Hyperkalemia (HK) is a common electrolyte disorder in chronic kidney disease (CKD), mainly in the advanced stages. A positive potassium balance due to reduced renal excretory capacity is likely the main pathogenic mechanism of HK. Research into the relative role of each pathogenic element in the development of HK in CKD may help to implement more suitable therapies.
ObjectiveTo investigate renal potassium handling in advanced CKD patients, and to determine the differences between patients with or without HK.
Material and methodsCross-sectional observational study in adult patients with stage 4–5 CKD pre-dialysis. Selection criteria included clinically stable patients and the ability to collect a 24h urine sample correctly. Blood and urinary biochemical parameters were analyzed including sodium and potassium (K). Fractional excretion of K (FEK) and K load relative to glomerular filtration (Ku/GFR) were calculated. HK was defined as a serum K concentration ≥5.5mmol/l.
ResultsThe study group consisted of 212 patients (mean age 65±14 years, 92 females) with a mean GFR of 15.0±4.2mL/min/1.73m2. 63 patients (30%) had HK. Patients with HK had lower mean bicarbonate levels with respect to patients with normal K levels (NK) (20.3±3.1 vs. 22.8±3.2 mEq/l, P<.0001), but no differences were noted in total urinary sodium and K excretion. While mean FEK values were lower in patients with HK (32.1±12.1% vs. 36.4±14.3%, P=.038), Ku / GFR values were significantly greater with respect to the NK subgroup (4.2±1.5 vs. 3.7±1.4mmol/mL/min, P=0.049). FEK showed a strong linear correlation with Ku / GFR (R2=0.74), and partial linear regressions demonstrated that at a similar Ku/GFR level, the FEK of patients with HK was lower than that of NK patients. By multivariate linear and logistic regression analyses, both FEK and Ku/GFR were shown to be the main determinants of K serum levels and HK.
ConclusionsAlthough the K load relative to glomerular filtration (Ku / GFR) is an important determinant of HK in advanced CKD, the most noteworthy characteristic associated with HK in these patients was the limitation of compensatory urinary K excretion, as indicated by lower FEK.
La hipercaliemia (HK) es un hallazgo frecuente en la enfermedad renal crónica(ERC), sobre todo en sus estadios más avanzados. El mecanismo patogénico más comúnde esta alteración es la ingesta-absorción de potasio que sobrepasa la capacidad excretorarenal. La investigación sobre el papel relativo de cada uno de los elementos patogénicos enel desarrollo de HK podría ayudar a su tratamiento.
ObjetivoAnalizar el manejo renal de potasio en pacientes con ERC avanzada prediálisis, yestablecer qué diferencias existen entre los que presentan o no HK.
Material y métodosEstudio transversal de observación en pacientes adultos con ERC estadio4–5 prediálisis. Entre los pacientes incidentes en la consulta ERCA se seleccionaron aque-llos clínicamente estables con capacidad para recoger adecuadamente la orina de 24 horas. Se midieron parámetros bioquímicos en sangre y orina que incluyeron las concentracio-nes de sodio y potasio (K). Se calculó la fracción de excreción de K (FEK) y la carga de Krelativa al filtrado glomerular (Ko/FG). Se definió la HK como una concentración de K sérico≥ 5,5 mmol/l.
ResultadosSe incluyeron 212 pacientes (edad 65 ± 14 a˜nos, 92 mujeres) con un FG15,0 ± 4,2 ml/min/1,73 m2. Sesenta y tres pacientes (30%) presentaban HK. Los pacientescon HK tenían un bicarbonato sérico más bajo (20,3 ± 3,1 vs. 22,8 ± 3,2 mEq/l, p < 0,0001),y un menor filtrado glomerular (14,1 ± 3,3 vs. 15,4 ± 4,4 ml/min/1,73 m2, p = 0,028), pero nomostraban diferencias en la excreción urinaria total de sodio o K. La FEK era inferior enlos pacientes con HK con respecto a los que presentaban normocaliemia (32,1 ± 12,1% vs.36,4 ± 14,3%, p = 0,038), mientras que la Ko/FG fue mayor (4,2 ± 1,5 vs. 3,7 ± 1,4 mmol porcada ml/min, p = 0,049). Existía una fuerte correlación lineal entre Ko/FG y FEK (R2= 0,74), yen regresiones parciales se observó que a igual carga de K, la FEK era inferior en los pacientescon HK. Mediante regresión lineal y regresión logística multivariable, tanto la FEK como laKo/FG fueron los principales determinantes del K sérico y de la HK.
ConclusionesAunque la carga de K relativa a la función renal (Ko/FG) se asocia de formarelevante a la HK de la ERC, la principal característica asociada a esta alteración bioquímicaes la incompleta excreción renal compensatoria de K, expresada como una menor FEK.
Hyperkalemia (HK) is a common electrolyte disorder in chronic kidney disease (CKD), especially advanced stages of CKD.1–5
The main pathogenic mechanisms involved in the development of HK in CKD are6–12: an intake with intestinal absorption of K that exceeds the excretion capacity by the kidneys, which may be limited by an interference in the mechanisms of tubular adaptation to a high load of K, especially those related to the concentration and / or action of aldosterone in the distal tubule. In addition, a redistribution of intracellular K in the case of metabolic acidosis, especially the mineral form — not the one caused by an excess organic acids.13,14
Despite the accumulation of factors predisposing to the development of HK, the kidney is able to maintain the K balance until the glomerular filtrate decreases below 10–15ml/min.8–11 Adaptations to normalize K levels include an increase in tubular secretion of K that eventually leads to a new equilibrium state of K levels, which often remain higher than normal (chronic HK).6–12 An increase in intestinal excretion of K is another adaptive mechanism that develops gradually in patients with CKD.6,15,16
The information on the management of K in CKD comes mainly from the translation of experimental research6–12; results from this investigation do to always answer questions frequently asked by physicians in the daily clinical practice, such as: is the HK in CKD solely the result of a greater (dietary) load of K?, how much is the safe daily load of K in relation to a given glomerular filtration?, what compensation levels of renal excretion of K can be achieved in advanced CKD? What are the main determinants of chronic HK in these patients? Research on these issues could be useful to develop strategies for the management of HK's in advanced CKD.
To investigate on the management of potassium under real clinical conditions in patients with advanced CKD, a cross-sectional study was conducted, in which the main differences between those who present or not HK are analyzed.
Material and methodsThis is an observational cross-sectional study in a cohort of adult predialysis patients with CKD stage 4–5. Among the incident patients in the advanced CKD outpatient clinic during the period from September 2009 to May 2015, we selected those >18 years, with estimated glomerular filtration rate <30mL/min/1.73m2, and able to correctly collect the 24-hour urine (see the definition of this criterion below).
Patients not clinically stable (heart, liver or respiratory failure, cancer, active infection, hypercatabolic states or severe gastrointestinal disorders), with a recent acute renal failure, on treatment with K-sparing diuretics (spironolactone or eplerenone), corticosteroids, fludrocortisone or cation exchange resins were excluded.
Included patients attended regularly the advanced CKD outpatient clinic. In addition to the conventional biochemical blood parameters - urea, creatinine, albumin, sodium, chloride, potassium and bicarbonate - creatinine, urea, sodium and potassium concentrations were also determined in all patients in urine collected during the 24h prior to the collection of blood samples.
Biochemical determinations were performed by conventional laboratory methods (Advia Chemistry, Siemens Healthcare Diagnostics).
Glomerular filtration rate was estimated using the abbreviated formula MDRD.17
The concentration of K was measurement in serum, not plasma. To detect possible interferences and errors in the measurement of serum K, a systematic review was performed by hemolytic index (HI), and all samples with a value considered as significant interference (HI>40) were discarded.
To verify the reliability of the 24-hour urine collection, the measured total excretion of creatinine was compared with that expected according to the anthropometric characteristics according to the formula of Ix et al.18 As a selection criterion for inclusion in the study, total creatinine in 24h urine should not differ ± 20% from the estimated creatinine excretion.
Through the total urinary excretion of urea nitrogen, the protein catabolism rate was calculated using the formula of Maroni et al.19
The amount of K collected in the 24-hour urine was taken as a total K load, this parameter referring to the approximate amount of the K ingested-absorbed, minus the amount excreted in the intestine, assuming in all patients, as a constant, both the amount excreted by the intestine and the endogenous balance.
The fractional excretion of K (FEK) was calculated according to the formula:
and the K load relative to glomerular filtration (Ku/GFR):Ku/GFR=(K in 24h urine)/ GFR, where GFR was measure by the Brochner-Mortensen formula20:
The net endogenous acid generation was also calculated according to the formula of Frassetto et al.21
HK was defined as a concentration of serum K≥5.5mEq/l.
The data were recorded at the first visit to the advanced CKD clinic, before the patients were instructed to make any diet restriction, although it is worth to mention the tendency to restrict some foods rich in K by the own patients or following recommendations by other nephrologists or primary care doctors.
Study design and statistical analysisCross-sectional study in which the data collected in the selected patients were analyzed, comparing the parameters of interest in patients with or without HK.
For the descriptive comparison of the continuous variables, and depending on their characteristics, parametric or non-parametric tests were used. Chi-square test was used for the comparison of categorical variables. The Kolmogorov-Smirnov test was used to determine whether the distribution of a quantitative variable followed a normal distribution.
In the total group, the best determinants of the existence of HK were analyzed by multivariate logistic regression. Simple linear regression analysis was used to establish the existence of an association between continuous variables and to represent it graphically. Multiple linear regression was used to investigate the best determinants of K levels (as a continuous variable). To determine the goodness of fit of the model, the coefficient of determination (R2) was calculated. To avoid overfitting in multivariate models, the variables were forced to enter with at least a significance of P≤0.01, with automatic backward conditional selection. A multicollinearity test was performed calculating the variance inflation factor (VIF). It was considered that some of the variables included in the multivariate models had significant collinearity if the VIF was >10.
Data are presented as mean±standard deviation. A P<0.05 indicated statistical significance. Statistical analysis and graphics were performed using the SPSS version 24.0 program (IBM Corp. Armonk, USA).
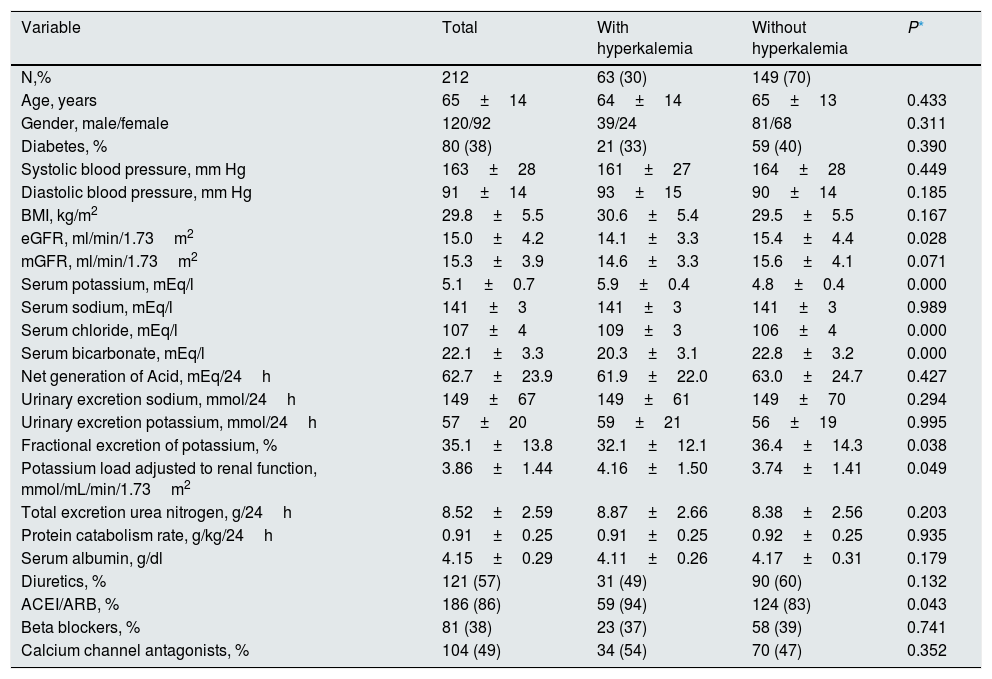
ResultsDuring the inclusion period, 608 patients were studied in the advanced CKD outpatient clinic, of which 54 were excluded due to clinical instability or recent acute renal failure, 17 were exclude because samples had high hemolytic index, 27 were not considered because of treatment with cation exchange resins, 10 were on treatment with diuretics sparing K and 288 were not included because they failed to meet the criteria for correct 24-hour urine collection. Thus, the patients finally included in the study were 212 with the demographic, clinical and biochemical characteristics shown Table 1.
Demographic, clinical and biochemical characteristics of the total study group, and according to the presence of hyperkalemia.
| Variable | Total | With hyperkalemia | Without hyperkalemia | P* |
|---|---|---|---|---|
| N,% | 212 | 63 (30) | 149 (70) | |
| Age, years | 65±14 | 64±14 | 65±13 | 0.433 |
| Gender, male/female | 120/92 | 39/24 | 81/68 | 0.311 |
| Diabetes, % | 80 (38) | 21 (33) | 59 (40) | 0.390 |
| Systolic blood pressure, mm Hg | 163±28 | 161±27 | 164±28 | 0.449 |
| Diastolic blood pressure, mm Hg | 91±14 | 93±15 | 90±14 | 0.185 |
| BMI, kg/m2 | 29.8±5.5 | 30.6±5.4 | 29.5±5.5 | 0.167 |
| eGFR, ml/min/1.73m2 | 15.0±4.2 | 14.1±3.3 | 15.4±4.4 | 0.028 |
| mGFR, ml/min/1.73m2 | 15.3±3.9 | 14.6±3.3 | 15.6±4.1 | 0.071 |
| Serum potassium, mEq/l | 5.1±0.7 | 5.9±0.4 | 4.8±0.4 | 0.000 |
| Serum sodium, mEq/l | 141±3 | 141±3 | 141±3 | 0.989 |
| Serum chloride, mEq/l | 107±4 | 109±3 | 106±4 | 0.000 |
| Serum bicarbonate, mEq/l | 22.1±3.3 | 20.3±3.1 | 22.8±3.2 | 0.000 |
| Net generation of Acid, mEq/24h | 62.7±23.9 | 61.9±22.0 | 63.0±24.7 | 0.427 |
| Urinary excretion sodium, mmol/24h | 149±67 | 149±61 | 149±70 | 0.294 |
| Urinary excretion potassium, mmol/24h | 57±20 | 59±21 | 56±19 | 0.995 |
| Fractional excretion of potassium, % | 35.1±13.8 | 32.1±12.1 | 36.4±14.3 | 0.038 |
| Potassium load adjusted to renal function, mmol/mL/min/1.73m2 | 3.86±1.44 | 4.16±1.50 | 3.74±1.41 | 0.049 |
| Total excretion urea nitrogen, g/24h | 8.52±2.59 | 8.87±2.66 | 8.38±2.56 | 0.203 |
| Protein catabolism rate, g/kg/24h | 0.91±0.25 | 0.91±0.25 | 0.92±0.25 | 0.935 |
| Serum albumin, g/dl | 4.15±0.29 | 4.11±0.26 | 4.17±0.31 | 0.179 |
| Diuretics, % | 121 (57) | 31 (49) | 90 (60) | 0.132 |
| ACEI/ARB, % | 186 (86) | 59 (94) | 124 (83) | 0.043 |
| Beta blockers, % | 81 (38) | 23 (37) | 58 (39) | 0.741 |
| Calcium channel antagonists, % | 104 (49) | 34 (54) | 70 (47) | 0.352 |
eGFR: estimated glomerular filtration rate (MDRD); mGFR: measured glomerular filtration (Bröchner-Mortensen equation).
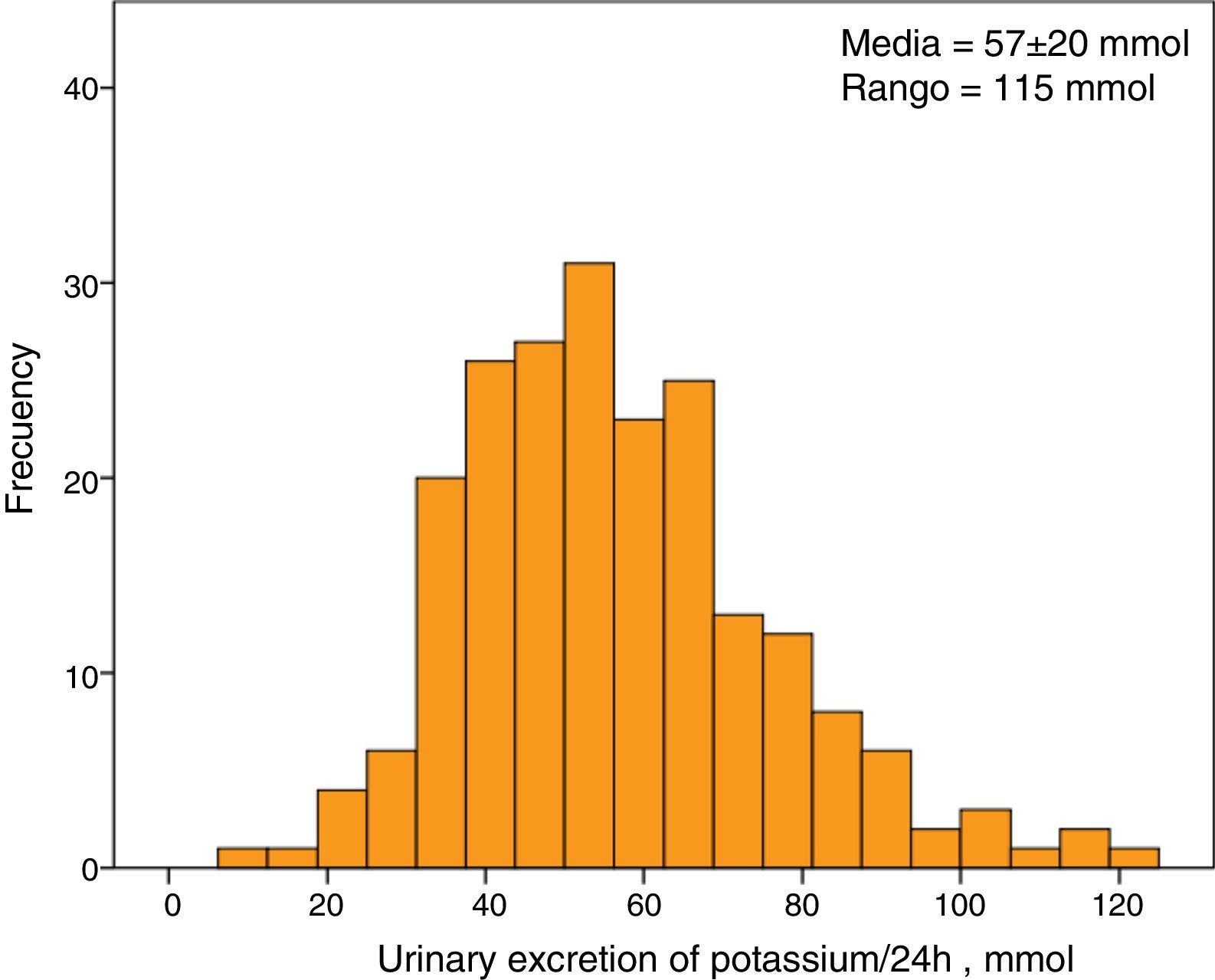
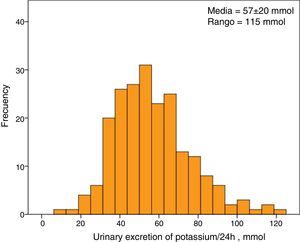
The average serum K concentration of the study group was 5.1±0.7mmol/l. A 30% of patients (63 patients) showed HK. The average excretion of K in 24-hour urine was 57±20mmol, with a minimum value of 8mmol and a maximum of 123mmol. The distribution of urine K excretion values followed a normal distribution (Fig. 1).
Male showed a significantly higher urinary excretion of both K (62±20 vs. 49±17mmol/24h, p<0.0001) and sodium (168±71 vs. 123±53mmol / 24h, P<0.0001) as compared with females.
The main differences between those with and without HK were: a slightly more reduced renal function, with more metabolic acidosis and more frequently treated with inhibitors of the renin-angiotensin system (ACEI/ARB).
No differences in absolute urinary excretion of K and sodium were observed between patients with or without HK. However, the total amount of K collected in urine adjusted to the degree of renal insufficiency, that is, the load of K per ml/min/1.73m 2 of glomerular filtration rate (Ku/FG), was greater in patients with HK than in those with normal K (Table 1).
The values of fractional excretion of K (FEK) were distributed over a wide range, from a minimum of 5% to a maximum of 88%. The mean FEK was significantly lower in patients with HK (Table 1).
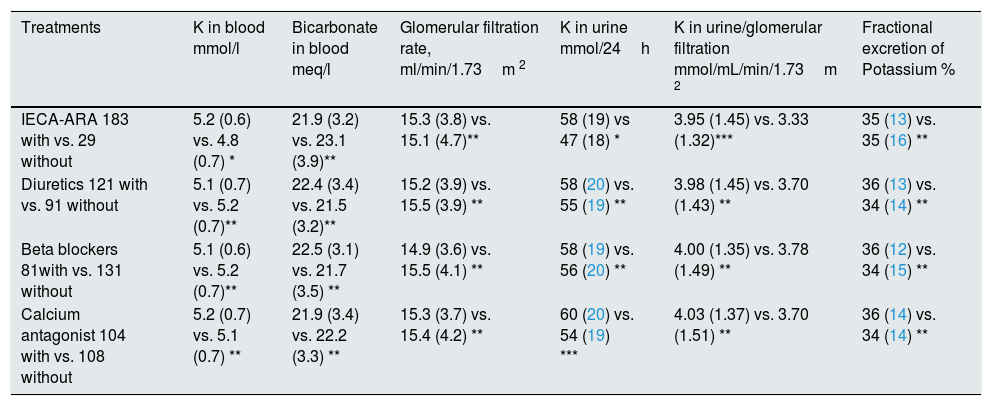
The differences in the main study parameters according to the prescribed medication are shown in Table 2.
Differences in the main study parameters according to the medication prescribed to the patients.
| Treatments | K in blood mmol/l | Bicarbonate in blood meq/l | Glomerular filtration rate, ml/min/1.73m 2 | K in urine mmol/24h | K in urine/glomerular filtration mmol/mL/min/1.73m 2 | Fractional excretion of Potassium % |
|---|---|---|---|---|---|---|
| IECA-ARA 183 with vs. 29 without | 5.2 (0.6) vs. 4.8 (0.7) * | 21.9 (3.2) vs. 23.1 (3.9)** | 15.3 (3.8) vs. 15.1 (4.7)** | 58 (19) vs 47 (18) * | 3.95 (1.45) vs. 3.33 (1.32)*** | 35 (13) vs. 35 (16) ** |
| Diuretics 121 with vs. 91 without | 5.1 (0.7) vs. 5.2 (0.7)** | 22.4 (3.4) vs. 21.5 (3.2)** | 15.2 (3.9) vs. 15.5 (3.9) ** | 58 (20) vs. 55 (19) ** | 3.98 (1.45) vs. 3.70 (1.43) ** | 36 (13) vs. 34 (14) ** |
| Beta blockers 81with vs. 131 without | 5.1 (0.6) vs. 5.2 (0.7)** | 22.5 (3.1) vs. 21.7 (3.5) ** | 14.9 (3.6) vs. 15.5 (4.1) ** | 58 (19) vs. 56 (20) ** | 4.00 (1.35) vs. 3.78 (1.49) ** | 36 (12) vs. 34 (15) ** |
| Calcium antagonist 104 with vs. 108 without | 5.2 (0.7) vs. 5.1 (0.7) ** | 21.9 (3.4) vs. 22.2 (3.3) ** | 15.3 (3.7) vs. 15.4 (4.2) ** | 60 (20) vs. 54 (19) *** | 4.03 (1.37) vs. 3.70 (1.51) ** | 36 (14) vs. 34 (14) ** |
With vs. without: number of patients being treated with or without the drug under study. Levels of statistical significance: * P<0.01. ** P>0.05. *** P<0.05.
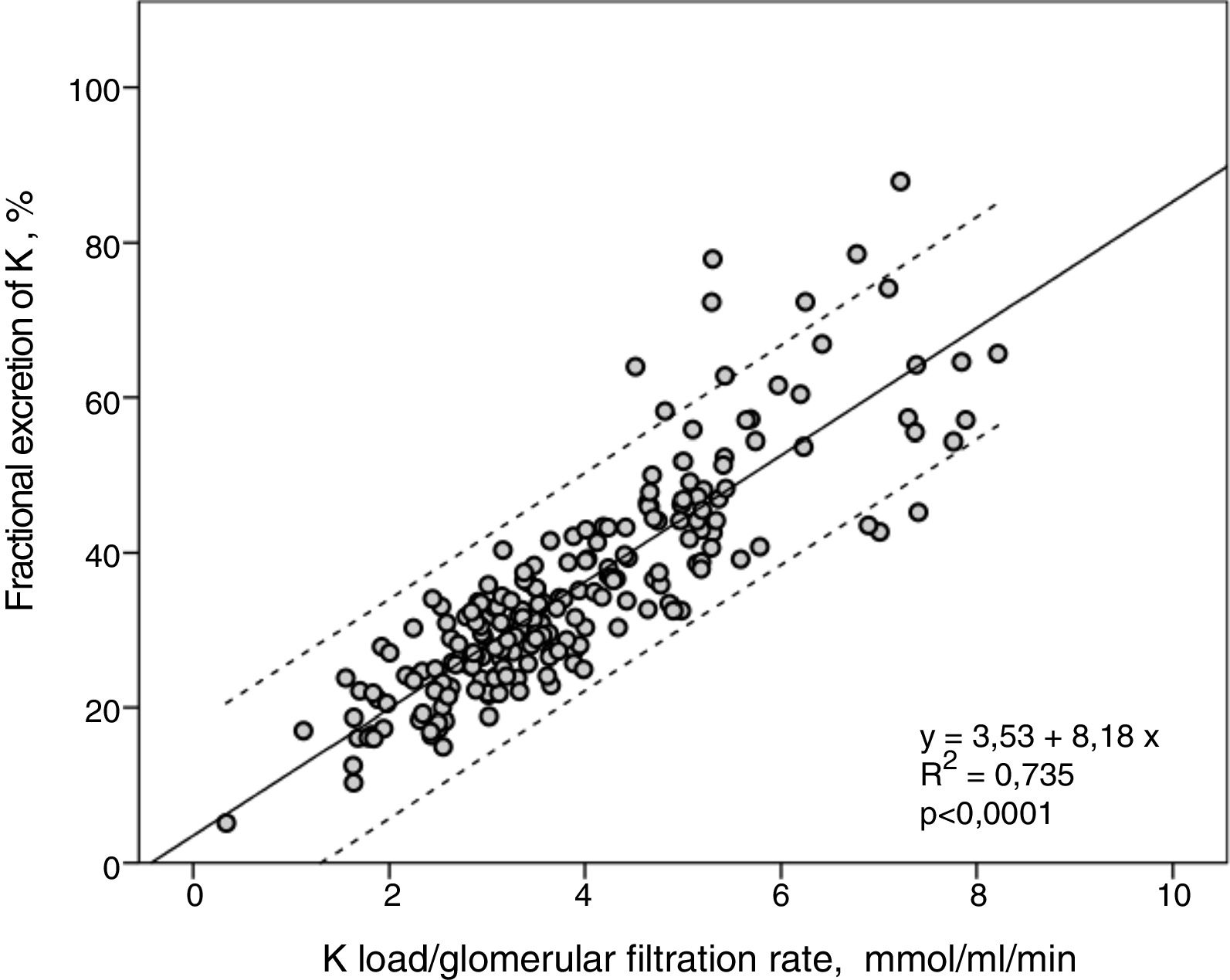
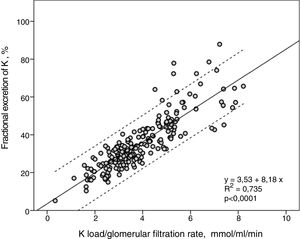
FEK and Ku / GFR showed a strong linear correlation (R2=0.74) (Fig. 2), so that the higher the K load per unit of renal function the greater the FEK.
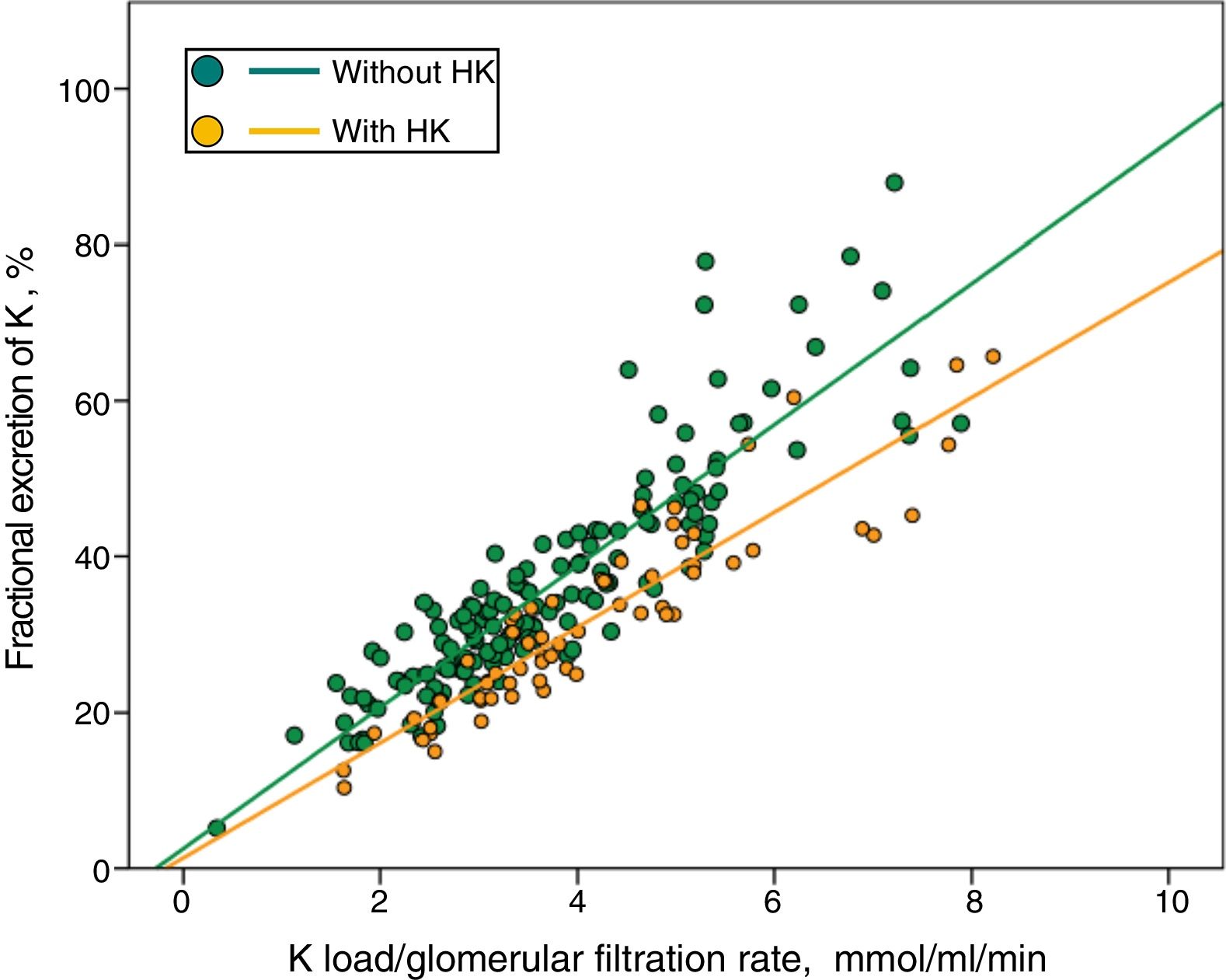
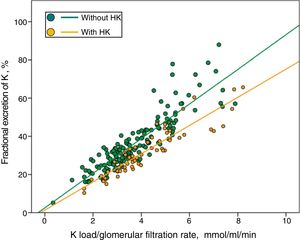
In Fig. 3 patients with or without HK were divided into separate regressions. It was observed that for the same Ku / GFR load, the FEK was lower in those patients with HK.
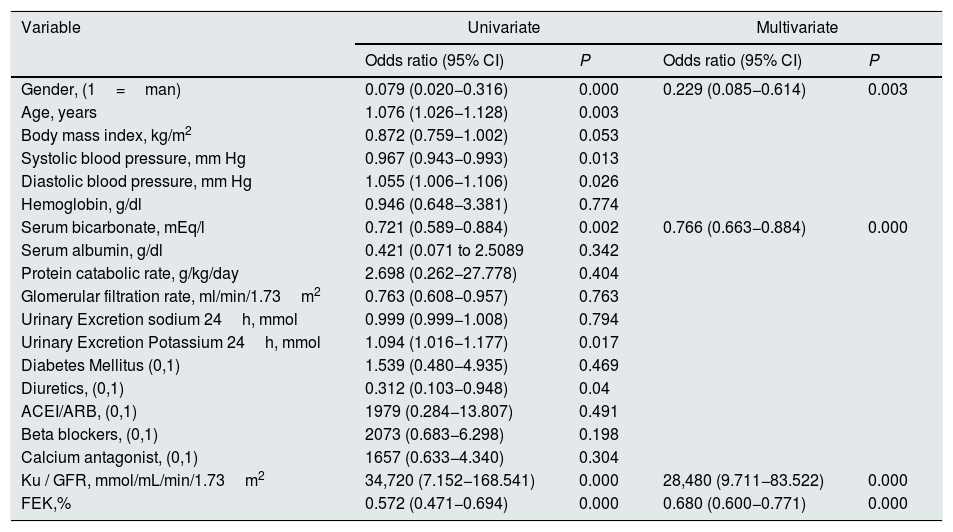
By multivariable logistic regression the main determinants of the presence of HK in this group of patients were (Table 3): gender (men would show a lower probability for the development of HK if the rest of the variables are taken into account), serum bicarbonate (inverse relationship with the risk of HK), but especially the load of K per unit of renal function (Ku/GFR) and the FEK.
Logistic regression on the determinants of the presence of hyperkalemia in the study group.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | |
| Gender, (1=man) | 0.079 (0.020−0.316) | 0.000 | 0.229 (0.085−0.614) | 0.003 |
| Age, years | 1.076 (1.026−1.128) | 0.003 | ||
| Body mass index, kg/m2 | 0.872 (0.759−1.002) | 0.053 | ||
| Systolic blood pressure, mm Hg | 0.967 (0.943−0.993) | 0.013 | ||
| Diastolic blood pressure, mm Hg | 1.055 (1.006−1.106) | 0.026 | ||
| Hemoglobin, g/dl | 0.946 (0.648−3.381) | 0.774 | ||
| Serum bicarbonate, mEq/l | 0.721 (0.589−0.884) | 0.002 | 0.766 (0.663−0.884) | 0.000 |
| Serum albumin, g/dl | 0.421 (0.071 to 2.5089 | 0.342 | ||
| Protein catabolic rate, g/kg/day | 2.698 (0.262−27.778) | 0.404 | ||
| Glomerular filtration rate, ml/min/1.73m2 | 0.763 (0.608−0.957) | 0.763 | ||
| Urinary Excretion sodium 24h, mmol | 0.999 (0.999−1.008) | 0.794 | ||
| Urinary Excretion Potassium 24h, mmol | 1.094 (1.016−1.177) | 0.017 | ||
| Diabetes Mellitus (0,1) | 1.539 (0.480−4.935) | 0.469 | ||
| Diuretics, (0,1) | 0.312 (0.103−0.948) | 0.04 | ||
| ACEI/ARB, (0,1) | 1979 (0.284−13.807) | 0.491 | ||
| Beta blockers, (0,1) | 2073 (0.683−6.298) | 0.198 | ||
| Calcium antagonist, (0,1) | 1657 (0.633−4.340) | 0.304 | ||
| Ku / GFR, mmol/mL/min/1.73m2 | 34,720 (7.152−168.541) | 0.000 | 28,480 (9.711−83.522) | 0.000 |
| FEK,% | 0.572 (0.471−0.694) | 0.000 | 0.680 (0.600−0.771) | 0.000 |
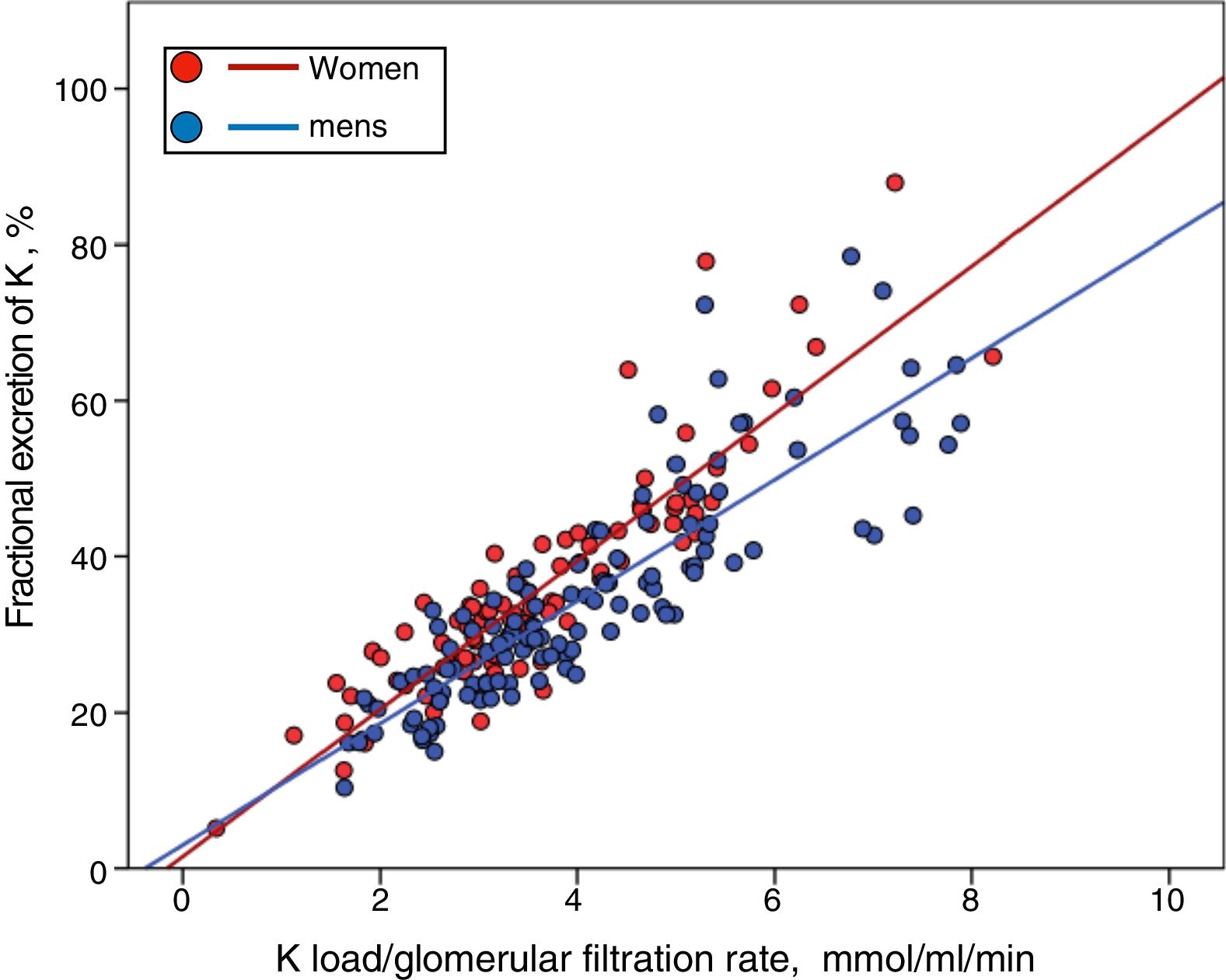
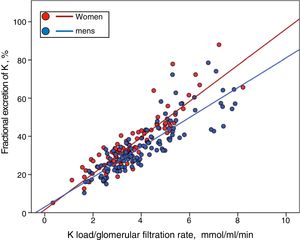
Although men showed a higher, but not significant, percentage of HK than women (33% vs. 26%), they also had a greater K load per unit of renal function (Ku/GFR 4.06±1. 51 vs. 3.61±1.33mmol/mL/min/1.73m2 ; P=0.027), but a similar FEK (34.7±13.6% vs. 35.7±14.1 %, P=0.581). Thus, if the relationship between the Ku/GFR and the FEK is represented separately according to gender, it is observed that for a given load of K the compensatory response is less in men than females (Fig. 4) (for a more extensive explanation of this finding, see section "Discussion").
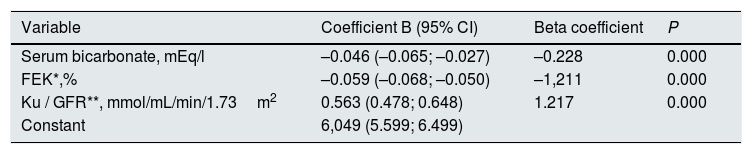
Through multiple linear regression, the main determinants of serum K concentration were serum bicarbonate, Ku/FG and FEK (Table 4).
Best determinants of serum K concentration by multiple linear regression analysis.
| Variable | Coefficient B (95% CI) | Beta coefficient | P |
|---|---|---|---|
| Serum bicarbonate, mEq/l | –0.046 (–0.065; –0.027) | –0.228 | 0.000 |
| FEK*,% | –0.059 (–0.068; –0.050) | –1,211 | 0.000 |
| Ku / GFR**, mmol/mL/min/1.73m2 | 0.563 (0.478; 0.648) | 1.217 | 0.000 |
| Constant | 6,049 (5.599; 6.499) |
Variables outside the best prediction equation: age, BMI, SBP, DBP, hemoglobin, albumin, protein catabolism rate, GFR, total urinary excretion of sodium, total urinary excretion of K, diabetes, diuretics, ACEI/ARA, blockers beta, calcium antagonists.
R2 model=0.542. The variance inflation factor of the variable serum bicarbonate in the model is 1.056; FEK of 3.987 and Ku/GFR of 3.937. FEK: Fractional excretion of potassium; Ku/GFR: potassium load per unit of renal function.
Despite the strong correlation between Ku/GFR and FEK, multicollinearity was ruled out in this multiple linear regression model by analysis of the variance inflation factor (Table 4).
DiscussionThe present study, on kidney control of K in daily clinical practice in pre-dialysis patients with advanced CKD, shows that the main determinants of the maintenance of serum K levels are: the load of K (intake and absorption less extrarenal excretion) relative to renal function, and FEK, the latter as an expression of the degree of adaptive compensation. In addition, serum bicarbonate was also shown as an independent determinant of HK and serum K levels in the patients studied.
The total amount of K excreted in the 24h urine reflects, in a stable clinical situation, the amount of K ingested and absorbed from the diet minus the amount excreted by the extrarenal route, mainly intestinal.7–9,11 Assuming a balance of endogenous K (i.e., K released by cell lysis equal to that used by new cells), and a constant intestinal excretion, urinary K (Ku) could be taken as an approximation of K ingested in the diet.
This parameter, here referred to as the K load, presented a normal distribution with a wide distribution range. Taking into account that the absorption of ingested K is 90%, and that the intestinal excretion in patients with CKD is approximately 15mmol/day, it can be estimated that the average intake of K in this group of patients was about 78mEq/day (3.04g).
Although the recommendations on the content of K that should have a healthy diet are about 4.7g/day,22 the measurements obtained in some studies in the general population indicate an average daily intake of K is approximately 3g,23 similar to the value estimated in our patients. Thus, the incidence of HK, which was 30%, was obtained assuming that this group of advanced CKD patients were not on a intentional dietary restriction of K or on any type of intestinal captor of K. However, HK was not strictly related to the absolute load of K, but to the K load relative to its glomerular filtration rate (Ku/ GFR).
Male patients showed a significantly greater load of both K and sodium as compared to female. This data is consistent with other studies in which the highest incidence of HK in men is attributed to a higher dietary intake of this K.23–25
With a greater K load and a significantly higher K load relative to renal function (Ku/ GFR), the incidence of HK was slightly higher in men than in women, although the difference was not significant. But, it was paradoxical that the men had a lower FEK than expected for their Ku/GFR. These results could explain the inclusion of male sex as an independent variable and significant determinant of the HK in the logistic regression model; a biological interpretation of this finding could be a more efficient extrarenal management of potassium in men than in women.
The strong linear correlation between the load of K relative to renal function (Ku/GFR) and FEK allowed us to uncover one of the main characteristics of patients with HK, as was the smaller slope of this line that could indicate the limitation of physiological process of compensation of renal excretion in patients with higher serum K concentration. In this group patients with HK the correlation between compensatory increase of FEK against high K loads was maintained, which points out that the determinants of a limitation in renal excretion of K (e.g., angiotensin inhibitors, hypoaldosteronism, etc.) act only incompletely by promoting a higher threshold of Kaliemia (chronic HK), above which there is still a capacity to increase renal excretion of K and avoid more extreme elevation of serum K levels. This finding is consistent with the physiological mechanisms of tubular handling of K,10,11,26 which can maintain an effective excretion of K even in situations of aldosterone blockade, and that despite promoting chronic HK, the serum K levels rarely reach extreme high levels.
Serum bicarbonate levels was found to be another of the main determinants of the HK. This close association is somewhat surprising because the direct pathophysiological mechanism between HK and metabolic acidosis is through redistribution of intracellular K.13,14 Such a situation occurs in cases of non-organic acid retention (mineral acidosis). A possible explanation for this finding could be the bidirectional relationship between HK and acidosis, that is, acidosis could cause a certain degree of redistribution of K, but at the same time HK could also reduce renal acidification mechanisms (decreased generation of ammonia).27 Moreover, inhibitors of the renin-angiotensin system or situations of low aldosterone secondary to low renin could also limit K excretion and development of tubular acidosis.13,14
This study has limitations. Due to the transversal and retrospective design, definite causal relationships cannot be established, and the interpretation of these static data are being extrapolated to theoretical dynamic responses in individuals. The criteria for inclusion of patients were fixed with the objective of obtaining reliable urine samples. It is possible that due to these criteria, more compliant and rigorous patients were selected which could cause selection bias. Nevertheless, the incidence of HK was similar to that observed in our ERCA population.
The assumptions of the endogenous K balance and constant K intestinal excretion in each individual may not be real, however the exclusion of patients with factors that predispose to an imbalance in K makes unlikely that this assumptions could alter the results and final estimations of this study.
In conclusion, although the K load relative to glomerular filtration rate (Ku/GFR) is a determinant of the probability of development of HK in patients with advanced CKD, the limitation of a compensatory renal excretion of K expressed as a lower FEK characterizes this electrolyte alteration. However, in these patients there could be exceptional mechanisms playing a role on K excretion that can effectively prevent extreme increase serum K levels.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Caravaca-Fontán F, Valladares J, Díaz-Campillejo R, Barroso S, Luna E, Caravaca F. Manejo renal del potasio en la enfermedad renal crónica avanzada: diferencias entre pacientes con o sin hipercaliemia. Nefrologia. 2020;40:152–159.