14.0%) vs. placebo (6.9%), but no adverse events related to hyperkalemia were fatal and only a small proportion led to permanent discontinuation of treatment (1.7% vs. 0.6%) or hospitalization (0.9% vs. 0.2%, respectively). Patients receiving finerenone had a moderate reduction in mean systolic blood pressure compared to patients receiving placebo at 4 months of treatment (–3.2 vs +0.5 mmHg). No greater number of cases of gynecomastia were reported with finerenone.54 Finally, different real-life studies with finerenone in patients with T2DM and CKD have reported results consistent with clinical trials.55,56 Regarding practical management, the starting dose will be 20 mg/day in case of eGFR ≥ 60 ml/min/1.73 m2 and 10 mg daily if eGFR 25−59 ml/min/1.73 m2. In the case of serum potassium increasing over 5.5 mmol/l, treatment should be interrupted and subsequently reconsider starting with the 10 mg dose, while if the initial dose was 10 mg and potassium remains ≤ 4.8 mmol/l, a rise to the 20 mg dose could be considered, if eGFR presented changes by < 30%. With respect to control analyses, serum potassium and glomerular filtration rate should be measured basally and at 4 weeks after initiation, to restart or increase of finerenone dose.21
Main results of Phase III clinical trials with finerenone in patients with T2DM and CKD.
| FIDELIO DKD (n = 5734) | FIGARO DKD (n = 7437) | FIDELITY (n = 13,171) | |
|---|---|---|---|
| Study characteristics | |||
| Design | Phase III, multicenter, randomized, double-blind, placebo-controlled clinical trial. | Pooled analysis FIDELIO DKD and FIGARO DKD | |
| Inclusion criteria. | Age ≥ 18 years, T2DM, maximum tolerated dose ACEIs/ARBs, serum potassium ≤ 4.8 mmol/l, CKD | ||
| CKD Definition. |
|
| |
| Primary variable. | Time to renal failure, sustained GFR ≥ 40% decline from baseline levels or renal death | Time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for HF. | Renal variable: time to renal failure, sustained eGFR decrease ≥ 57% from baseline levels or renal death |
| CV variable: time to CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for HF | |||
| Secondary variable. | Time to CV death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for HF | Time to renal failure, sustained eGFR decline ≥ 40% from baseline or renal death | |
| Baseline characteristics | |||
|---|---|---|---|
| Age, years | 65.6 | 64.1 | 64.8 |
| Sex (male), % | 70.2 | 69.4 | 69.8 |
| HbA1c, % | 7.7 | 7.7 | 7.7 |
| eGFR, ml/min/1.72 m2 | 44.3 | 67.8 | 57.6 |
| uACR ≥ 300 mg/g, %. | 87.5 | 50.7 | 66.7 |
| ACEI or ARBs, % | 100 | 100 | 100 |
| SGLT2 inhibitors, % | 4.6 | 8.4 | 6.7 |
| GLP-1 receptor agonists, % | 6.9 | 7.5 | 7.2 |
| Results | |||
|---|---|---|---|
| Median follow-up, years | 2.6 | 3.4 | 3.0 |
| Renal failure, sustained decrease eGFR ≥ 40% from baseline levels or renal death. | RR: 0.82 (IC 95%: 0.73−0.93) | RR: 0.87 (IC 95%: 0.76−1.01) | RR: 0.85 (IC 95%: 0.77−0.93). |
| Renal failure, sustained eGFR ≥ 57% decrease from baseline levels or renal death | RR: 0.68 (IC 95%: 0.55−0.82) | RR: 0.77 (IC 95%: 0.60−0.99) | RR: 0.77 (IC 95%: 0.67−0.88) |
| Renal insufficiency | RR: 0.87 (IC 95%: 0.72−1.05) | RR: 0.72 (IC 95%: 0.49−1.05) | RR: 0.84 (IC 95%: 0.71−0.99) |
| End-stage renal disease | RR: 0.86 (IC 95%: 0.67−1.10) | RR: 0.64 (IC 95%: 0.41−0.99) | RR: 0.80 (IC 95%: 0.64−0.99). |
| CV death, nonfatal myocardial infarction, nonfatal stroke, hospitalization IC | RR: 0.86 (IC 95%: 0.75−0.99) | RR: 0.87 (IC 95%: 0.76−0.98) | RR: 0.86 (IC 95%: 0.78−0.95). |
ARBs: angiotensin II receptor antagonists; uACR: albumin creatinine ratio; CV: cardiovascular; T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; FIDELIO-DKD: Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease; FIGARO-DKD: Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease; GLP-1: glucagon-like peptide type 1; HbA1c: glycosylated hemoglobin; HF: heart failure; ACEIs: angiotensin-converting enzyme inhibitors; SGLT2 inhibitors: sodium-glucose cotransporter 2 inhibitors; RR: relative risk.
Cardiovascular benefits of finerenone in different subgroups, in the FIDELITY study.
| Category | Finerenone (n = 6519. | Placebo (n = 6507) | RR (IC 95%) | p interaction |
|---|---|---|---|---|
| n/N (n/100PY) | n/N (n/100PY) | |||
| FGe ml/min/1.73 m2 | ||||
| <25 | 11/81 (5.2) | 23/81 (12.2) | 0.48 (0.22−1.03) | 0.14 |
| 25–<45 | 321/2.117 (5.7) | 331/2.115 (5.8) | 0.94 (0.81−1.10) | |
| 45–<60 | 197/1.717 (4.0) | 247/1.717 (5.1) | 0.80 (0.66−0.97) | |
| ≥60 | 295/2.603 (3.6) | 337/2.592 (4.2) | 0.87 (0.74−1.01) | |
| CAC mg/g | ||||
| <30 | 10/120 (2.4) | 15/110 (4.3) | 0.59 (0.24−1.45) | 0.41 |
| 30–<300 | 260/1.076 (3.8) | 292/2.023 (4.4) | 0.86 (0.73−1.02) | |
| ≥300 | 554/4.321 (4.7) | 631/4.371 (5.4) | 0.89 (0.79−1.00) | |
| iSGLT-2 baseline | ||||
| No | 786/6.081 (4.4) | 887/6.068 (5.1) | 0.87 (0.79−0.96) | 0.41 |
| Yes. | 39/438 (3.0) | 52/439 (4.1) | 0.63 (0.40-< 1.00) | |
| Basal GLP-1 agonist. | ||||
| No | 767/6.022 (4.4) | 875/6.060 (5.0) | 0.87 (0.79−0.96) | 0.63 |
| Yes. | 58/497 (3.8) | 64/447 (4.9) | 0.76 (0.52−1.11) | |
uACR: albumin creatinine ratio; eGFR: estimated glomerular filtration rate; GLP-1: glucagon-like peptide type 1; SGLT2 inhibitors: sodium-glucose cotransporter 2 inhibitors; PY: patient-years; RR: relative risk.
For all these reasons, finerenone is positioned as an important drug in the overall strategy for the comprehensive treatment of patients with T2DM and CKD with albuminuria, since it is the only one that specifically affects inflammation and fibrosis.
Compared to conventional treatment, the combination of SGLT2 inhibitors, GLP-1 receptor agonists and finerenone in patients with T2DM and albuminuria reduces major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke or cardiovascular death) by 35% (RR: 0.65; CI 95%: 0.55−0.76), highlighting the importance of multifactorial holistic treatment.57
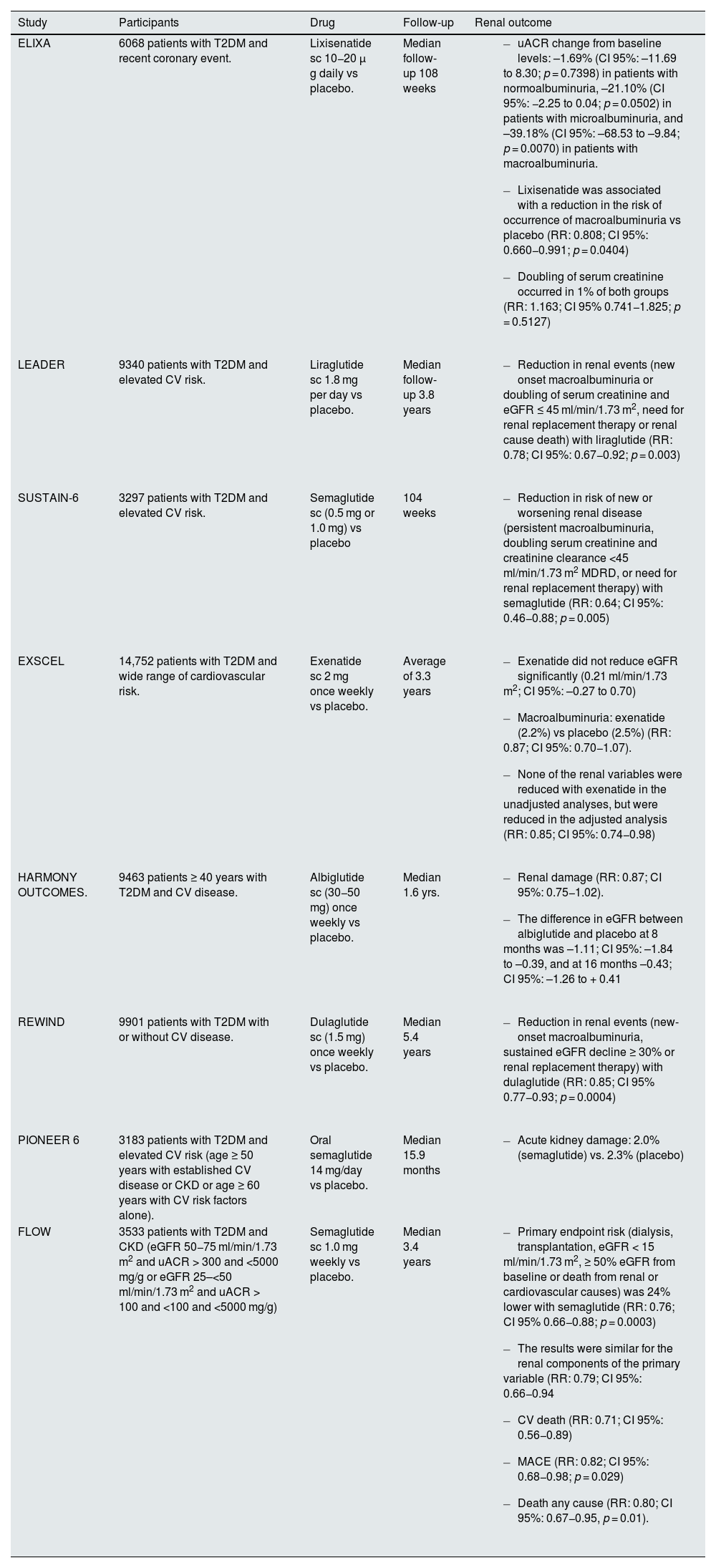
GLP-1 receptor agonistsSome GLP-1 receptor agonists have shown beneficial effects at the renal level in patients with T2DM (Table 6).58–65 However, except for the FLOW study, performed specifically in the T2DM and CKD population, the rest of the clinical trials have been performed in patients with T2DM, perhaps with elevated cardiovascular risk, but only some with CKD.58–64
Effect of GLP-1 receptor agonists on renal events in clinical trials in patients with T2DM.
| Study | Participants | Drug | Follow-up | Renal outcome |
|---|---|---|---|---|
| ELIXA | 6068 patients with T2DM and recent coronary event. | Lixisenatide sc 10−20 μ g daily vs placebo. | Median follow-up 108 weeks |
|
| LEADER | 9340 patients with T2DM and elevated CV risk. | Liraglutide sc 1.8 mg per day vs placebo. | Median follow-up 3.8 years |
|
| SUSTAIN-6 | 3297 patients with T2DM and elevated CV risk. | Semaglutide sc (0.5 mg or 1.0 mg) vs placebo | 104 weeks |
|
| EXSCEL | 14,752 patients with T2DM and wide range of cardiovascular risk. | Exenatide sc 2 mg once weekly vs placebo. | Average of 3.3 years |
|
| HARMONY OUTCOMES. | 9463 patients ≥ 40 years with T2DM and CV disease. | Albiglutide sc (30−50 mg) once weekly vs placebo. | Median 1.6 yrs. |
|
| REWIND | 9901 patients with T2DM with or without CV disease. | Dulaglutide sc (1.5 mg) once weekly vs placebo. | Median 5.4 years |
|
| PIONEER 6 | 3183 patients with T2DM and elevated CV risk (age ≥ 50 years with established CV disease or CKD or age ≥ 60 years with CV risk factors alone). | Oral semaglutide 14 mg/day vs placebo. | Median 15.9 months |
|
| FLOW | 3533 patients with T2DM and CKD (eGFR 50−75 ml/min/1.73 m2 and uACR > 300 and <5000 mg/g or eGFR 25–<50 ml/min/1.73 m2 and uACR > 100 and <100 and <5000 mg/g) | Semaglutide sc 1.0 mg weekly vs placebo. | Median 3.4 years |
|
uACR: albumin creatinine ratio; CV: cardiovascular; T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; GLP-1: glucagon-like peptide type 1; 95% CI: 95% confidence interval; RR: relative risk.
The FLOW study included 3533 patients with T2DM and CKD, defined by an eGFR of 50 to 75 ml/min/1.73 m2 and a urinary uACR of >300 and <5.000 mg/g or an eGFR of 25 to <50 ml/min/1.73 m2 nd a urinary uACR of >100 and <5000 mg/g. Patients were randomized to receive subcutaneous semaglutide (1.0 mg weekly) or placebo. Semaglutide reduced uACR by 32%. The primary study endpoint was severe kidney disease events, consisting of a combination of occurrence of renal failure (dialysis, transplantation, or eGFR of <15 ml/min/1.73 m2), a ≥50% reduction in eGFR from baseline, or death from renal or cardiovascular causes. After a median follow-up of 3.4 years, semaglutide reduced the risk of the primary endpoint by 24%, the composite endpoint of renal events by 21%, cardiovascular mortality by 29%, risk of MACE by 18%, and death from any cause by 20%. The risk of serious adverse effects was lower in the semaglutide group, although the risk of discontinuation due to adverse effects was more frequent with semaglutide compared to placebo (13.2% vs. 11.9%), due to a higher number of gastrointestinal side effects (4.5% vs. 1.1%).65
The beneficial renal effects of GLP-1 receptor agonists would be independent of their glycemic effect. Thus, these drugs exert beneficial effects on several cardiovascular risk factors, such as arterial hypertension, lipid metabolism or weight loss, which, together with natriuresis, would have a reducing effect on albuminuria. In addition, inhibition of the sodium-hydrogen 3 exchanger (NHE3) at the proximal level would have an effect on natriuresis and secondarily, blood pressure. GLP-1 receptor agonists would also improve renal hemodynamic function by reducing glomerular hyperfiltration through activation of tubuloglomerular feedback. Similarly, anti-inflammatory, antioxidant and anti-ischemic properties have been described with GLP-1 receptor agonists.66–68
Furthermore, given that adiposity plays a central role in the etiopathogenesis of cardio-reno-metabolic syndrome, GLP-1 receptor agonists would be of particular interest in this clinical context, since they have been shown to be protective at the renal, cardiovascular and metabolic levels, in addition to reducing appetite, weight loss and improved glycemic control.16
Finally, small clinical trials and observational studies are showing modest benefits at the glycemic level and on body weight, both in patients with end-stage renal disease and in renal transplant patients. In any case, it will take more studies to assess the effects in these populations.69,70
Current perspective and recommendations for improvementThe 2025 American Diabetes Association (ADA) guidelines advise, with a level of recommendation A, the use of finerenone in patients with T2DM, CKD (eGFR ≥ 25 ml/min/1.73 m2) and albuminuria to reduce cardiovascular events and progression of CKD,11 whereas the 2024 Spanish Society of Nephrology consensus for the management of diabetic nephropathy suggests the use of finerenone in patients with T2DM, an eGFR ≥ 25 ml/min/1,73 m2, normal serum potassium concentration (≤5.1 mmol/l) and albuminuria (uACR ≥ 30 mg/g), despite the maximum tolerated doses of a RAASi, with a level of recommendation 1A.12
In short, the treatment of patients with CKD and T2DM should follow a comprehensive strategy, which requires a global approach that includes both the control of the different cardiovascular risk factors and the use of those drugs that have demonstrated a benefit at the renal and cardiovascular level, mainly ACEI or ARBs, SGLT2 inhibitors, finerenone and GLP-1 receptor agonists. In addition, the combination of these treatments should not be delayed in time, since this would allow the progression of CKD, as well as the risk of presenting cardiovascular complications. Instead, clinicians should start treatment early, including in combination, in an individualized manner, according to the clinical characteristics of the patients.38,71–80
On the other hand, although it is recommended that albuminuria is routinely assessed in patients with DM, independently of eGFR values at least once a year,11,12 this practice is uncommon, which leads to underdiagnosis of CKD.7
Measures of great interest and help to confirm albuminuria in clinical practice would be to standardize screening with uACR and eGFR measurement in primary care, the implementation of specific training and educational activities, and the use of new technologies.80,81 Regarding the latter, the use of the Nefroconsultor mobile app is a clinical decision support system that can improve appropriate referrals by 28%, as well as determine albuminuria at the time of referral.80,81
Another important measure would be to establish joint protocols with different hospital specialties involved in the management of this population (endocrinology, nephrology, internal medicine, cardiology), emphasizing the importance of continuity of care between different levels of care, where the roles of each professional and the criteria for referral to each of the specialties involved are clearly defined in protocols.5,12,82–85
Finally, to improve the management of patients with T2DM and CKD with albuminuria, we propose 10 recommendations, summarized in Table 7.
Recommendations for the improvement in the integrated management of people with T2DM and CKD with albuminuria.
| 1. Importance of albuminuria | Albuminuria is a crucial marker of renal impairment and cardiovascular risk in people with diabetes, requiring appropriate assessment and management to prevent associated complications |
| 2. Early diagnosis | Regular testing for albuminuria in people with diabetes is recommended, using the urine albumin/creatinine ratio as the standard method of assessment. The use of the mobile app Nefroconsultor could be useful. |
| 3. Multidisciplinary approach and referral criteria | Collaboration between all levels of care should be encouraged for an adequate integrated management, recommending that primary care should act as coordinator and manager of chronicity. |
| 4. Effective pharmacological interventions | Comprehensive treatment with ACEI/ARBs, SGLT2 inhibitors, GLP-1 receptor agonists and finerenone are advised as effective treatments to reduce the progression of renal disease and cardiovascular protection in patients with diabetes and chronic kidney disease, in accordance with the recommendations of clinical practice guidelines |
| 5. Vascular risk management | Control of blood pressure and lipids is essential, as well as the promotion of healthy lifestyle habits, including a balanced diet and regular exercise. |
| 6. Patient education | Educational programs should be implemented to address diabetes, renal health and the use of drugs with cardiorenal protection, empowering patients in their self-management and management of the disease. |
| 7. Adherence to treatment | The importance of promoting adherence to treatment through self-management strategies and close follow-up by the healthcare team should be emphasized. |
| 8. Continuous evaluation | Success criteria should be established to evaluate the effectiveness of treatment, including reduction of albuminuria and improvement in renal function. |
| 9. Use of technology | The implementation of technologies for monitoring albuminuria, renal function and early detection of clinical events is recommended. Tools such as remote monitoring devices and mobile applications can facilitate patient follow-up and communication with the healthcare team. |
| 10. Research and updating | The medical community should be encouraged to promote ongoing research on new therapies and approaches in the management of diabetes with albuminuria, updating clinical practices based on recent findings |
GLP-1: glucagon-like peptide type 1; ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin II receptor antagonists; T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; SGLT2: sodium-glucose cotransporter 2.
DM is a chronic disease that, when accompanied by albuminuria, significantly increases the risk of presenting renal, cardiovascular and metabolic complications. Therefore, the detection of albuminuria is a basic element from the beginning of the diagnosis of DM. Holistic treatment, which allows management of different factors involved in the development of diabetic kidney disease, is the best approach to reduce the risk of progression of kidney disease and the development of cardiovascular complications. The use of ACEIs or ARBs, SGLT2 inhibitors, finerenone and GLP-1 recept agonists with proven renal benefit constitutes the main management of patients with T2DM, CKD and albuminuria.
FundingContent Ed Net provided editorial assistance in the writing of this article with funding from Bayer Hispania.
RFO has participated in consultancies with Novartis, Sanofi, Amgen, Amarin, and has received honoraria for papers from Novo Nordisk, Amarin, Novartis, Sanofi, Organon.
JCFG has received speaking fees from Novo Nordisk, Lilly, Boehringer-Ingelheim, Menarini, Esteve, and AstraZeneca.
JCF-G was supported by the Intensification Research Program (INT21/00078, ISCIII, Spain; cofunded by the Fondo Europeo de Desarrollo Regional FEDER).
The authors have participated in an Advisory Board funded by Bayer, in which scientific and clinical aspects related to the use of finerenone in the management of chronic kidney disease and heart failure were discussed. They have received fees from Bayer for their participation in the Advisory Board. However, Bayer has not influenced the writing, analysis, or interpretation of the content of this manuscript.